ISSN: 0973-7510
E-ISSN: 2581-690X
Lovastatin is a naturally produced 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase enzyme inhibitor- used for treating hypercholesterolemia. It was the first statin drug which was approved by the United States Food and Drug Administration (USFDA). In the current study, endophytic fungus Fusarium nectrioides (MH173849) isolated from Euphorbia hirta L. was used for the production of lovastatin. Four different culture media indicated as M1, M2, M3 and M4 were used for the initial production of lovastatin. Liquid cheese whey was used as nitrogen source. Growth morphology of fungi was investigated using Scanning Electron Microscopy analysis. Also, parameters like temperature, pH, inoculum size, incubation time, and RPM were optimized for the obtaining highest lovastatin production. Among the four media, M4 was found to produce the maximum concentration of lovastatin. Parameters such as temperature of 28°C, pH 6, RPM – 180 rpm and inoculum size of 5 x107 spores/mL were optimal for the production of lovastatin by F. nectrioides (MH173849).
Fusarium nectrioides, Lovastatin, Endophytic Fungus, Euphorbia hirta (Linn), Liquid Cheese Whey, Optimization
Diseases like hypercholesterolemia, i.e., increased blood cholesterol levels, have been on the rise due to changes in the diet and lifestyle across the globe. Elevated cholesterol levels in the blood pose a significant threat as it increases the likelihood of a person developing atherosclerosis and ischemic heart disease in the long run. Research indicated that it could also increase the chances of getting diseases such as dementia, Alzheimer’s, Ischemic heart stroke, obesity, diabetes, and certain cancers. The liver predominantly synthesizes cholesterol in a series of more than 25 enzyme-driven steps, and the rest is derived from the food we consume.9,12 Biosynthesis of cholesterol in the body takes place through the mevalonic acid pathway. The predominant enzyme targeted for controlling the cholesterol level is 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting and regulatory enzyme in cholesterol biosynthesis. The group of compounds that inhibit the enzyme HMG-CoA reductase is the statin group of molecules.9,32
Lovastatin is a statin group of a drug molecule and was the first anti-cholesterol drug approved by the United States Food and Drug Administration (USFDA). It is involved in the inhibition of cholesterol biosynthesis. Lovastatin also works as an in vivo competitive inhibitor of HMG-CoA reductase for humans and animals as well as in plants and is hence used to treat hypercholesterolemia2,9,10,19,20,31,32 Lovastatin is available in the lactone form but converted to β-hydroxy acid invivo, the active state. Also, Lovastatin is the precursor of simvastatin.2 Studies have shown that lovastatin can also increase good cholesterol or high-density lipoproteins in the blood, thereby preventing plaque formation.19 Apart from its anti-cholesterol activity, lovastatin has anti-inflammatory, anti-cancer, neuroprotective, antimicrobial properties. Studies have indicated that it could be effective against osteoporosis, Alzheimer’s disease, and multiple sclerosis.20,32
Literature has shown that fungal fermentation has yielded several biologically important active metabolites. Several fungal species can produce lovastatin by solid-state, liquid surface, and submerged fermentation. Lovastatin is produced intracellularly and accumulates in the mycelia. Fugal species such as Aspergillus terreus, Aspergillus flavus, Penicillium spp., Monascus ruber, Monascus purpureus, Trichoderma spp are commonly employed for the production of lovastatin. Also, Scopolariopsis, Paecilomyces, Phoma, Gymnoascus, Doratomyces, Pleurotus, Phythium, and Hypomyces have shown potential to produce lovastatin. Mushrooms like Agaricus bisporus, Pleurotus ostreatus, and P. citrinopileatus have been used to produce lovastatin.2,9,12,30,31
Endophytic fungi exist in the tissues of living plants and aid in the growth of plants. These fungi protect the plant from biotic and abiotic stresses and don’t cause any harm to the plants. In turn, the plant provides these fungi with nutrition and shelter. Endophytic fungi produce secondary metabolites and bioactive compounds that are structurally unique with diverse pharmacological properties. So, research has been focused on producing such bioactive products from endophytes so that it may be used in the treatment of diseases3,6,7,13,15,18,21,26 In the present study, we did a preliminary investigation on the potential of endophytic fungus Fusarium nectrioides for the production of lovastatin.
