ISSN: 0973-7510
E-ISSN: 2581-690X
There are an estimated 150 million cases of urinary tract infection (UTIs) reported each year, putting it among the most prevalent infectious diseases. The study aimed to estimate the prevalence of AmpC β-lactamase and Extended spectrum β-lactamase (ESBL) production amidst E. coli and Klebsiella pneumonia strains from UTI cases. A total of 406 non-repetitive urine samples (203 isolates of E. coli and K. pneumoniae each) were analyzed. The study revealed significant prevalence of MDR strains, with 157 isolates (77%) of E. coli and 171 isolates (84%) of Klebsiella showing resistance to multiple antibiotics. 195 isolates (96%) of E. coli and 179 isolates (88%) of K. pneumoniae showed highest levels of resistance to Ciprofloxacin and Cefoxitin, respectively. Production of ESBL was found in 128 isolates (63%) of E. coli and 75 isolates (37%) of K. pneumoniae by Disc diffusion method, while Putative AmpC was seen in 103 isolates (51%) of E. coli and 120 isolates (59%) of K. pneumoniae by Combination disc test. These findings highlight the immediate need for robust antibiotic stewardship practices and effective strategies for infection control to manage UTIs, particularly against MDR strains, adopting targeted antibiotic treatment based on patient-specific factors and local resistance pattern.
Urinary Tract Infections, ESBL, AmpC, E. coli, Klebsiella
Infections caused by Multidrug-resistant (MDR) Enterobacteriaceae pose a significant public threat due to their association with high mortality rates.1 One of the most common bacterial infections in both community and hospital settings is Urinary Tract Infection (UTI). UTIs are a serious public health issue in India, where they greatly increase hospital admissions and outpatient morbidity. According to Maharashtra studies, the prevalence of UTIs among patients who come with urinary symptoms ranges from 20% to 35%, higher rates are seen in females, the elderly, and those who are catheterized.2 Urine bag management, aseptic insertion technique and catheterization duration have a significant impact on the incidence of Catheter Associated Urinary Tract Infections (CAUTIs).3
β-lactam antibiotics, which are a class of drugs that contain β-lactam ring in their chemical structure, are used for treatment of UTIs.4 Effective treatment of UTIs has become more challenging due to the rising incidence of antibiotic resistance, which is mainly caused by the production of AmpC β-lactamases and Extended Spectrum β-lactamases (ESBL). By granting resistance to a range of β-lactam antibiotics, including monobactams, penicillins, and third-generation cephalosporins, these enzymes restrict the range of available treatments.5,6 The empirical and irrational use of broad-spectrum antibiotics in conjunction with inadequate infection control practices further facilitates the selection and spread of multidrug-resistant bacteria in hospital settings.7 Furthermore, many laboratories frequently neglect the routine detection of ESBL and putative AmpC producers because of lack of funding, standardized testing procedures, or clinician awareness.8
There are few studies that focus on individual regions, despite the availability of national-level data on increases in antibiotic resistance. There is a dearth of current local surveillance data on the prevalence and resistance mechanisms of uropathogens, especially in smaller cities like Karad and Satara. Clinicians are forced to use antiquated or generic empirical medicines in the absence localized antibiograms and resistance profiles, which increases treatment failure rates and healthcare expenses.9
In the light of this, the current study was conducted to ascertain the frequency of Klebsiella pneumoniae and E. coli that produce AmpC β-lactamases and ESBL that were isolated from urine samples in a tertiary care hospital in Karad, Maharashtra. Based on local resistance trends, the results should help guide logical antimicrobial therapy and help build effective infection control methods.
The current study on the “Prevalence of Extended spectrum β-lactamase and AmpC β-lactamase among Escherichia coli and Klebsiella pneumoniae isolated in Urinary tract infections” was executed over a span of two years from June 2022 to June 2024 at the Microbiology Department of Krishna Institute of Medical Sciences, KVVDU, Karad, District – Satara, Maharashtra, India.
Sample size calculation
As per the study undertaken by Gupta et al.,10 in the department of Medical Microbiology, Government Medical College, Chandigarh, among 150 isolates of E. coli isolated from urine samples, ESBL production was seen in 52.6% of isolates and AmpC production was seen in 8% of isolates. With this reference, the sample size required in this study is calculated.
Calculation was done using the formula
n = 4pq/L2
Where, p = Proportion of ESBL and AmpC production
q = 100 – p
L = Precision
Therefore, Sample size = 4 x 52.6 x 47.4 /72 = 203
Thus, a minimum of 203 urinary isolates of E. coli and Klebsiella pneumoniae, each making a total of 406, were taken in this study.
