ISSN: 0973-7510
E-ISSN: 2581-690X
In Iraq, people prepare turshi (fermented Iraqi vegetable pickles) from different vegetables such as cucumber, turnip, eggplants, cabbage, carrot and pepper. This study investigated the effect of adding probiotic bacteria (Lactobacillus acidophilus), synbiotic (Lactobacillus acidophilus + inulin) to Iraqi turshi product, on lactic acid bacteria counts, total count, yeasts and molds, pH values, organoleptic characteristics and the antagonistic activity of turshi against the pathogenic bacteria. Lactic acid bacteria counts were higher in synbiotic turshi log 9.68 cfu/ml comparing with log 9.54 cfu/ml and log 3.97 cfu/ml for probiotic and control turshi samples at the end of study period, respectively. Total count for control sample was higher (log 6.99 cfu/ml) comparing with probiotic and synbiotic samples (log 6.90 cfu/ml and log 6.52 cfu/ml) respectively after 30 days. It was observed that yeasts and molds counts for control sample were higher (log 2.39 cfu/ml) after 30 days, while probiotic and synbiotic samples were log 1.21 cfu/ml and log 0.71 cfu/ml respectively. pH values were close for both synbiotic (3.36) and probiotic (3.73) samples, while it was higher for control sample (4.53) after 30 days. The organoleptic characteristics were more acceptable for synbiotic sample followed by probiotic and control samples, respectively. The antagonistic activities of turshi samples against Escherichia coli and Staphylococcus aureus were higher for synbiotic sample followed by probiotic and control samples, respectively. It was clear that synbiotic turshi was more desirable in all studied characteristics comparing with probiotic and control turshi and this finding reveals that synbiotic turshi could be used as a potential healthy product.
Turshi, pickles, synbiotic, probiotic, L. acidophilus, inulin.
Fermentation, one of the oldest and safest ways to conserve food (Chavan and Kadam 1989). Fermented foods provide beneficial bacteria to our digestive tract to maintain the integrity and health of the digestive system. In addition, it allows the naturally beneficial bacteria to do the fermentation through which the vegetables will develop sour taste that is acceptable and rich in vitamins. The fermentation of lactic acid is the only way to preserve all natural plant components and improve the quality, smell and taste (Bamforth, 2005).
Fermented food have different kinds such as dairy, meat and vegetables. Vegetables fermentation is not familiar commercially as the other Fermented foods since it doesn’t have standard ingredients and its composition varied depending on geographic conditions and climate. Different fermented vegetable products are produced in different places in the world such as sauerkraut. The wide spread fermented vegetables in Turkey are cucumber, olive, beet, pepper, celery root, eggplant, garlic, apple and cabbage. Turshi can be produced by using one kind of vegetables or a mixture of vegetables (Aktan et al., 1998; Erten and Tang ler, 2010)
Vegetables are a good source of antioxidants like flavonoids, vitamins, phenolic compounds, carotenoids, dietary fibres and minerals (Sun et al., 2009; Kusznierewicz et al., 2010). Lactic acid bacteria existed in pickled garlic have good inhibition ability against some food-borne pathogens (Sadeghi, 2016).
Lactic acid fermentations have widely known for years and used in various food industries such as fruit and vegetable processing, production of rye bread and fermented milk beverages (McMurtrie, 2016; Gorzelany et al., 2018; McMurtrie and Johanningsmeier, 2018).
USA regulations define pickles as a kind of low-acid food to which acid is added, water activity above 0.85 and the final equilibrium pH is 4.6 or below (Acosta et al., 2015).
Probiotic came from (Pro Bios) the Greek word that means “for life”. In ancient time the people knew the positive effect of fermented food on host health such as fermented dairy which known as an excellent medication without knowing the mechanism of its action (Neish, 2009).
Moreover, probiotic properties could be added to the product by microorganisms used in fermentation. There have been many studies found that the consumption of probiotic is useful in the treatment of lactose intolerance, immune function, cholesterol, diarrhoea, blood pressure, colon cancer, Irritable bowel syndrome, colitis, inflammation and absorption of minerals (Montalto et. al., 2006; Upadrasta and Madempudi, 2016; Levri et. al., 2005; Simons et. al., 2006; Billoo et. al., 2006; Geier et. al., 2006; Bengmark, 2007; De Preter et. al., 2011)
Many antimicrobials produced by lactic acid bacteria such as (formic, acetic, lactic acids), hydrogen peroxide, ethanol, hydrogen peroxide, reuterin and diacetyl, have the ability to stop molds spoilage. Sorbic acid, propionic acid and benzoic acid are regarded as chemical food additives and are applied in pickles preservation, so the using of probiotic vegetable fermentation could be serve as an alternative to the chemical additives preservation (Leroy and Vuyst, 2004).