Isolation of Fusarium nectrioides (MH173849) from E. hirta
Fusarium nectrioides (MH173849), an endophytic fungus was isolated from the medicinal plant, Euphorbia hirta. Fusarium nectrioides was investigated for lovastatin production. The isolated pure cultures were maintained in potato dextrose agar culture medium (PDA).
Inoculum Preparation
Mycelial agar plug (1cm x 1cm) of F. nectrioides was taken from PDA plates. It was then suspended in 5ml of saline containing 0.2% Tween 80 solution and was filtered using Whatmann (No.1) filter paper (Dobranic et al. 2011). Filtered mycelium suspension (5×107spores mL-1) was utilized as an inoculum for the fermentation process. 1mL of spore suspension was inoculated in the seed media. Seed media was prepared using Glucose – 50 g/L, Yeast extract – 20 g/L, Magnesium sulphate – 0.04 g/L and Lactose – 25 g/L. The pH of the media was maintained at pH 6. The inoculated flasks were incubated at 28˚C for 40 hrs in a shaking incubator at 180 rpm.1,28
Production Medium
In order to study the production of lovastatin, F. nectrioides was inoculated in four different media. The four media are Potato Dextrose Broth (M1), Potato Dextrose Broth with Liquid Cheese Whey (M2), Modified media (M3) and modified media with Liquid Cheese Whey (M4). Whey is a liquid by-product generated from cheese manufacturing. It is composed of approximately 0.3% fat, 0.8% protein, 4.9% lactose, and 0.5% minerals. Liquid Cheese Whey is used as both carbon and nitrogen source. Spore inoculum in seed media (5×107 spore per mL) was inoculated in the M1-M4 production media in triplicates under sterile condition. The pH of the media was maintained at pH 6. All the flasks were incubated at 28°C at 180 rpm for 15 days.4,8,11,14
The composition of the four media used are as follows:
- M1 media – Potato Dextrose Broth – 250 g of peeled, sliced potatoes were boiled in 1 L distilled water for 30 min and filtered afterwards. With the 1 L of filtrate, 20g of dextrose was added.
- M2 media – Potato Dextrose Broth with Liquid Cheese Whey – 20g of acidified Liquid Cheese Whey was added to M1 medium.
- M3 medium – Modified Media -contained: glucose (20g/L), lactose (20g/L), yeast extract (20g/L), histidine (4g/L), potassium di hydrogen phosphate (4g/L), Magnesium Sulphate (0.4g/L), Calcium chloride (0.4g/L), Ferrous sulphate heptahydrate (0.2g/L) was dissolved in 1000ml distilled water.
- M4 medium – Modified Media with Liquid Cheese Whey – Lactose from M3 medium was re-placed with Liquid Cheese whey.
Extraction and Detection of Lovastatin
Fermentation of the F. nectrioides in the four different media was carried out for about 16 days. The media was filtered using sterile filter paper to separate the mycelia from the media. Then, equal volumes of ethyl acetate were added to the filtered media, and the solution was acidified from pH 6 to pH 2. The solution was kept in a shaker at 100 rpm for 2hrs at room temperature. After the extraction cycle, the mixture was centrifuged at 1500 rpm for 20 min, and the organic phase was collected. It was then concentrated using a rotary evaporator. The extract was then analysed for lovastatin production using UV spectrometry at 238nm.33
SEM Analysis of Fungal Growth on Four Media
The various growth morphology of F. nectrioidesin the four different culture media M1, M2, M3 and M4 were investigated using a Scanning Electron Microscope (SEM). Biomass separated from the culture broth by centrifugation was washed with sterile distilled water and stored in lyophilizer at -20°C. Lyophilized samples were dissolved in dimethyl sulfoxide (DMSO) and used for analysis.