Inclusion criteria
Non-repetitive midstream urine samples were collected from patients who presented with symptoms of UTI, either from inpatient or outpatient departments. Only those samples that showed growth of E. coli and Klebsiella pneumoniae on culture were included in this study.
Exclusion criteria
Isolates from the same specimen and patient were not included in order to prevent duplication.
Statistical analysis
MS Excel Software was used to fill data. Subsequently, Graph Pad Instant software was used to run chi-square test to present the analyzed data as a percentage and p-value. An association or difference is considered significant if the probability is less than 0.05.
Sample collection
Non-repetitive midstream urine samples received in the Microbiology Department from patients presenting with symptoms of UTI, whether from the out-patient and in-patient departments were processed. After being collected, the samples were sent within an hour to the laboratory.
Bacterial Isolation
Bacterial isolates were obtained from urine samples using standard microbiological techniques. The samples were cultured on suitable media such as MacConkey agar and Blood agar to facilitate the growth and identification of organism.
Bacterial identification
Isolated colonies were subjected to Gram staining to determine Gram reaction and morphology. Preliminary identification was performed based on Oxidase and Catalase tests. This was followed by a series of biochemical tests (Figure 1a and 1b), including Indole production, Citrate utilization, Urease activity, Triple Sugar Iron (TSI) reaction, Methyl red test, and Nitrate reduction test. Identification of E. coli and Klebsiella pneumoniae was performed in accordance with standard microbiology protocols.11
Figure 1. (a) Biochemicals of E. coli – Indole produced, Citrate not utilised, Urease not hydrolysed, TSI showing acidic slant with acidic butt, Positive Methyl red test, Positive Nitrate reduction; (b) Biochemicals of K. pneumoniae – Indole not produced, Citrate utilised, Urease hydrolysed, TSI showing acidic slant with acidic butt, Negative Methyl red test, Positive Nitrate reduction
Antibiotic susceptibility profile
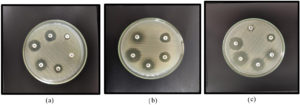
In accordance with CLSI standards 2024,12 Mueller-Hinton agar was used for antimicrobial susceptibility testing employing the Kirby-Bauer disc diffusion technique. After being adjusted to the 0.5 McFarland turbidity standard, a suspension of test and control organisms were inoculated onto Mueller-Hinton Agar plates. Antibiotic discs were set 20 mm apart and plates underwent 37 °C incubation for 16-22 hours. Antibiotics include Amikacin (30 µg), Ceftazidime (30 µg), Cefepime (30 µg), Cefotaxime (30 µg), Cefuroxime (30 µg), Cefoxitin (30 µg), Ciprofloxacin (5 µg), Ertapenem (10 µg), Fosfomycin (200 µg), Gentamicin (10 µg), Imipenem (10 µg), Meropenem (10 µg), Nalidixic Acid (30 µg), Nitrofurantoin (300 µg), Norfloxacin (10 µg), Co-trimoxazole (25 µg). The diameter of inhibition zones (Figure 2) were documented and analyzed in accordance with CLSI guidelines.
Isolates exhibiting an inhibition zone of ≤22 mm for Ceftazidime and ≤27 mm for Cefotaxime were selected for further investigation as potential ESBL producers.12,13 Similarly, isolates with a zone of ≤14 mm diameter for Cefoxitin were identified as Putative AmpC producers.14 These findings were further confirmed through phenotypic testing methods.
Figure 2. (a) Antibiotic susceptibility testing for Ceftazidime (CAZ), Cefoxitin (CX), Nalidixic acid (NA), Norfloxacin (NX), Nitrofurantoin (NIT). (b) Antibiotic susceptibility testing for Gentamicin (GEN), Fosfomycin (FO), Ciprofloxacin (CIP), Cotrimoxazole (COT). (c) Antibiotic susceptibility testing for Cefuroxime (CXM), Imipenem (IPM), Amikacin (AK), Cefepime (CPM), Meropenem (MRP)
Disc Diffusion method for ESBL Detection
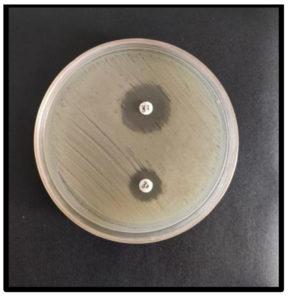
The test inoculum was prepared and modified to match the 0.5 McFarland turbidity, then inoculated onto the Mueller-Hinton Agar plates. A Ceftazidime (30 µg) disc and a Ceftazidime/Clavulanic acid (30 µg/10 µg) disc were positioned 20 mm part on MHA plates. The zone diameter increased by approximately 5 mm or more by Clavulanic acid as opposed to Ceftazidime alone indicated ESBL production (Figure 3).
