ISSN: 0973-7510
E-ISSN: 2581-690X
Elizabethkingia meningoseptica a rare pathogen in earlier times has been accused to infect the immunocompromised, preterm neonates, the patients exposed to longterm antibiotics and intensive care units. The apparent resistance of the multidrug Elizabethkingia meningoseptica affects the selection of appropriate antibacterial agents against it. The current study attempts to determine the prevalence of E. meningoseptica infections and consider the sensitivity pattern in a tertiary care hospital. A prospective study of prevalence of E. meningoseptica in a tertiary care hospital from March 2020 to March 2021 i.e over a period of 1 year. Patient clinical data as well as ABST patterns were collected and analyzed. Out of total 1813 patient’s samples E. meningoseptica was isolates from 21 cases (1.15%). Average age was 61.42 years, with males most likely to be infected (52.38%). All the adult patients had underlying diseases, obstructive gall bladder diseases (n=7, 33.33%) which included choledocholithiasis, obstructive jaundice, cholangitis and carcinoma gallbladder. Urinary tract diseases (n=8, 38.09%) which include chronic kidney disease (CKD) and acute kidney disease (AKD), pneumonia (n=11, 52.38%) including urosepsis and other diseases AML (n=1, 04.7%). Susceptibility tests showed 100% in vitro against few antimicrobials like cefepime, meropenem and amikacin which can be utilized to treat most common Gram-negative bacterial infections. Isolates are usually the completely sensitive to minocycline. E. meningoseptica is a rising microbe in intensive care setup due to its resistance pattern.
Elizabethkingia meningoseptica, Immunocompromised, Multidrug resistance, intensive care unit
Elizabethkingia meningoseptica is a ubiquitous, Gram-negative, non fermentative bacillus.1,2 It is considered as potential pathogen to patients in intensive care units because of its resistance to various drugs and its adjustment to biospheres such as disinfectants, water supplies and clinical gadgets. Biofilms production, intracellular invasion and the capacity to live long in humid environments are important in its survival.3-7
Patient categories most affected include preterm neonates, patient with long ICU stay and broad spectrum antibiotics, intravenous catheters and other clinical gadgets and orthopedic implants.8,9 E. meningoseptica has resistance which is both chromosomal and plasmid mediated, which results in resistance of common antibiotics like aminoglycosides, carbapenems and beta-lactams but this organism shows special sensitivity to minocycline, trimethoprim-sulfamethoxazole, fluoroquinolone, piperacillin-tazobactam. Until now no antimicrobial regimen for empiric therapy has been prescribed.10,-12 For this rare opportunistic pathogen few studies are there along with antimicrobial susceptibility pattern and because of raising infection trend this need to be given utmost importance, particularly in developing country like India, where hospital infection is a major concern.19 Gram-negative nosocomial infections are used to treat the most common antibiotics but, E. meningoseptica is clinically quite difficult to decide on powerful antibiotics due to the fact no interpretive minimum inhibitory concentration (MIC) breakpoints of antibiotics against this organism and reports of antimicrobial have been reported. The treatment outcomes are also rare.
The SENTRY antibacterial drug monitoring program showed that piperacillin-tazobactam, trimethoprim-sulfamethoxazole, rifampicin and quinolone are the most effective drugs against E. meningoseptica. The programme was held from 1997 to 2001.13
There are six species are assigned to Elizabethkingia genus, Elizabethkingia meningoseptica, Elizabethkingia miricola, Elizabethkingia anophelis, Elizabethkingia bruuniana, Elizabethkingia ursingii, with the first 3 considered to be medically important. The rest three generally possess low virulence and are not medically important.14-17
Elizabethkingia is the name of its pioneer Elizabeth O. King Prior Classified in CDC Iia group, chryseobacterium (1994) and Flavobacterium (1959) classified as Elizabethkingia under the parent Flavobacteriaceae in 2005 based on 16s rRNA phylogenetic studies. Removal of E. meningoseptica resistant(R) in many Beta- lactams whenever possible due to longer class A Beta-lactamases (ESBLs) and class B Metallo Beta-lactamases (MBLs).18 The antimicrobial effect of Elizabethkingia may likewise also go depending at the species.