Effect of Culture Conditions (Temperature, pH, Inoculum Size, Incubation Time, and RPM) on Fungal Growth and Lovastatin Production
Based on preliminary analysis, Modified media with liquid cheese whey (M4) gave a high yield of lovastatin. This was then chosen for further optimization studies. Effect of different temperatures, pH, inoculum size, incubation time, and RPM in the shaker flask level fermentation was studied to enhance the Lovastatin production.22
Production, Extraction, and Confirmation of Lovastatin
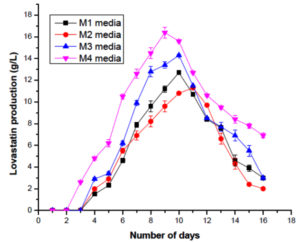
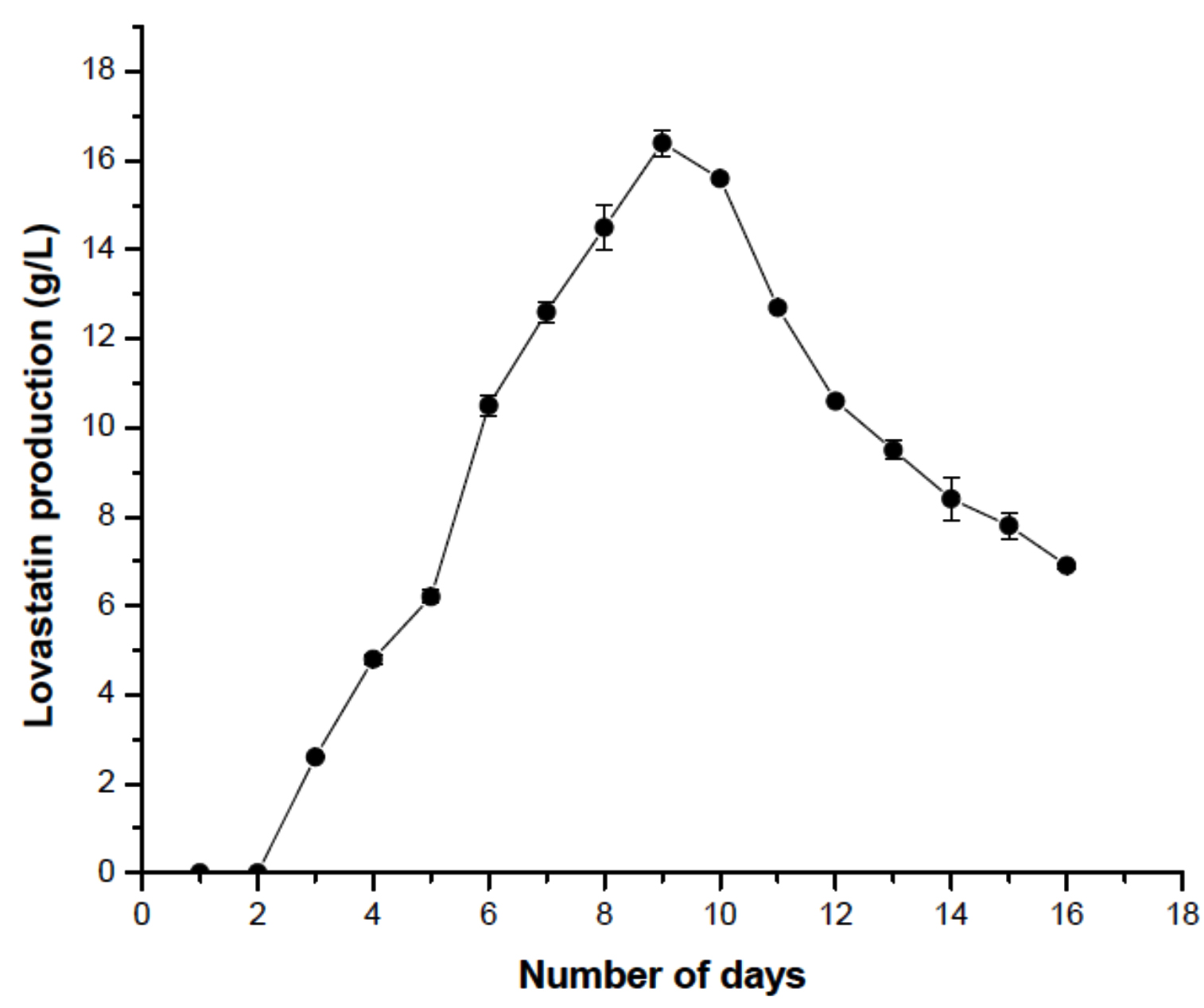
Fusarium nectrioides isolated from Euphorbia hirta were investigated for the production of Lovastatin. The pure cultures maintained in PDA were initially inoculated in the seed medium. The F. nectrioides were inoculated in four different media from the seed media, i.e., M1 –M4. The cultures were allowed to grow in the production media for about 16 days, and the production was monitored till the 16th day of fermentation. After which, ethyl acetate was added to the fermentation broth to extract lovastatin. After the extraction, the presence of lovastatin was confirmed in all four media by UV spectrophotometry at 238nm.33 The quantitative estimation was done by comparing the values with the lovastatin standard curve. In M1 and M3 media, the maximum concentration of lovastatin produced was 12.7 g/L and 14.3 g/L, respectively, on the 10th day of incubation. In M2 media, maximum lovastatin production was observed on the 11th day when titers reached 11.3 g/L lovastatin. Among the four media, the maximum production was observed in M4 media, which yielded 16.4 g/L lovastatin on the 9th day of incubation. So lovastatin production was focused on M4 media containing liquid cheese whey for further optimization studies (Figure 1).
SEM Analysis of Fungal Growth on Four Media