Prebiotics are carbohydrates which pass through the small intestine without any digestion, after that they reach the colon and stimulate the function and the growth of beneficial bacteria, especially bifidobacteria and lactobacilli. Inulin is a chicory extract prebiotic consists of long-chain fructooligosaccharides (Vandenplas, 2002)
Inulin, fructooligosaccharides, Galactooligosaccharides and b-glucans are the main types of prebiotics, and all are nutrients for probiotics (Roberfroid, 1993).
Adding probiotics, prebiotics or synbiotics into food is desirable for intestinal microbiota and they may be consumed as raw fruit and vegetables, vegetable pickles or dairy products (Markowiak and Slizewska, 2017)
Synbiotic consists of probiotic and prebiotic and created to overcome on some difficulties in probiotics survival in gastrointestinal tract (Rioux et. al., 2005). Therefore, a suitable mixture of both probiotic and prebiotic in a single product should ensure an appropriate effect comparing to the activity of probiotic or prebiotic alone (Bengmark, 2005; Panesar et al., 2009)
Study aims to investigate the effect of adding probiotic and synbiotic properties to Iraqi turshi during storage period and improving the organoleptic characteristics of the product.
Probiotic Bacteria
L. acidophilus strain was purchased from LGGTM (Finland).
Prebiotic
Inulin was purchased from NOW Foods products (USA).
Production of Iraqi Turshi
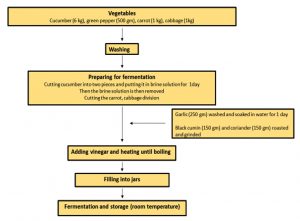
Cucumber, green pepper, carrot and cabbage, garlic, black cumin, coriander and vinegar were purchased from Basrah local market. The Iraqi traditional method was used in turshi production as shown in Fig. 1. After completing the production of Iraqi turshi, it was divided into 3 jars. First jar is a control, L. acidophilus (109 cfu/g) 1% w/v was added into the second jar (probiotic), and L. acidophilus (109 cfu/g) 1% w/v and inulin 1% w/v was added into the third jar (synbiotic).
Microbiological analysis
Lactic acid bacteria (LAB), total count, yeasts and molds counts were evaluated during storage periods (0, 15 and 30 days). Ten milliliters of turshi sample transferred aseptically into 90 ml of 0.1% peptone plus 0.85% sodium chloride solution and homogenized. Serial dilutions were made. The enumeration of LAB (MRS agar) at 37°C for 24-48 hrs. anaerobically (Harrigan, 1998), total count (Nutrient agar) at 37°C for 24-48 hrs., yeast and mold (PDA agar ) at 25°C for 5 days (Tournas et. al., 1998).
Determination of pH
The pH of turshi samples were measured by using pH meter (Pye Unicam-Model 292MK. England).
Organoleptic Characteristics
For Organoleptic Characteristics, ten specialists from food science department evaluated turshi products according to Rajablou et al. (2012) for taste, color, texture and over all acceptability.
Antagonistic activity of turshi (pickled vegetables)
The antagonistic activity of turshi samples against Escherichia coli and Staphylococcus aureus was done by using the agar well diffusion assay on Mueller Hinton agar (Herreros et al., 2005). Each indicator bacteria were spread and then turshi sample (vinegar) were added to 5-mm diameter well on these Petri dishes. The indicator bacteria counts were the same (100µl, 108 cfu/ml) prepared from broth culture of bacteria according to 0.5 McFarland standard. The inhibition zone diameter of turshi samples was measured after 48 hrs. of an aerobic incubation at 37°C.
Statistical analysis
Triplicate Complete Randomized Design was used in data analysis of the studied characteristics by using SPSS (2009) version17. The averages were compared using less significant difference test rate within the program.