Combination Disc test for Putative AmpC Detection
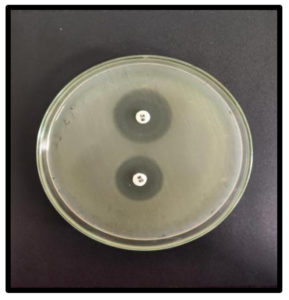
Before being inoculated onto the Mueller-Hinton Agar plates, the test inoculum was prepared and modified to match the 0.5 McFarland turbidity. Cefoxitin-resistant strains were confirmed using a combination disc method with Cefoxitin and Cefoxitin/Boronic acid (BA) disc. BA solution was made by combining 120 mg of phenylboronic acid in 3 ml Dimethy sulfoxide and 3 ml distilled water and 20 µl was dispensed onto Cefoxitin discs. A ≥5 mm increase in the extent of the inhibition zone for Cefoxitin when BA is present as opposed to Cefoxitin alone indicated AmpC production (Figure 4).
A total of 2,653 urine samples were collected from patients who exhibited clinical signs of urinary infection and yielding significant bacterial growth (≥105 CFU/mL). Of these, only isolates of E. coli and Klebsiella pneumoniae were selected for further analysis, in accordance with the study’s inclusion criteria. This resulted in the identification of 406 isolates, comprising 203 E. coli and 203 K. pneumoniae. Table 1 shows the highest prevalence of UTIs was observed in the 41-60 year age group (36.2%), followed by 61-80 years (35.4%). Females were commonly affected than males across all age groups, accounting for 54.2% of total cases. The least affected age group was <20 years (2.7%). Overall, UTIs were most frequent among middle-aged and elderly females.
Table (1):
Age and Gender wise distribution of UTI patients
Age Group |
Female N (%) |
Male N (%) |
Total N (%) |
|---|---|---|---|
8 (2) |
3 (0.7) |
11 (2.7) |
|
21-40 years |
51 (12.6) |
37 (9) |
88 (21.6) |
41-60 years |
75 (18.5) |
72 (17.7) |
147 (36.2) |
61-80 years |
77 (19) |
67 (16.4) |
144 (35.4) |
>80 years |
9 (2.2) |
7 (1.7) |
16 (3.9) |
Total |
220 (54.2) |
186 (45.8) |
406 (100) |
N = Number of isolates, % = Percentage of isolates
Among the inpatient department samples, 79% of E. coli and 92% K. pneumoniae were found while 8% of Klebsiella and 21% of the E. coli isolates were identified from outpatient department samples, with majority found in Inpatient department.
Among Inpatient department (Table 2), majority of the isolates were found in ICUs (49% E. coli and 50% Klebsiella) followed by Medicine ward (29% Escherichia and 20% Klebsiella), Surgery ward (22% and 15% of Klebsiella pneumoniae and E. coli respectively), Obstetrics and gynecology ward (4% of each isolate), Orthopedics ward (2% of E. coli isolates and 3% by Klebsiella), and Pediatrics department (1% of each isolate).
Table (2):
Distribution of E. coli and K. pneumoniae isolates across different Inpatient department settings
Inpatient Department |
E. coli N (%) |
K. pneumoniae N (%) |
|---|---|---|
Medicine |
47 (29) |
37 (20) |
Surgery |
24 (15) |
41 (22) |
Orthopedics |
4 (2) |
6 (3) |
Obgyn |
6 (4) |
7 (4) |
Pediatrics |
1 (1) |
1 (1) |
ICU |
79 (49) |
95 (50) |
Total |
161 (100) |
187 (100) |
N = Number of isolates, % = Percentage of isolates
Within the Outpatient department samples (Table 3), Medicine and Surgery department each reported 38% E. coli isolates, while Obstetrics and gynecology reported 24% of isolates. For K. pneumoniae, the Medicine department had 56% isolates, Surgery had 31% and obstetrics and gynecology had 13% of isolates.