The most pathogenic Elizabethkingia sp. infects the cerebrospinal skin, fluid, blood, respiratory system, soft tissues, other sites and capable of infecting human beings.20 Several classes of important antibiotics such as beta-lactams, clindamycin, teicoplanin, chloramphenicol, carbapenems, erythromycin, aminoglycosides and tetracyclines, are reported to be non effective as the microrganism exhibits inherent resistance.
Over the past decade, there has been an increase in the number of patients with E. meningoseptica.21 Scarcely any studies of E. meningoseptica are proposed from India.
This study is done to find prevalence of Elizabethkingia meningoseptica in a tertiary care setup. Along with it the organism’s infectious potential, co-morbidity associated with infected patients, and other predisposing factors and antibiotic resistance pattern are also investigated.
The study was conducted in the Department of Microbiology, IMS & SUM hospital; a tertiary care hospital in Eastern Odisha. A clinical specimen was blood which was processed according to standard protocol. Patient having fever and symptoms of sepsis were included in the study. All blood culture was processed by automated blood culture system Bact/Alert 3D (BioMerieux). Detail history of patients were collected from whom E. meningoseptica was isolated from March 2020 to March 2021 were included in this study. Patient’s information such as hospital data was included including age, gender, antibiotic treatment, comorbidity and hospital indications has been recorded. In addition, records of present ailments and characteristic related signs, the type of infection, the use of invasive methods, procedures, and device was obtained and analyzed.
The colonies of E. meningoseptica are usually smooth circular with regular margins 1-2 mm colony. They show slight yellow pigmentation in nutrient agar. In blood agar showed same morphology in grayish-white non hemolytic colonies. Usually, no growth seen in MacConkey agar.
The colonies are oxidase positive, indole-positive and catalase positive, OF glucose shows oxidative pattern, urease negative (contrast E.miricola which is urease positive), mannitol positive, non-nitrate reducing saprophytic bacilli. It oxidatively utilises glucose, maltose does not reduce nitrate. Utilisation of maltose but not xylose and sucrose were presumed to be E. meningoseptica .
ABSTs for segregates were also performed using the Kirby Bauer disc diffusion method. Interpretation of ABSTs was performed in accordance with the requirements for non –Enterobacteriaceae set with the guide of using the CLSI. Identification and antibiotic sensitivity of tigecycline and colistin was done by automated broth microdilution method in Vitek 2 compact system (Biomerieux) using GN 271 card.
In this 1-year retrospective study from March 2020 to March 2021 E. meningoseptica. Total 21 were isolated in this period. Out of total 1813 positive blood culture. The mean age of the patients was 61.42 years, and ranged from 25 to 90 years. Among adult patients there were 11 males (52.38%) and 10 females (47.61%) (Table 1, 2 & 3).
Table (1):
Total number of blood culture positive (n=1813).
Total number of positive blood cultures |
1813 |
E.meningoseptica isolated from patients |
21 (1.15%) |
Others |
1792 |
Table (2):
Gender distribution of E.meningoseptica patients (n=21).
Gender |
Male |
Female |
|---|---|---|
Number of patients |
11 (52.38%) |
10 (47.61%) |
Table (3):
Age and sex wise distribution of E. meningoseptica isolated from patients (n=21).
| Age group | Male | Female | |
|---|---|---|---|
| <5 yrs | 0 | 0 | |
| 6-15yrs | 0 | 0 | |
| 16-30 yrs | 1 | 1 | |
| 31-45 yrs | 0 | 0 | |
| 46-60 yrs | 2 | 6 | |
| >60 yrs | 8 | 3 | |
| Total | 11(52.38%) | 10(47.61%) | |
All the adult patients had underlying diseases, obstructive gall bladder diseases (n=7, 33.33%) which included choledocholithiasis, obstructive jaundice, cholangitis and carcinoma gallbladder. Urinary tract diseases (n=8, 38.09%) including chronic kidney disease (CKD) and acute kidney disease (AKD), pneumonia (n=11, 52.38%) including urosepsis and other diseases AML (n=1, 04.7%) and other blood dyscrasias. All patients developed fever only after 48 hours of hospitalization (Table 4).