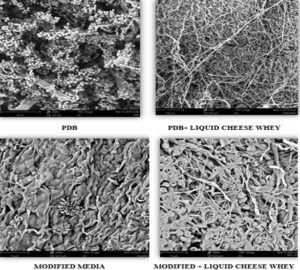
The morphological growth pattern of F. nectrioides in all the four culture media was analysed under the Scanning electron microscope and is represented in Figure 2. Compared to other media, M4 media showed dense growth of fungus and higher biomass which designates that the components of modified media, in combination with liquid cheese whey supported the fungus in terms of their growth and product formation.
Effect of Culture Conditions on Fungal Growth and Lovastatin Production
Growth of fungus and lovastatin production was observed in the M4 culture medium. Specific parameters of M4 culture media such as: temperature, pH, RPM, inoculum size, and the incubation period were monitored. Based on the optimization studies, maximum lovastatin production was observed at a temperature of 28°C, pH of 6, RPM of 180, inoculum size of 5 x107 spores/mL on the 9th day.
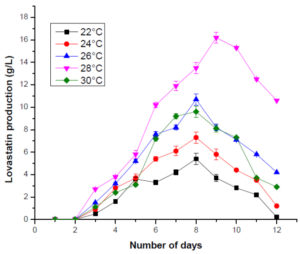
For optimizing the temperature, the temperature of M4 media varied between 22°C and 30°C. The maximum production of lovastatin was observed at 28°C on the 9th day. Literature suggests that when the temperature in the production media was maintained outside the optimum temperature range, it impacted the metabolic activities of the microorganisms. At temperatures lower or higher than the optimum temperature, the yield of the lovastatin was low. The decreased production of lovastatin at high temperatures could be attributed to the inactivation or the denaturation of lovastatin, but not only. Also, when the incubation period is prolonged, lovastatin production decreases. This might be possibly due to the cultures reaching the end-point of fermentation. These results coincide with previously published data on lovastatin production by Monascu sruber, Monascuspurpureus and Aspergillus terreus5,23,24,25,27(Figure 3).