Table (3):
Distribution of E. coli and K. pneumoniae isolates across different Outpatient department settings
Outpatient Department |
E. coli N (%) |
K. pneumoniae N (%) |
|---|---|---|
Medicine |
16 (38) |
9 (56) |
Surgery |
16 (38) |
5 (31) |
Obgyn |
10 (24) |
2 (13) |
Total |
42 (100) |
16 (100) |
N = Number of isolates, % = Percentage of isolates
The antibiotic susceptibility of the isolates showed that 96% of E. coli are resistant to Ciprofloxacin, with the highest resistance, whereas 96% E. coli are susceptible to Fosfomycin, showing highest sensitivity. On the other hand, 88% of K. pneumoniae isolates show the highest level of resistance to Cefoxitin, whilst 58% of isolates show the highest level of sensitivity to Fosfomycin (Table 4). The statistical test indicates (c2 = 1797.3, p value <0.0001) significant findings. Multidrug-resistance is described as resistance to upto three minimum antibiotics from various classes of antimicrobial drugs.15 R0 – No resistance to antibiotics, R1 – Resistant to a single class, R2 – showing resistance to two classes, R3 – having resistance to three classes, R4 – having resistance to four classes, ≥R5 – Resistant to five or more classes. Among E. coli, there were 2 isolates with R0, 6 with R1, 9 with R2, 29 with R3, 50 with R4, and 107 with ≥R5, resulting in 157 isolates (77%) exhibiting MDR. For Klebsiella pneumoniae, there were 5 isolates with R0, 5 with R1, 8 with R2, 14 with R3, 17 with R4, and 154 with ≥R5, resulting in 171 isolates (84%) showing MDR.
Table (4):
Antibiotic susceptibility profile of E. coli and K. pneumoniae isolates Antibiotics
| E. coli | K. pneumoniae | |||
|---|---|---|---|---|
| Sensitive N (%) | Resistant N (%) | Sensitive N (%) | Resistant N (%) | |
| Amikacin | 40 (20) | 163 (80) | 91 (45) | 112 (55) |
| Cefepime | 41 (20) | 162 (80) | 31 (15) | 172 (85) |
| Cefoxitin | 77 (38) | 126 (62) | 24 (12) | 179 (88) |
| Ceftazidime | 13 (6) | 190 (94) | 20 (10) | 183 (90) |
| Cefuroxime | 16 (8) | 187 (92) | 16 (8) | 187 (92) |
| Ciprofloxacin | 8 (4) | 195 (96) | 22 (11) | 181 (89) |
| Cotrimaxazole | 82 (40) | 121 (60) | 73 (36) | 130 (64) |
| Ertapenem | 74 (36) | 129 (64) | 63 (31) | 140 (69) |
| Fosfomycin | 195 (96) | 8 (4) | 118 (58) | 85 (42) |
| Gentamicin | 90 (44) | 113 (56) | 67 (33) | 136 (67) |
| Imipenem | 62 (30) | 141 (70) | 63 (31) | 140 (69) |
| Meropenem | 62 (30) | 141 (70) | 63 (38) | 140 (62) |
| Nalidixic acid | 17 (8) | 186 (92) | 23 (11) | 180 (89) |
| Nitrofurantoin | 71 (35) | 132 (65) | 78 (38) | 125 (62) |
| Norfloxacin | 38 (19) | 165 (81) | 34 (17) | 169 (83) |
N = Number of isolates, % = Percentage of isolates
According to Table 5, 63% of the E. coli isolates were positive for ESBL and 37% to be ESBL negative. However, sixty-three percent of K. pneumoniae were found to be ESBL negative and 37% to be ESBL positive. This shows that E. coli has higher rate of ESBL production.
Table (5):
Production of ESBL among E. coli and K. pneumoniae isolates
Organism |
ESBL Producers N (%) |
Non-ESBL producers N (%) |
|---|---|---|
E. coli |
128 (63) |
75 (37) |
K. pneumoniae |
75 (37) |
128 (63) |
N = Number of isolates, % = Percentage of isolates
Of the total E. coli, 51% emerged to be AmpC producing, on the contrary, majority of the isolates of K. pneumoniae that tested positive for AmpC was 59% (Table 6).