Table (4):
Clinical profile of 21 patients with E.Meningoseptica infections.
Age |
Sex |
Underlying disease |
|---|---|---|
51 |
Female |
Choledocholithiasis |
76 |
Male |
Anemia in CKD |
58 |
Female |
CaGB |
79 |
Male |
Urosepsis, HTN, AKD |
85 |
Male |
Obstructive jaundice |
67 |
Male |
R/L pneumonia |
62 |
Male |
CRTI, CKD |
50 |
Male |
Pneumonia |
26 |
Male |
Fever, AKDS |
70 |
Male |
CCM Post covid pneumonia |
60 |
Male |
CKD |
58 |
Female |
CaGB |
60 |
Female |
AKD, sepsis, T2DM, HTN |
26 |
Female |
Cholangitis |
67 |
Male |
R/L pneumonia |
80 |
Female |
CKD, T2DM |
67 |
Female |
AML |
72 |
Male |
CCM Fever, AKDS |
67 |
Male |
R/L Pneumonia |
58 |
Female |
CaGB |
51 |
Female |
Choledocholithiasis |
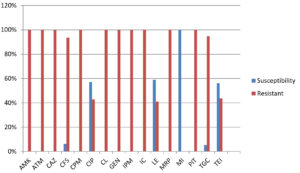
Susceptibility of the E. meningoseptica isolates to amikacin, aztreonam, ceftazidime, cefepime and colistin were 100% resistant following were meropenem (100%), Tigecycline(94.7%) & cefatrizine (93.7%). Minocycline was the most sensitive drug (100%) susceptibility (Table 5, Fig. 1).
Table (5):
Antibiotic susceptibility pattern of Elizabethkingia meningoseptica.
Antibiotic |
Susceptibility |
Resistant |
|---|---|---|
Amikacin (AMK) |
0 (0%) |
20 (100%) |
Aztreonam (ATM) |
0 (0%) |
14 (100%) |
Ceftazidime (CAZ) |
0 (0%) |
18 (100%) |
Cefatrizine (CFS) |
1 (6.25%) |
15 (93.7%) |
Cefepime (CPM) |
0 (0%) |
17 (100%) |
Ciprofloxacin (CIP) |
8 (57.1%) |
6 (42.8%) |
Colistin (CL) |
0 (0%) |
17 (100%) |
Gentamicin (GEN) |
0 (0%) |
18 (100%) |
Imipenem (IPM) |
0 (0%) |
16 (100%) |
Imipenem-cilastatin (IC) |
0 (0%) |
6 (100%) |
Levofloxacin (LE) |
10 (58.8%) |
7 (41.1%) |
Meropenem (MRP) |
0 (0%) |
19 (100%) |
Minocycline (MI) |
19 (100%) |
0 (0%) |
Piperacillin/tazobactam (PIT) |
0 (0%) |
18 (100%) |
Tigecycline (TGC) |
1 (5.26%) |
18 (94.7%) |
Teicoplanin (TEI) |
9 (56.2%) |
7 (43.7%) |
Fig. 1. Antibiotic susceptibility pattern of Elizabethkingia meningoseptica (Amikacin (AMK), Aztreonam (ATM), Ceftazidime (CAZ), Cefatrizine (CFS), Cefepime (CPM), Ciprofloxacin (CIP), Colistin (CL), Gentamicin (GEN), Imipenem (IPM), Imipenem-cilastatin (IC), Levofloxacin (LE), Meropenem (MRP), Minocycline (MI), Piperacillin/tazobactam (PIT), Tigecycline (TGC), Teicoplanin (TEI).
In the present study which was under taken in Department of Microbiology from March 2020 to March 2021. Total 1813 patients were tested for positive blood culture. Out of which 21 were isolated from E. meningoseptica patients.
In our study E. meningoseptica was obtained 21 patients. The average age of the patients was 61.42 years, with 52.38% being male patients and 47.61% being female patients. In an study in Central Taiwan by Chang YC et al, the average age of the patients was 71.09 years (except for 2 neonates), with 81.8% of males.22 Mostly the elderly age groups are affected due to associated co-morbidities.