Microbiological content of turshi vegetables pickled
Results obtained in Table 1 show that LAB counts in control sample were low during the periods of the study which ranged between log (3.97-4.58) cfu/ml in contrast with LAB counts in probiotic and synbiotic samples which were higher at zero-time log (9.82 and 9.87) cfu/ml respectively, decreased after 15 days, and reduced a little after 30 days of the study period to reach log (9.54 and 9.68) cfu/ml respectively. Synbiotic sample was higher after 30 days in LAB counts over probiotic and control samples respectively and this may be attributed to the availability of inulin which provided a suitable environment to the growth of LAB on the account of other bacteria.
Table (1):
Microbiological content of Control, Probiotic and Synbiotic turshi samples.
| LAB | Total Count | Yeast & Mold | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | 0 | 15 | 30 | 0 | 15 | 30 | 0 | 15 | 30 |
| Control turshi | 4.58 | 4.31 | 3.97 | 7.27 | 7.07 | 6.99 | 1.75 | 2.28 | 2.39 |
| Probiotic turshi | 9.82 | 9.74 | 9.54 | 7.45 | 7.24 | 6.90 | 1.79 | 1.52 | 1.21 |
| Synbiotic turshi | 9.87 | 9.81 | 9.68 | 7.44 | 7.14 | 6.52 | 1.77 | 1.44 | 0.71 |
| LSD | 5.24 | 0.07 | 0.15 | 0.01667* | .07333* | .08667* | NS | 0.073 | 0.49333* |
Total count as shown in Table 1 indicates that probiotic sample at zero-time was higher log (7.45) cfu/ml followed by synbiotic sample log (7.44) cfu/ml and control sample log (7.27) cfu/ml respectively. After 15 days, a high reduction happened in synbiotic and probiotic samples. Synbiotic sample was the higher in its total count reduction followed by probiotic sample and control sample respectively. At the end of the study period (30 days) there was a sharp reduction in synbiotic sample total count log (6.52) cfu/ml and probiotic sample log (6.90) cfu/ml, and there was a slight reduction in control sample log (6.99) cfu/ml, this may be attributed to the activity of LAB in synbiotic and probiotic samples and the support of inulin in synbiotic samples to produce acids and by-products which inhibited the growth of undesirable microorganisms. Yeast and molds counts as shown in table (1) were close in all study samples at zero-time. The counts increased gradually in control sample to reach log 2.39 cfu/ml at the end of the study period, while it decreased in probiotic and synbiotic samples to reach log (1.21 and 0.71) cfu/ml respectively at the end of the study period. The growing of LAB and producing acids reduced the pH and affected negatively on the growth of yeasts and molds.
The viability and activity of probiotic bacteria during preparation and storage are very important for their industrial applications. They can be added to probiotic products as fresh or lyophilized cells. High population levels, between 106-108 microbial cells/ml should be present in probiotic products. It should be also explained that during food processing, bacteria subjected to stress conditions such as freezing, drying and concentration stress and these are also decreasing the viability of LAB (Kos et al., 2008).
Both probiotic and synbiotic turshi samples of this study are considered as probiotic products as the probiotic levels were log (9.54 – 9.68) cfu/ml respectively, and this agreed with Beganovic et. al. (2011) who showed that L. plantarum L4 and L. mesenteroides LMG 7954 strains in sauerkraut pickles are considered as probiotic products because probiotic cells in final product was determined higher than 106 cfu/g. Same results reported by ַetin (2011) who found that the product kept its probiotic level during the 60-day storage period.
The addition of probiotic bacteria affected positively on the LAB counts and decreased the counts of total bacteria, yeasts and molds and these results agreed with etin (2011) who reported that L. plantarum had a positive effect on LAB counts in turshi samples .
Turshi (Pickled vegetables) pH value
The fermentation process’ success depends on the pH which gives an indicator to the fermentation. pH values in Table 2 show that all samples were close in pH values at zero-time. There was a clear decrease in synbiotic and probiotic samples after 15 and 30 days while there was a slight decrease in control sample pH values. The study results agreed with Blanc (1996) who found that the pH was high at the beginning of the study period and because of acid formation, dropped quickly. The results came close with Pundir and Jain (2010) who reported that sauerkraut brine pH ranged between (3-4) and the pH showed a decreasing trend from the day of preparation till the end of preservation period.