Table (6):
Putative Production of AmpC among E. coli and K. pneumoniae isolates
Organism |
AmpC Producers N (%) |
Non-Ampc Producers N (%) |
|---|---|---|
E. coli |
103 (51) |
100 (49) |
K. pneumoniae |
120 (59) |
83 (41) |
N = Number of isolates, % = Percentage of isolates
Analyzing the prevalence of AmpC and ESBL production by Klebsiella pneumoniae and E. coli as well as evaluating their antibiotic susceptibility profiles and resistance trends were the purpose of this study. A total of 406 urine samples, comprising 203 of E. coli and Klebsiella each, were acquired during the study period. Among the total isolates, the most affected age group was 41-80 years ago. Similar findings were reported by Goyal et al.16 who observed that majority were from were in the 41-80 age group. Studies by Mohammed et al.,17 Mofolorunsho,18 Jalil,19 and Azab20 observed predominance of females. The ratio of male to female in the present study was 1:1.27 with 44% males and 56% females showing E. coli isolates. It was similar to the study by Mofolorunsho18 that shows 43.8% males and 56.2% females were affected. Conversely, K. pneumoniae was seen in 55% males and 45% females. This correlates with the findings of Mohammed17 that indicate 50% males and 50% females were affected. The predominance of females can be explained by the urethra that is shorter and closer to the rectum.
In the study, 92% of Klebsiella and 79% E. coli isolates were from Inpatient department, while 8% Klebsiella and 21% of E. coli came from Outpatient section. This data indicates majority of the isolates were from inpatient department. Due to prolonged hospital stays, the use of broad-spectrum antibiotics, and frequent exposure to invasive procedures such as catheterisation, hospital patients particularly those in intensive care units (ICU) or those undergoing surgical procedures are more susceptible to healthcare-associated infections (HAI). These factors all contribute to the acquisition of nosocomial pathogens, including multidrug-resistant Enterobacteriaceae.21,22 Furthermore, inpatients are more likely than outpatients to undergo routine microbiological examinations, which raises the likelihood of isolate identification in this population. On the other hand, there are fewer isolation reports from outpatients since they frequently have less serious infections and might be treated empirically without microbiological confirmation.23
Antimicrobial resistance is growing global concern in both community and healthcare settings. Chander24 reported E. coli resistance to Ceftriaxone (100%) and Ceftazidime, which aligns with the present study findings of resistance towards Ceftriaxone and Ceftazidime (89% and 94% respectively). However, Chander also noted high resistance to Nalidixic acid (95%), whereas this study showed that E. coli had 92% resistance towards Nalidixic acid, and K. pneumoniae showed 89% resistance towards Nalidixic acid. Here, Ciprofloxacin showed a higher rate of resistance of 96% consistent with the results of Sageerabanoo.25 Sageerabanoo showed high level resistance towards Gentamicin and Cotrimaxazole, while our study showed resistance of 56% to Gentamicin and 60% to Cotrimaxazole by E. coli and 67% to Gentamicin and 64% to Cotrimaxazole by Klebsiella. Our study found 96% and 58% sensitivity to Fosfomycin in E. coli and Klebsiella pneumoniae, respectively, mirroring recent clinical evidence that highlights Fosfomycin’s continued effectiveness against uropathogenic strains and supports its utility in treating multidrug-resistant UTIs.26 The 2023-2024 IDSA guidance further endorses oral Fosfomycin as an alternative for uncomplicated cystitis caused by ESBL-producing E. coli, while advising caution in its use against Klebsiella pneumoniae due to the presence of the fosA gene that can confer resistance.27
This study shows MDR E. coli (77%) and Klebsiella (84%) that are close to the results by Shatalov28 who observed 74% MDR E. coli and 91% MDR Klebsiella. Chander24 reported findings of 92% and 87% MDR E. coli and Klebsiella, while Eshetie,29 found 92% MDR E. coli and 95% MDR Klebsiella. Studies by Tekele30 recorded MDR rates of 69% and 82% by E. coli and K. pneumoniae. Although molecular methods are the most effective for detecting beta-lactamase enzymes like ESBL and AmpC, they are often inaccessible in developing countries due to limited resources and infrastructure. Therefore, employing various phenotypic techniques is necessary to detect AmpC and ESBL in Gram-negative bacteria. The present findings of ESBL in E. coli (63%) and Klebsiella pneumoniae (32.4%) correlate with the study conducted by Vijayvergia31 showing ESBL production rates of 67.5% and 32.4% among E. coli and Klebsiella pneumoniae. Similarly, study from Cameroon on urinary pathogens reported comparable ESBL rates in Klebsiella pneumoniae, reinforcing the relevance of the present study findinds to broader epidemiological patterns.32 The 63% prevalence in E. coli are comparable to rates observed in healthcare settings within India and Southeast Asia, where ESBL burden is notably elevated. For instance, a recent study from northeastern Thailand found ESBL production in 96.5% of Cephalosporin-resistant E. coli, with CTX-M-15 as the predominant gene.33 These variations reflect regional differences in antibiotic usage, infection control practices and diagnostic capacity.