All patients in our study had a serious underlying disease; which includes pneumonia (52.38%), kidney diseases (acute chronic) (38.09%), obstructive gallbladder diseases (33.33%), and AML (04.7%). In a study conducted in a medical Kasturba, dept. of Microbiology, by Hsu MS et al, the most widely recognized hidden sicknesses was DM (09.5%), COPD (36.4%), Malignancy (22.3%) and Chronic urinary tract illness (22.3%).10 All patients in our study had developed fever after 48 hours of admission. Which may indicate the hospital acquired nature of the infection.
In our study 8 patients had concomitant bacteria. It is supported by finding of Venkatesh et al. but the pathogenic role is difficult to explain in these cases. These 8 cases were not included in our study.23
As per our knowledge, this is the primarily observed to report that chronic liver disease could be a capability threat for thing E. meningoseptica infection. Cirrhosis because of any aetiology disrupts the homeostatic function of liver in the body. Patients with chronic liver disease have been shown to have hypoalbuminemia, accelerated need for invasive techniques, and special nutritional requirements making them an immunocompromised group. Cirrhosis-Associated Immune Dysfunction (CAID’s) is a condition which refers to each immunodeficiency and systemic inflammation that arise in cirrhosis.24 Variations inside the nearby and systemic immunity in patients with CAID’s could lead to changes in both innate and acquired immunity. Following which immune paralysis can set in being marked by rise in anti-inflammatory cytokines and decrease of pro-inflammatory cytokines.24,25 predisposing the individual to infection. In our case also it has been same thing gall bladder disease (33.33%).
The susceptibility action of E. meningoseptica antibiotics varies in detail. The organism is mostly insensitive to most β-lactam antibiotics which include aztreonam and carbapenems, aminoglycoside compounds of drugs and chloramphenicol but was defenseless against drugs such as piperacillin, minocycline, tigecycline, fluoroquinolones and cotrimoxazole.7,9,10,22,26,27
Studies investigating resistance of E. meningoseptica found that resistance to antimicrobial effects is due to the synthesis of metallo-β-lactamases recorded by the genes Bla (GOB) and BlaB, which has the potential to lower the β-lactam antibiotic limit, consequently controlling their convenience selection.28 This high level antimicrobial ineffectiveness can be the reason for the presence of this creature in patients who were all on broad range anti-infection agents.
E. meningoseptica has identified numerous putative genes conferring antibiotic resistance was conducted by whole genome research. However, It have been further investigated only a few homologs. It has explored the association between fluoroquinolone reistance and target gene mutations in E. meningoseptica found in recent two studies. Quinolone-resistance-determining regions (QRDRs) of DNA gyrase subunit A (GyrA) and subunit B (GyrB) were detected in the several point mutations. Amino acid alterations Ser83Ile/pro95Ser in GyrA and Ser452Arg/Glu470Asp in GyrB were significantly associated with levofloxacin resistance.
The use of combination therapy rather than use of monotherapy for successful treatment of the patients is recommended.29,30 Although the in-vitro activity of newer tigecycline, rifampicin fluoroquinolones, and minocycline is good, it has been shown that E. meningoseptica infections respond well, when these include antibiotic combinations example are given; vancomycin plus rifampicin, piperacillin-tazobactam plus rifampicin or a fluoroquinolone combined with rifampicin and vancomycin.29,31
The ABSTs by KirbyBauer disc diffusion method revealed 100% resistance to aztreonam, amikacin, ceftazidime, imipenem ,colistin ,gentamicin, cefepime, meropenem, piperacillin/tazobactam, imipenem-cilastatin followed by cefatrizine(93.7%), tigecycline(94.7%), ciprofloxacin (42.8%),and teicoplanin(43.7%),who come to resistance respectively. The susceptibilities of our isolates to minocycline and levofloxacin were noted to be 100% and 58.8% individually. In earlier reports showed that minocycline is the most effective drugs are 100% susceptibility, which may be the drugs of choice for empiric therapy.1,22 Similar finding is found in automated system also.