Table (2):
pH of Control, Probiotic and Synbiotic turshi samples.
| pH | |||
|---|---|---|---|
| Day Sample | 0 | 15 | 30 |
| Control turshi | 5.533 | 4.833 | 4.533 |
| Probiotic turshi | 5.433 | 4.633 | 3.73 |
| Synbiotic turshi | 5.467 | 4.267 | 3.367 |
| LSD | NS | 0.2000* | 0.3667* |
pH values of this study were in contrast with ַetin (2011) who noticed that there was a similar change in pH values between probiotic and control turshi samples during the storage period, he noticed at the end of storage period that the pH levels of both samples increased. Susilowati et al. (2018) found that the lowest pH value was observed in pickled ginger prepared using 2.5% salt for 10 days was (3.33) and this result was close to our study result for synbiotic turshi after 30 days.
The rapid decrease in turshi pH for probiotic and synbiotic samples was because of LAB (probiotic) throughout the production of lactic acid as a main catabolite (Giraffa et al., 2010).
Organoleptic Characteristics
Results of organoleptic characteristics illustrated in table (3) show that synbiotic sample was more acceptable followed by probiotic and control samples respectively. This may be attributed to the taste, flavor and odor produced by probiotic bacteria (L. acidophilus) added to the probiotic and synbiotic turshi samples, and inulin which provided a suitable substrate to LAB. Results came in agree with etin (2011) who observed that the organoleptic value of the samples had been improved by adding of L. plantarum which increased desired flavour, taste and odour of turshi.
Table (3):
Organoleptic Characteristics of Control, Probiotic and Synbiotic turshi samples.
Taste |
Colour |
Texture |
Over all acceptability |
|
|---|---|---|---|---|
Control turshi |
7.50 |
6.90 |
7.00 |
7.80 |
Probiotic turshi |
8.70 |
7.50 |
7.90 |
8.40 |
Synbiotic turshi |
9.10 |
7.80 |
8.30 |
8.70 |
LSD |
1.20 |
NS |
0.89 |
NS |
Antagonistic activity of turshi
All turshi samples showed antimicrobial activity against potentially pathogenic gram-negative and gram-positive bacteria (E. coli and S. aureus). This activity may be attributed to bacteriocin production by LAB (Giraffa et al., 2010).
The highest antimicrobial activity was for synbiotic turshi sample followed by probiotic and control turshi samples respectively as shown in Table 4.
Table (4):
Antagonistic activity (mm) of Control, Probiotic and Synbiotic turshi samples against E. coli and S. aureus.
E. coli |
S. aureus |
|
|---|---|---|
Control Pickled |
12.40 |
11.06 |
Probiotic Pickled |
12.90 |
11.36 |
Synbiotic Pickled |
13.33 |
11.60 |
LSD |
0.50 |
0.30 |
Fermented pickled vegetables have a high microbial load since it could be a vector in transporting pathogenic bacteria from the farm and can’t be pasteurized. So, LAB could reduce the number of undesirable microorganisms in these vegetables (Tamang et. al., 2009).
Adding a protective culture is considered as a safety factor guaranties the stability of food microorganisms and reduces the risk of growing pathogenic and food spoilage microoganisms (Holzapfel et al., 1995; Inatsu et al., 2005; Rabie et. al., 2011).
The results of this study showed that probiotic and synbiotic turshi samples have probiotic product properties in regard of LAB counts, more desirable organoleptic characteristics and good antagonistic activity against the pathogenic bacteria, These data lead to potential probiotic and synbiotic products that have the ability to be a good environment and carrier for the beneficial probiotic bacteria and keeping them for a long time comparing with dairy or other products which suffer from deterioration because the bad preservation methods.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SGA and WAS conceived and designed the analysis, collected the data, Contributed data or analysis tools. SGA and AAH performed the analysis. SGA wrote the manuscript. All authors read and approved the manuscript for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
The authors confirm that the data supporting the findings of this study are available within the article.
- Acosta, O.G., Vermeylen, F.M., Noel, C. and Padilla-Zakour, O.I. Modelling the effects of process conditions on the accumulated lethality values of thermally processed pickled carrots. Food Control, 2015; 51: 390-396.
Crossref - Aktan, N., Y cel, U. and Kalkan, H. Tursu Teknolojisi (Pickled Technology), Ege University Press, ׀zmir, Turkey, 1998.