Singhal6 showed 6.97% and 6.18%, while results of Sageerabanoo et al.,25 showed 46.6% and 12.8% AmpC in E. coli and Klebsiella, respectively. Ugwu et al.34 showed 21% AmpC by E. coli and 13% by K. pneumoniae. Inamdar35 observed 76.2% and 20.1% AmpC production among E. coli and Klebsiella pneumoniae, respectively. In 2022, Perera et al.36 showed 24% and 35% production of AmpC among the isolates. This study showed 51% putative AmpC production among E. coli, which coincides with findings by Vandana et al.,37 having 58.5% AmpC production among E. coli, whereas 59% Klebsiella pneumoniae were putative AmpC producers in our study which is comparatively higher than other studies, but Vandana37 shows a higher rate of AmpC production of 82%.
According to recent studies, ESBL/AmpC co-producers significantly reduce the alternatives for β-lactam therapy, highlighting the worldwide clinical challenge these enzymes present.38 A recent study reported that 6.5% of ESBL-producing E. coli and Klebsiella isolates also produced AmpC enzymes, co-resistance to non-β-lactam like Fluoroquinolones, Aminoglycosides and Sulphonamides was extremely high, underscoring the diminishing efficacy of alternative classes of antibiotics.39 These findings emphasize the need for enhanced microbiological detection, antimicrobial stewardship, and tailored infection-control strategies.
Advances in rapid diagnostics-such as the GeneXpert ESBL-AmpC PCR assay-have shown promise in accelerating detection directly from urine, potentially reducing time to targeted therapy and curbing unnecessary carbapenem use.40 Additionally, a 2025 review highlights the complementary roles of phenotypic methods and cutting-edge molecular tools (e.g., NGS, MALDI-TOF, PCR) in overcoming limitations of conventional testing-especially important in resource-limited settings. Collectively, these studies support our conclusion: that integrated diagnostic strategies, sustained antimicrobial surveillance, and robust stewardship are vital to containing the spread of ESBL/AmpC-producing multidrug-resistant uropathogens and guiding effective empirical therapy.
The present study outlines antimicrobial resistance levels, including detection of beta-lactamase production, among E. coli and K. pneumoniae isolates from urinary specimens. The study shows that E. coli was common in patients among 61-80 years old (37%), while Klebsiella pneumoniae were more common in the 41-60 years age group (40%). Females showed higher prevalence of E. coli (56%), whereas Klebsiella pneumoniae were more prevalent in males (55%).
Both organisms were predominantly isolated from Inpatient section, with the ICU reporting the highest number of isolates. Antibiotic susceptibility testing revealed high resistance rates to Ciprofloxacin in E. coli (96%) and Cefoxitin in Klebsiella pneumoniae (88%). E. coli showed high sensitivity to Fosfomycin (96%), while Klebsiella pneumoniae demonstrated sensitivity to Tigecycline (82%). The study also highlighted significant rates of ESB and AmpC beta-lactamase production in both the isolates. The frequency of ESBL was 63% by E. coli and 37% by Klebsiella pneumoniae isolates, while AmpC enzyme was seen in 51% of E. coli and 59% Klebsiella pneumoniae. MDR was observed in 77% of E. coli and 84% of Klebsiella pneumoniae with notable percentage co-producing ESBL and AmpC enzymes. These findings underscore the urgent need for robust antibiotic stewardship practices to manage UTIs effectively, particularly in opposition to multidrug-resistant strains.
The research highlights the pressing need for effective infection prevention measures as well as prudent drug use in tackling the growing threat of antibiotic-resistant UTI. Enhanced hygiene practices, rigorous catheter care protocols, and proactive surveillance are crucial in preventing and managing outbreaks of resistant UTI. Moreover, adopting antibiotic stewardship programs that prioritize targeted antibiotic treatment based on patient specific factors and local resistance patterns are essential. Collaborative efforts and strong public health policies are essential to protect treatment options and enhance outcomes for individuals with UTI.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The study was approved by the Institutional Ethical and Research Committee, Krishna Institute of Medical Sciences, Deemed to be University, Karad, with protocol number 255/2021-2022.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Arumugam K, Karande GS, Patil SR. Prevalence of Carbapenemase Production Among Klebsiella and Escherichia coli Isolated From Urinary Tract Infections. Cureus. 2024;16(10):e70918.