In summary, E. meningoseptica is a rising and resistant microbe, especially in patients with comorbidities. Prompt diagnosis and proper treatment, dedicated infection controls are key in preventing this organism to become a potential hospital colonizer. Along with health care workers screening for probably carrier should be done. Routine invasive equipment cleaning and sterilization should be emphasized. The above measures be implemented along with clinical awareness and energetic surveillance with continued research of the organism to which will prevent the outbreaks of E. meningoseptica infections.
ACKNOWLEDGMENTS
The authors would like to thank the technical staff at the Department of Microbiology, Institute of Medical Sciences & Sum Hospital, Bhubaneswar, India for their support.
CONFLICT OF INTEREST
All the authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AD collected, analyzed and interpreted the data. SK and KKS conceived and designed the study. AD and DK drafted the manuscript. DK revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Bloch KC, Nadarajah R, Jacobs R. Chryseobacterium meningosepticum: An emerging pathogen among immunocompromised adults. Medicine (Baltimore). 1997;76(1):30-41.
Crossref - Henriques IS, Araujo S, Azevedo JSN, et al. Prevalence and diversity of carbapenem-resistant bacteria in untreated drinking water in Portugal. Microb Drug Resist.2012;18(5):531-537.
Crossref - Lin PY, Chen HL, Huang CT, Su LH, Chiu CH. Biofilm production, use of intravascular indwelling catheters and inappropriate antimicrobial therapy as predictors of fatality in Chryseobacterium meningosepticum bacteraemia. Int J Antimicrob Agents. 2010;36(5):436-440.
Crossref - Jiang X, Wang D, Wang Y, Yan H, Shi L, Zhou L. Occurrence of antimicrobial resistance genes sul and dfrA12 in hospital environmental isolates of Elizabethkingia meningoseptica. World J Microbiol Biotechnology. 2012;28(11):3097-3102.
Crossref - Ceyhan M, Yildirim I, Tekeli A, et al. A Chryseobacterium meningosepticum outbreak observed in 3 clusters involving both neonatal and non-neonatal pediatric patients. Am J Infect Control. 2008;36(6):453-457.
Crossref - Hoque SN, Graham J, Kaufmann ME, Tabaqchali S. Chryseobacterium (Flavobacterium) meningosepticum outbreak associated with colonization of water taps in a neonatal intensive care unit. J Hosp Infect. 2001;47(3):188-192.
Crossref - Ghafur A, Vidyalakshmi PR, Priyadarshini K, Easow JM, Raj R, Raja T. Elizabethkingia meningoseptica bacteremia in immunocompromised hosts: The first case series from India. South Asian J Cancer. 2013;2(4):211-215.
Crossref - Tak V, Mathur P, Varghese P, Misra MC. Elizabethkingia meningoseptica: An emerging pathogen causing meningitis in a hospitalized adult trauma patient.Indian J Med Microbiol. 2013;31(3):293-295.
Crossref - Ratnamani MS, Rao R. Elizabethkingia meningoseptica: Emerging nosocomial pathogen in bedside hemodialysis patients. Indian J Crit Care Med. 2013;17(5):304-307.
Crossref - Hsu MS, Liao CH, Huang YT, et al. Clinical features, antimicrobial susceptibilities, and outcomes of Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) bacteremia at a medical centre in Taiwan, 1999-2006. Eur J ClinMicrobiol Infect Dis. 2011;30(10):1271-1278.
Crossref - Lin YT, Chiu CH, Chan YJ, et al. Clinical and microbiological analysis of Elizabethkingia meningoseptica bacteremia in adult patients in Taiwan. Scand J Infect Dis.2009;41(9):628-634.
Crossref - Lin JN, Lai CH, Yang CH, Huang YH. Elizabethkingia infections in humans: from genomics to clinics. Microorganisms. 2019;7(9):295.
Crossref - Kirby JT, Sader HS, Walsh TR, Jones RN. Antimicrobial susceptibility and epidemiology of a worldwide collection of chryseobacterium spp: report from the SENTRY antimicrobial surveillance program (1997- 2001). J Clin Microbiol. 2004;42(1):445-448.