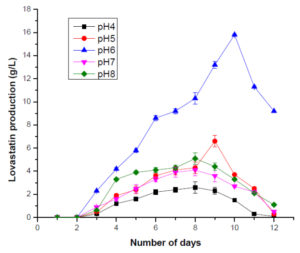
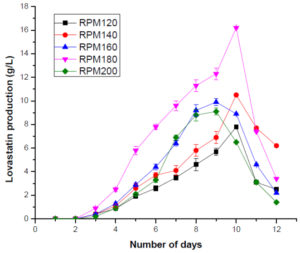
For optimizing the pH, the pH of M4 media ranged between 4 and 8. The maximum production of lovastatin was observed at pH=6 on the 11th day. pH plays an important role in sustaining all biochemical processes and therefore, it can affect the growth of fungi and its capability to produce metabolites. pH has a major influence on the production of lovastatin. Literature suggests that pH between 5.8 and 7.5 are optimal for the production of lovastatin whereas the acidic pH of the media inhibits lovastatin production. The pH that was used in the current study i.e., pH 6 aids in the production of lovastatin (Figure 4). The rpm of M4 media was varied between 120 and 200 rpm to optimize the rpm. The maximum production of lovastatin was observed at 180 on the 11th day. It was observed that the rpm less than 180 provided insufficient oxygen to the fungal cells. When the rpm is increased, the cells might have experienced shear stress thereby there, a decrease in the production of lovastatin was observed (Figure 5).
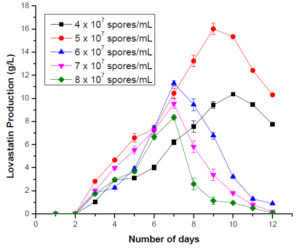
The inoculum concentration plays an essential role in lovastatin production. The maximum lovastatin production was observed at 5 x 107 spores/mL on the 9th day. As the inoculum size increased, a decrease in lovastatin production was observed. An increase in the inoculum size may have shifted the dynamics towards growth rather than lovastatin production. It was observed that when the inoculum concentration was more, the lovastatin production was around the 7th day. But the yield of lovastatin was low (Figure 6). To estimate the optimum incubation period, fermentation flasks were incubated for 16 days. The production of lovastatin was observed from day 2 of incubation. A steady increase in lovastatin production was observed up to day 9; after that, a decline in lovastatin production was seen. They indicated that 9 days of incubation was sufficient for the maximum lovastatin production. Previous reports indicated that incubation between 6 and 10 days was optimum for lovastatin production by different fungi (Figure 7).
For enhancing the production of lovastatin, four different culture media have been formulated by replacing the lactose with a Liquid cheese Whey as one of the carbon sources, and the morphological growth variations of F. nectrioides were investigated using SEM analysis. In addition to the morphological variations, the culture conditions of the chosen fungi in medium M4 were found to be best at a temperature of 28°C, pH of 6, and RPM of 180 for the growth of F. nectrioides (MH173849). Due to its wide applications in medicinal fields, demand for this compound increased many folds and required a strong science base, cheap raw materials, and effective fungal isolates to meet the requirement for commercial production. So, the novel idea of utilizing an industrial waste, such as Liquid Cheese Whey has been emerged for the cost-effective production of lovastatin at large scale using F. nectrioides.