- Bamforth, C.W. Introduction. Food, Fermentation and Microorganisms, Blackwell Publishing, UK, 2005; 14-16.
Crossref - Beganovic J., Pavunc A.L., Gjuracic K., Spoljarec M., Suskovic J. and Kos B. Improve sauerkraut production with probiotic strain Lactobacillus plantarum LA and Leuconostoc mesenteroides LMG 7954. J. Food Sci., 2011; 76(2): 124-129.
Crossref - Bengmark, S. Bioecological control of inflammatory bowel disease. Clin. Nutr., 2007; 26: 169–181.
Crossref - Bengmark, S. Bioecological control of the gastrointestinal tract: The role of flora and supplemented probiotics and synbiotics. Gastroenterol. Clin. N. Am., 2005; 34: 413–436.
Crossref - Billoo, A.G., Memon, M.A., Khaskheli, S.A., Murtaza, G., Iqbal, K., Saeed Shekhani, M., and Siddiqi, A.Q. Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhoea. World J. Gastroenterol., 2006; 12: 4557–4560.
Crossref - Blanc, P.J. Characterization of tea fungus metabolites. Biotechnol. Letters, 1996; 18(2): 139-142.
Crossref - ַCetin, B. Production of probiotic mixed pickles (Tursu) and microbiological properties. African Journal of Biotechnology, 2011; 10(66).
Crossref - Chavan, J.K. and Kadam, S.S. Critical reviews in food science and nutrition. Food Sci., 1989; 28: 348–400.
Crossref - De Preter, V., Hamer, H.M., Windey, K. and Verbeke, K. The impact of pre- and/or probiotics on human colonic metabolism: Does it affect human health? Mol. Nutr. Food Res., 2011; 55: 46–57.
Crossref - Erten, H. and Tang ler, H. Fermente Bitkisel r nler (in Gda Biyoteknolojisi Ed by Necla Aran), Nobel Yayn ve Datm Inc., Ankara, 2010.
- Geier, M.S., Butler, R.N. and Howarth, G.S. Probiotics, prebiotics and synbiotics: A role in chemoprevention for colorectal cancer? Cancer Biol. Ther., 2006; 5: 1265–1269.
Crossref - Giraffa, G., Chanishvili, N. and Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol., 2010; 161: 480-487.
Crossref - Gorzelany, J., Migut, D., Matlok, N., Balawejder, M., Kacaniova, M. The impact of the cucumber fruit preparation on their mechanical properties and microbial status of the souring brine during souring procedure. Econtechmod, 2018; 07(1): 11–17.
- Harrigan, W.F. Laboratory methods in food microbiology, (3rd Ed.), San Diego, California, USA, 1998.
- Herreros, M.A., Sandoval, H., Gonzalez, L., Castro, J.M., Fresno, J.M. and Tornadijo, M.E. Antimicrobial activity and antibiotic resistance of lactic acid bacteria isolated from Armada cheese (a Spanish goats’ milk cheese). Food Microbiology, 2005; 22: 455-459.
Crossref - Holzapfel, W.H., Geisen, R. and Schillinger, U. Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes.Int. J. Food Microbiol., 1995; 24: 343-362.
Crossref - Inatsu, Y., Bari M.L., Kawasaki, S. and Kawamoto, S. Effectiveness of Some Natural Antimicrobial Compounds in Controlling Pathogen or Spoilage Bacteria in Lightly Fermented Chinese Cabbage. J. Food Sci., 2005; 70(9): 393-397.
Crossref - Kos, B., Suskovic, J., Beganovic, J., Gjuracic, K., Frece, J., Iannaccone, C. and Canganella, F. Characterization of the three selected probiotic strains for the application in food industry. World Journal of Microbiology Biotechnology, 2008; 24: 699-707.
Crossref - Kusznierewicz, B., lewandowska, J., kruszyna, A., piasek, A., smiechowska, J., namiesnik, A. and bartoszek, A. The antioxidative properties of white cabbage (brassica oleracea var. Capitata f. Alba) fresh and submitted to culinary processing. J. Food Biochem., 2010; 34: 262-285.
Crossref - Leroy, F. and Vuyst, L.D. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol., 2004; 15: 67-78.