Crossref - Kim KK, Kim MK, Lim JH, Park HY, Lee ST. Transfer of chryseobacterium meningosepticum and chryseobacterium miricola to elizabethkingia gen. Nov. As elizabethkingia meningoseptica comb. Nov. And elizabethkingia miricola comb. Nov Int J Syst Evol Microbiol. 2005;55(3):1287-1293.
Crossref - Kampfer P, Matthews H, Glaeser SP, Martin K, Lodders N, Faye I. Elizabethkingia anophelis sp. Nov., isolated from the midgut of the mosquito anopheles gambiae. Int J Syst Evol Microbiol. 2011;61(11):2670-2675.
Crossref - Nicholson AC, Gulvik CA, Whitney AM, et al. Revisiting the taxonomy of the genus elizabethkingia using whole-genome sequencing, optical mapping, and maldi-tof, along with proposal of three novel elizabethkingia species: Elizabethkingia bruuniana sp. Nov., elizabethkingia ursingii sp. Nov., and elizabethkingia occulta sp. Nov. Antonie van Leeuwenhoek.2018;111(1):55-72.
Crossref - Lau SKP, Chow WN, Foo CH, et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci Rep. 2016;17(6):26-45.
Crossref - LinX-H,XuY-H,SunX-H,HuangY,LiJ-B. Genetic diversity analyses of antimicrobial resistance genes in clinical Chryseobacterium meningosepticum isolated from Hefei, China. Int J Antimicrob Agents. 2012; 40(2):186-188.
Crossref - Ramanan P, Razonable RR. Elizabethkingia species sepsis after lung transplantation: Case report and literature review. Transpl Infect Dis. 2013;15(6):E229-E234.
Crossref - Weaver KN, Jones RC, Albright R, et al. Acute emergence of Elizabethkingia meningoseptica infection among mechanically ventilated patients in a long-term acute care facility. Infect Control Hosp Epidemiol. 2010; 31(1):54-58.
Crossref - Chang YC, Lo HH, Hsieh HY, Chang SM. Identification and epidemiological relatedness of clinical Elizabethkingia meningoseptica isolates from central Taiwan. J Microbiol Immunol Infect. 2014;47(4):318-323.
Crossref - Rastogi N, Mathur P, Bindra A, et al. Infections due to Elizabethkingia meningoseptica in critically injures trauma patients: A seven-year study. J Hosp Infect. 2016;92(1):30-32.
Crossref - Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol. 2014;61(6):1385-1396.
Crossref - Noor MT, Manoria P. Immune dysfunction in cirrhosis. J Clin Transl Hepatol.2017;5(1):50-58.
Crossref - Pereira GH, Garcia DDO, Abboud CS, Barbosa VLD, Silva PS. Nosocomial infections caused by Elizabethkingia meningoseptica: An emergent pathogen. Braz J Infect Dis. 2013;17(5):606-609.
Crossref - Maraki S, Scoulica E, Manoura A, Papageorgiou N, Giannakopoulou C, Galanakis E. A Chryseobacterium meningosepticum colonization outbreak in a neonatal intensive care unit. Eur J Clin Microbiol Infect Dis. 2009;28(12):1415-1419.
Crossref - Gonzalez LJ, Vila AJ. Carbapenem resistance in Elizabethkingia meningoseptica is mediated by metallo-β-lactamase BlaB. Antimicrob Agents Chemother. 2012;56(4):1686-1692.
Crossref - Jean S, Lee WS,Chen FL, Ou TY,Hsueh PR. Elizabethkingia meningoseptica: An important emerging pathogen causing healthcare-associated infections. Journal of Hospital Infection. 2014;86(4):244-249.
Crossref - Moore LS, Owens DS, Jepson A, et al. Waterborne Elizabethkingia meningoseptica in adult critical care. Emerg Infect Dis. 2016;22(1):9-17.
Crossref - Yang YS, Chun JW, Koh Jw. Keratitis with Elizabethkingia meningoseptica occurring after contact lens wear: A case report. Korean J Ophthalmol .2013;27(2):133-136.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.