Lovastatin is a medication given to patients to manage high cholesterol levels. It exists in two forms. The active form of lovastatin is an open-ring β-hydroxy acid and the inactive form is the closed-ring β-lactone form. The properties of these two forms i.e. pharmaceutical and physicochemical properties are different. In general, the filamentous fungi secretes the lovastatin in the active open-ring β-hydroxy acid form in the culture media. But the lactone form of lovastatin is beneficial for quantification analyses. So, β-hydroxy acid form needs to be converted to lactone form. This is achieved by reducing the pH and lactonization form. Therefore, producing lovastatin in an acidic medium is required.37
The physical and chemical parameters that are maintained in growth media conditions have an effect on the production of secondary metabolites by the fungi. In general, the synthesis of secondary metabolites occurs in the late log phase or the stationary phase of the growth. This is owing to the fact that the essential nutrients get depleted in the late log phase. It cannot secrete or synthesize at the early growth stage of fungi. Also, the secretion of accumulated metabolites into the surrounding medium is necessary. The lovastatin production is highly influenced by slow metabolizing carbon sources like lactose, glycerol, and fructose when compared to glucose.34-36 Fungi produce lovastatin as a secondary metabolite. Lovastatin production in fungi uses the pathway which utilizes slow metabolizing carbon. Whereas, it uses carbon sources like glucose biomass production. Thus, lactose used in the medium favors the production of lovastatin.37
In the literature, many have reported on the production of lovastatin. Jaivel and Marimuthu, reported the production of lovastatin by A. terreus (JPM3).38 They have reported that A. terreus (JPM3), yielded 138.4 mg L−1 lovastatin. In another study, Pecyna and Bizukojc analysed lovastatin yield in submerged fermentation using the lactose-to-glycerol ratio. The yield of lovastatin was about 161.8 mg L−1.39 Sridevi and Charya, reported the isolation and production of lovastatin by A. terreus KSVL-SUCP-75.40 The maximum production of lovastatin of 360 mg L−1 was observed. A. terreus KPR12 produced maximum lovastatin yield of 450.79 mg L−1 which was reported by Srinivasan et al.37 Mahmoud and Hadi reported Laetioporus sulphureus which was reported to produce maximum lovastatin of about 280μg/ mL.16 Pushpa et al. reported of lovastatin production from various fungi as Shizophyllum commune and Pleurotus ostreatus which produced 38μg/ mL and 30μg/mL, respectively.33
From this study, culture medium M4 was found to work best at a temperature of 28°C, pH of 6 and RPM of 180 for the growth of F. nectrioides (MH173849). Hence, an industrial waste, liquid cheese whey could be used as one of the carbon and nitrogen source for the production of Lovastatin. Further studies need to be performed to further scale up the production.
ACKNOWLEDGMENTS
The authors are thankful to the Management of Bannari Amman Institute of Technology, Sathyamangalam for providing the necessary facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C. Mevinolin A Highly Potent Competitive Inhibitor of Hydroxymethylglutaryl-coenzyme A Reductase and a Cholesterol-lowering Agent. Proc Natl Acad Sci.1980;77(7):3957-3961.
Crossref - Al-Saman MA, Helmy MA, Abdella A, Wilkins MR, Gobba NA,Mahrous H. Optimization of lovastatin production by Aspergillus terreus ATCC 10020 using solid-state fermentation and its pharmacological applications.Biocatal Agric Biotechnol. 2021;31:101906.
Crossref - Batista BN, Matias RR, Oliveira RLE, Albuquerque PM. Hydrolytic enzyme production from acai palm (Euterpe precatoria) endophytic fungi and characterization of the amylolytic and cellulolytic extracts. World J Microbiol Biotechnol. 2022;38(2):30.
Crossref - Casas Lopez JL, Sanchez Perez JA, Fernandez Sevilla JM, Acien Fernandez FG, Molina Grima E,Chisti Y. Production of Lovastatin by Aspergillus terreus: effects of the C:N ratio and the Principal Nutrients on Growth and Metabolite Production. Enzyme Microb Technol. 2003;33(2-3):270-277.
Crossref - Chang YN, Lin YC, Lee CC, Liu BL, Tzeng YM. Effect of rice-glycerol complex medium on the production of lovastatin by Monascu sruber. Folia Microbiologica. 2002;47:677-684.