Crossref - Tankhiwale SS, Jalgaonkar SV, Ahamad S, Hassani U. Evaluation of extended spectrum beta lactamase in urinary isolates. Indian J Med Res. 2004;120(6):553-556.
- Jha T, Khaparde M, Parkhe TS, Purandare B, Lavate R. Incidence Risk Factors and Drug Resistance Patterns of Bacterial Isolates in Patients with Catheter-associated Urinary Tract Infections. Indian J Crit Care Med. 2025;29(4):338.
Crossref - Das D, Vohra P, Mane P, Shaozae CK. Extended spectrum, AmpC & metallo-β-lactamases producing Escherichia coli in urinary isolates: A prospective study in north India. Indian J Med Res. 2025;161(2):167.
Crossref - Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):90-101.
Crossref - Singhal S, Mathur T, Khan S, et al. Evaluation of methods for AmpC β-lactamase in gram negative clinical isolates from tertiary care hospitals. Indian J Med Microbiol. 2005;23(2):120-124.
Crossref - Laxminarayan R, Matsoso P, Pant S, Brower C, Rottingen JA, Klugman K, Davies S. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168-175.
Crossref - Manoharan A, Sugumar M, Kumar A, Jose H, Mathai D, ICMR-ESBL study group. Phenotypic & molecular characterization of AmpC β-lactamases among Escherichia coli, Klebsiella spp. & Enterobacter spp. from five Indian Medical Centers. Indian J Med Res. 2012;135(3):359-364.
- Taneja N, Sharma M. Antimicrobial resistance in the environment: The Indian scenario. Indian J Med Res. 2019;149(2):119-128.
Crossref - Gupta V, Rani H, Singla N, Kaistha N, Chander J. Determination of extended-spectrum β-lactamases and AmpC production in uropathogenic isolates of Escherichia coli and susceptibility to fosfomycin. J Lab Physicians. 2013;5(02):090-093.
Crossref - Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie & McCartney Practical Medical Microbiology. 14th ed. Edinburgh: Churchill Livingstone. 1996:131-49.
- CLSI: M100: Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute, Wayne, PA; 2024
- Latifpour M, Gholipour A, Damavandi MS. Prevalence of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in nosocomial and community-acquired urinary tract infections. Jundishapur J Microbiol. 2016;9(3).
Crossref - RaMaKRiShnan K, KALI A, SAH S, PAGAL S, KENCHAPPA P, SEETHA KS. Molecular Profile of Emerging Multidrug Resistant Klebsiella pneumoniae Clinical Isolates from Southern India. J Clin Diagn Res. 2019;13(3):12644;dc01-dc06.
Crossref - Goel IN, Sachin S, Kumar CP, Nikhil P, Suneet Y. Different phenotypic methods for detection of ESBL production in bacteria: A reiew. J Pharm Sci Innov. 2014;3(3):197-198.
Crossref - Goyal S, Beniwal V. Study of Multidrug Resistance Pattern among Escherichia coli isolated from patients with Urinary tract infection. Asian J Pharm Clin Res. 2016;9(6):157-160.
Crossref - Mohammed MA, Alnour TM, Shakurfo OM, Aburass MM. Prevalence and antimicrobial resistance pattern of bacterial strains isolated from patients with urinary tract infection in Messalata Central Hospital, Libya. Asian Pac J Trop Med. 2016;9(8):771-776.
Crossref - Mofolorunsho KC, Ocheni HO, Aminu RF, Omatola CA, Olowonibi OO. Prevalence and antimicrobial susceptibility of extended-spectrum beta lactamases-producing Escherichia coli and Klebsiella pneumoniae isolated in selected hospitals of Anyigba, Nigeria. Afr Health Sci. 2021;21(2):505-512.
Crossref - Jalil MB, Al Atbee MY. The prevalence of multiple drug resistance Escherichia coli and Klebsiella pneumoniae isolated from patients with urinary tract infections. J Clin Lab Anal. 2022;36(9):e24619.
Crossref - Azab K. Prevalence and relation of urinary tract infection bacterial pathogens to sex and ages among patients in three arab countries. Azhar J Pharm Sci. 2021;63(1):194-206.
Crossref - Weiner-Lastinger LM, Abner S, Edwards JR, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol. 2020;41(1):1-18.
Crossref - Tangden T, Pulcini C, Aagaard H, et al. Unavailability of old antibiotics threatens effective treatment for common bacterial infections. Lancet Infect Dis. 2018;18(3):242-244.