Crossref - Levri, K.M., Ketvertis, K., Deramo, M., Merenstein, J.H. and D’Amico, F. Do probiotics reduce adult lactose intolerance? J. Family Pract., 2005; 54: 613–620
- Markowiak, P. and Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Health. Human Nutrients, 2017; 9(9): 1021.
Crossref - McMurtrie, E.K. Quality of cucumbers fermented in acidified and non-acidified calcium chloride brines for reduced environmental impact of brining operations. Thesis of Master degree, North Carolina State University, Department of Food, Bioprocessing and Nutrition Sciences, 2016.
- McMurtrie, E.K. and Johanningsmeier, S.D. Quality of cucumbers commercially fermented in calcium chloride brine without sodium salts. Journal of Food Quality, 2018; 13.
Crossref - Montalto, M., Curigliano, V., Santoro, L., Vastola, M., Cammarota, G., Manna, R., Gasbarrini, A. and Gasbarrini, G. Management and treatment of lactose malabsorption. World J. Gastroenterol., 2006; 12: 187.
Crossref - Neish, A.S. Microbes in gastrointestinal health and disease. Rev. Bas. Clin. Gastroenterol., 2009; 136: 65–80.
Crossref - Panesar, P.S., Kaur, G., Panesar, R. and Bera, M.B. Synbiotics: Potential Dietary Supplements in Functional Foods; IFIS: Berkshire, UK, 2009.
- Pundir, R.K. and Jain, P. Changes in microflora of sauerkraut during fermentation and storage. World J. Dairy and Food Sci., 2010; 5(2): 221-225.
- Rabie, M.A., Siliha, H., Saidy, S., Badawy, A.A. and Malcata, F.X. Reduced biogenic amine contents in sauerkrautvia addition of selected lactic acid bacteria. Food Chem., 2011; 129: 1778-1782.
Crossref - Rajablou, S., Aminafshar, M., Jamalifar, H. and M.R F. Make pickles probiotic with using strain Lactobacillus plantarum native. Journal of Food Technology & Nutrition, 2012; 9(2): 65-72.
- Rioux, K.P., Madsen, K.L. and Fedorak, R.N. The role of enteric microflora in inflammatory bowel disease: Human and animal studies with probiotics and prebiotics. Gastroenterol. Clin. N. Am., 2005; 34: 465–482.
Crossref - Roberfroid, M. Dietary fiber, inulin, and oligofructose: a review comparing their physiological effects. Crit. Rev. Food Sci. Nutr., 1993; 33: 103-48.
Crossref - Sadeghi, A. In vitro Assessment of Some Probiotic Properties of Lactobacillus fermentum Isolated from Pickled Garlic. Journal of Food Quality and Hazards Control, 2016; 3: 67-72.
- Simons, L.A., Amansec, S.G. and Conway, P. Effect of Lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutr. Metab. Cardiovasc. Dis., 2006; 16: 531–535.
Crossref - SPSS. (SPSS) statistical package for window ver. 17. Chicago: SPSS, Inc., 2009.
- Sun, Y.P., Chou, C.C. and Yu, R.C. Antioxidant activity of lactic-fermented Chinese cabbage. Food Chem., 2009; 115(3): 912-917.
Crossref - Susilowati, S., Laia, S. and Purnomo, H. The effect of salt concentration and fermentation time on pH value, total acidity and microbial characteristic of pickled ginger (Zingiber officinale Rosc.). International Food Research Journal, 2018; 25(6): 2301-2306.
- Tamang, J.P., Tamang, B., Schillinger, U., Guigas, C. and Holzapfel, W.H. Functional properties of lactic acid bacteria isolated from ethnic fermented vegetables of the Himalayas. Int. J. Food Microbiol., 2009; 135(1): 28-33.
Crossref - Tournas, V., Stack, M.E., Mislivec, P.B., Koch H.A. and Bandler, R. Yeasts, molds and mycotoxins (Chapter 18), Bacteriological Analytical Manual (BAM, 8th edition), Food and Drug Administration, Gaithersburg, MD, USA, 1998.
- Upadrasta, A. and Madempudi, R.S. Probiotics and blood pressure: Current insights. Integr. Blood Press. Control, 2016; 9: 33–42.
Crossref - Vandenplas, Y. Oligosaccharides in infant formula. Br. J. Nutr.; 2002; 87: 293-6.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.