Crossref - Chen H, Chen J, Qi Y, et al. Endophytic fungus Cladosporium tenuissimum DF11, an efficient inducer of tanshinone biosynthesis in Salvia miltiorrhiza roots. Phytochemistry. 2022;194:113021.
Crossref - dos Santos IR, Mohamed TA, Borges LL, et al. The global tendency in the research of biological activity in endophytic fungi: a scientometric analysis. Curr Res Environ Appl Mycol. 2022;12(1):1-4.
Crossref - Dragone G, Mussatto SI, Almeida e Silva JB, Teixeira JA. Optimal Fermentation Conditions for Maximizing the Ethanol Production by Kluyveromyces fragilis from Cheese Whey Powder. Biomass Bioenerg. 2001;45(5):1977-1982.
Crossref - Easa SM, Mattar ZA, Khalaf MA, Khalil MF. Biosynthesis of Lovastatin by Gamma Irradiated Aspergillus terreus. J NuclSci Technol. 2021;9(1):19-31.
Crossref - El-Bondkly AA, El-Gendy MM, El-Bondkly A. Construction of efficient recombinant strain through genome shuffling in marine endophytic Fusarium sp. ALAA-20 for improvement lovastatin production using agro-industrial wastes. Arab J Sci Eng. 2021;46(1):175-90.
Crossref - Hajjaj H, Niederberger P, Duboc P. Lovastatin Biosynthesis by Aspergillus terreus in a Chemically Defined Medium. Appl Environ Microbiol. 2001;67(6):2596-2602.
Crossref - Hakiem AF, Mohamed NA, Ali HR. FTIR spectroscopic study of two isostructural statins: Simvastatin and Lovastatin as authentic and in pharmaceuticals. Spectrochim Acta A Mol Biomol Spectrosc. 2021;261:120045.
Crossref - Hridoy M, Gorapi M, Hossain Z, et al. Putative Anticancer Compounds from Plant-Derived Endophytic Fungi: A Review. Molecules. 2022;27(1):296.
Crossref - Karthika C, Sharmila G, Muthukumaran C, Krishnan M. Utilization of Whey Powder as an Alternate Carbon Source for Production of Hypocholesterolemic Drug by Aspergillus terreus MTCC 1281. Food Sci Biotechnol. 2013;22(5):1-7.
Crossref - Macias-Rubalcava ML, Garrido-Santos MY. Phytotoxic compounds from endophytic fungi. Appl Microbiol Biotechnol. 2022;106(3):1-20.
Crossref - Mahmoud OA, Abdel HadiSY. Extraction and Purification of Lovastatin from the Edible Mushroom Laetiporus sulphureus and its Antioxidant Activity. Egypt J Bot. 2022;62(1):169-175.
- Meng X, Fang Y, Ding M, et al. Developing fungal heterologous expression platforms to explore and improve the production of natural products from fungal biodiversity. Biotechnol Adv. 2022;54:107866.
Crossref - Nagarajan K, Ibrahim B, Bawadikji AA, et al. Recent Developments in Metabolomics Studies of Endophytic Fungi. J Fungi. 2022;8(1):28.
Crossref - Nata NA, Said FM, Shaarani SM, Nawi M, Harun N.Simulation of Lovastatin Production in Solid-State Fermentation via Oil Palm Frond. J Chem Technol Biotechnol. 2021;7(2):7-10.
Crossref - Oliveira MC, Paulo AJ, Lima CD, et al. Lovastatin producing by wild strain of Aspergillus terreus isolated from Brazil. Prep Biochem Biotechnol. 2021;51(2):164-172.
Crossref - Ortega HE, Torres-MendozaD, Caballero EZ,Cubilla-Rios L. Structurally uncommon secondary metabolites derived from endophytic fungi. J Fungi. 2021;7(7): 570.