Crossref - Allegranzi B, Nejad SB, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228-241.
Crossref - Chander A, Shrestha CD. Prevalence of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae urinary isolates in a tertiary care hospital in Kathmandu, Nepal. BMC Res Notes. 2013;6:487.
Crossref - Sageerabanoo S, Malini A, Mangaiyarkarasi T, Hemalatha G. Phenotypic detection of extended spectrum β-lactamase and Amp-C β-lactamase producing clinical isolates in a Tertiary Care Hospital: A preliminary study. J Nat Sci Biol Med. 2015;6(2):383.
Crossref - Dombach JL, Smith N, Kottiri T, Schiller A, Kamau E. Antimicrobial susceptibilities of clinical bacterial isolates from urinary tract infections to fosfomycin and comparator antibiotics determined by agar dilution method and automated micro broth dilution. Microbiol Spectr. 2025:e01860-24.
Crossref - Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Bonomo RA. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin Infect Dis. 2024:ciae403.
Crossref - Shatalov A. Prevalence and antibiotic resistance pattern of Escherichia coli and Klebsiella pneumoniae in urine tract infections at the La Paz Medical center, Malabo, Equatorial Guinea. Open J Med Microbiol. 2015;5(4):177-183.
Crossref - Eshetie S, Unakal C, Gelaw A, Ayelign B, Endris M, Moges F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral Hospital, Northwest Ethiopia. Antimicrob Resist Infect Control. 2015;4:12.
Crossref - Tekele SG, Teklu DS, Tullu KD, Birru SK, Legese MH. Extended-spectrum Beta-lactamase and AmpC beta-lactamases producing gram negative bacilli isolated from clinical specimens at International Clinical Laboratories, Addis Ababa, Ethiopia. PLoS One. 2020;15(11):e0241984.
Crossref - Vijayvergia V, Sahni A, Khan I, Lall M, Vijay K, Khan ID. Phenotypic detection of Extended Spectrum Beta-Lactamases and AmpC Beta-Lactamases among nosocomial isolates in a tertiary care hospital. Bangladesh J Med Sci. 2013;12(4):378-384.
Crossref - Bayaba S, Founou RC, Tchouangueu FT, et al. High prevalence of multidrug resistant and extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolated from urinary tract infections in the West region, Cameroon. BMC Infect Dis. 2025;25(1):115.
Crossref - Chaisaeng S, Chopjitt P, Kasemsiri P, et al. High prevalence of ESBL-producing E. coli phylogroup B2 clinical isolates in northeastern Thailand. BMC Microbiol. 2024;24(1):425.
Crossref - Ugwu MC, Shariff M, Nnajide CM, Beri K, Okezie UM, Iroha IR, Esimone CO. Phenotypic and molecular characterization of β-lactamases among enterobacterial uropathogens in southeastern Nigeria. Can J Infect Dis Med Microbiol. 2020;2020(1):5843904.
Crossref - Inamdar DP, Anuradha B. Phenotypic methods for detection of AmpC β-lactamases in gram negative clinical isolates of a tertiary care hospital. Indian J Microbiol Res. 2020;7(2):125-129.
Crossref - Perera PDVMP, Gamage S, De Silva HSM, et al. Phenotypic and genotypic distribution of ESBL, AmpC β-lactamase and carbapenemase-producing Enterobacteriaceae in community-acquired and hospital-acquired urinary tract infections in Sri Lanka. J Global Antimicrob Resist. 2022;30:115-122.
Crossref - Vandana KE, Honnavar P. AmpC beta lactamases among ESBL producing Escherichia coli and Klebsiella pneumoniae-If you don’t look, you won’t find. J Clin Diagn Res. 2009;3(4):1653-1656
- Nasrollahian S, Graham JP, Halaji M. A review of the mechanisms that confer antibiotic resistance in pathotypes of E. coli. Front Cell Infect Microbiol. 2024;14:1387497.
Crossref - Garba Z, Bonkoungou IJO, Somda NS, et al. Fecal carriage of carbapenemase and AmpC-β-lactamase producers among extended spectrum β-lactamase-producing E. coli and Klebsiella spp. isolates in patients attending hospitals. BMC Infect Dis. 2025;25(1):109.
Crossref - Tops SC, Coolen JP, Tenover FC, et al. The diagnostic accuracy of the GeneXpert ESBL-ampC prototype assay for rapid PCR-based detection of extended-spectrum beta-lactamase genes directly from urine. Microbiol Spectrum. 2023;11(6):e03116-23.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.