Crossref - Ozmihci S, Kargi F. Fermentation of Cheese Whey Powder Solution to Ethanol in a Packed-Column Bioreactor: Effects of Feed Sugar Concentration.J Chem Technol Biotechnol. 2009;84(1):106-111.
Crossref - Panda BP, Javed S, Ali M. Optimization of fermentation Parameters for higher lovastatin production in Red mold rice through co-culture of Monascus purpureus and Monascus ruber. Food Bioprocess Technol. 2008;3:373-378.
- Pansuriya RC,Singhal RS. Response surface methodology for optimization of production of lovastatin by solid state fermentation. Braz J Microbiol. 2010;41:164-172.
Crossref - Pie-Lian WEI, Zhi-nan XU, Pei-Lin CEN.Lovastatin production by Aspergillus terreus in Solid-State fermentation. J Zheijan Univ. 2007;9:1521-1526.
Crossref - Rigobelo EC, Baron NC. Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology. 2022;13(1):39-55.
- Siamak M, Moazami N, Haghighi S, Mohseni F, Mirdamadi S,Bakhtiari M.Screening of lovastatin production by filamentous fungi. Iranian Biomed J. 2003;7:29-33.
- Su YC, Wang JJ, Lin TT, Pan TM. Production of the secondary metabolites g-aminobutyric acid and monacolin K by Monascus. J Ind Microbiol Biotechnol. 2003;30(1):41-46.
Crossref - Tsiantas K, Tsiaka T, Koutrotsios G, et al. On the identification and quantification of ergothioneine and lovastatin in various mushroom species: Assets and challenges of different analytical approaches. Molecules. 2021;26(7):1832.
Crossref - Upendra RS, Khandelwal P.Recent advancements in fermentation studies for lovastatin biosynthesis. Microbial Biotechnology in Food and Health 2021:251-288.
Crossref - Veernala PK, Yugandhar NM. Isolation and Screening of Lovastatin production using fungal
isolate from soil samples under solid state fermentation. AJIR Publisher, 2021.
Crossref - Xie L, Zhu G, Shang J, et al. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell Signal. 2021;87:110122.
Crossref - Pushpa H, Priyata H, Nomita Devi K, Onya N, Vijayalakshmi A, Ramesh DH. Screening of lovastatin (HMG-CoA reductase inhibitor) from edible wild mushrooms. Curr Res Environ Appl Mycol. 2016;6(3):190-196.
Crossref - Bizukojc M, Ledakowicz S. Physiological, morphological and kinetic aspects of lovastatin biosynthesis by Aspergillus terreus. Biotechnol J. 2009;4:647-664.
Crossref - Mulder KC, Mulinari F, Franco OL, Soares MS, Magalhaes BS, Parachin NS. Lovastatin production: from molecular basis to industrial process optimization. Biotechnol Adv. 2015;33:648-665.
Crossref - Lopez JC, Perez JS, Sevilla JF, Fernandez FA, Grima EM, Chisti Y. Production of lovastatin by Aspergillus terreus: effects of the C:N ratio and the principal nutrients on growth and metabolite production. Enzyme Microb Technol. 2003;33(2-3):270-277.
Crossref - Srinivasan N, Thangavelu K, Uthandi S. Lovastatin production by an oleaginous fungus, Aspergillus terreus KPR12 using sago processing wastewater (SWW). Microbial Cell Fact. 2022;21(1):1-4.
Crossref - Jaivel N, Marimuthu P. Isolation and screening of lovastatin producing microorganisms. Int J EngSci Technol. 2010;2:2607-2611.
- Pecyna M, Bizukojc M. Lovastatin biosynthesis by Aspergillus terreus with the simultaneous use of lactose and glycerol in a discontinuous fed-batch culture. Biotechnol J. 2011;151:77-86.
Crossref - Sridevi B, Charya MAS. Isolation, identification and screening of potential cellulase-free xylanase producing fungi. Afr J Biotechnol. 2011;10:4624-4630.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.