ISSN: 0973-7510
E-ISSN: 2581-690X
Mucormycosis is an angioinvasive opportunistic fungal infection, but these have become emerging pathogens, especially in conditions with underlying predisposing risk factors in a favourable setting. With the exponential rise in COVID-19 cases, there was an increase in the number of mucormycosis cases among them. The global prevalence rate of mucormycosis in COVID-19 globally varies from 0.005 to 1.7 per million population and in India, it is approximately 0.14 cases/1000. The objective of this study is to detect the prevalence of mucormycosis with the antifungal susceptibility pattern among COVID-19 patients admitted in our hospital. A total of 347 COVID-19 and post-COVID-19 patients with symptoms suggestive of mucormycosis were included in this study. Nasal scrapings, debrided necrotic tissue, unhealthy tissue bits and biopsy tissues taken through FESS were processed for mycological examination under sterile conditions. Among the total 347 samples processed, 87(25%) were positive for fungal culture. Among the culture positves 7.8% (25) belong to mucorales. Among the total 87 fungal isolates, the majority of organism isolated was Aspergillus sp(68%), followed by Rhizopus sp (18%). Rhizopus/Aspergillus sp (5%), Mucor species (5%), Rhizomucor sp (2%), Mucor/Aspergillus sp(1%), Curvularia sp (1%) were the other fungi isolated. All the strains of Mucorales were sensitive to Posaconazole and one strain showed resistance to amphotericin B with MIC 8 µg/ml by microbroth dilution method based on CLSI M27 guidelines for Amphotericin B, and Posaconazole.
COVID-19, Mucormycosis, Antifungal susceptibility, Rhizopus sp
Mucormycosis is an angioinvasive opportunistic fungal infection caused by a group of fungi belonging to phylum Glomeromycota, family Mucoraceae and order Mucorales. These saprophytic fungi are found ubiquitously in the soil and environment on decaying organic matter. But these have become the emerging pathogens especially in conditions with underlying predisposing risk factors in a favourable setting.1,2
Infection in humans occurs predominantly by the inhalation of sporangiospores, occasionally by ingestion of contaminated food or by traumatic inoculation.3,4 Among the total pathogenic group of Mucorales, Rhizopus arrhizus is the most commonly isolated agent causing mucormycosis all over the world, followed by Lichtheimia, Apophysomyces, Rhizomucor, Mucor and Cunninghamella species.5
Immunocompromised patients, individuals with uncontrolled diabetes mellitus or haematological malignancies are at higher risk for acquiring this infection.6-8 The ongoing Coronavirus disease 2019 (COVID-19) was declared as a global pandemic by the World Health Organisation (WHO) in March 2020.9-12 The disease continued as a major public health concern with more than 162 million cases and more than 3 million deaths globally at the time of this study.13
With the exponential rise in COVID-19 cases, there was an increase in the vulnerability of the patients to secondary bacterial and fungal infections. There was a sudden surge in the number of mucormycosis cases in patients of COVID-19 in the Indian subcontinent. It was during the second wave of COVID-19 pandemic, India had faced an imminent threat in the form of emerging coronavirus disease-associated mucormycosis.14-17 A cascade of events including immune dysregulation, ciliary dysfunction, inflammation due to thrombosis, coagulation in microvasculature and other favourable factors like treatment with steroids, expression of GRP78 in endothelial cell surface in diabetics, availability of free iron all lead to the emergence of Mucormycosis as a complication in COVID-19 patients. Along with these, prolonged stay in hospital, emergency invasive procedures and mechanical ventilation add more risks for the patients to acquire the complication.18,19
The overuse of high-dose glucocorticoids and the administration of highly immunosuppressive drugs to treat patients with the coronavirus disease 2019 (COVID-19) are responsible in part for the increasing number of COVID-associated mucormycosis. Prolonged administration of Corticosteroids will lead to impaired migration, ingestion and phagolysosome fusion in macrophages.20,21
The global incidence rate of mucormycosis in COVID-19 globally varies from 0.005 to 1.7 per million population.22 The prevalence in India is approximately 0.14 cases/1000[23]. This study aims to detect the prevalence of mucormycosis with the antifungal susceptibility pattern among COVID-19 patients admitted to our hospital.
Aim & Objectives
- To determine the prevalence of Mucormycosis among COVID-19 patients
- To isolate and identify the fungal etiological agents of Mucormycosis
- To perform antifungal susceptibility tests for the mucorales group of fungi isolated.
- To analyse the risk factors associated with the disease and follow up of patients.
The study was conducted in the Department of Microbiology, Stanley Medical College, Chennai, for a period of six months from May 2021 to October 2021. A total of 347 patients with COVID-19 or in post-COVID-19 period for up to three months after COVID with clinical symptoms and signs suggestive of Mucormycosis were included in the study. After obtaining Institutional Ethics clearance samples received from suspected cases were processed. Nasal scrapings, debrided necrotic tissue, unhealthy tissue bits and biopsy tissues taken through FESS were all processed in Biosafety cabinet IIA with standard and contact precautions. Based on the nature of the sample, 10% or 20% Potassium hydroxide was used to see the fungal elements in wet mount. The smear was examined after 15 minutes under low and high power microscopy and observed for the presence of fungal elements. The same sample was inoculated onto two tubes of Saboraud’s Dextrose Agar and the tubes were incubated at 37 °C and 25 °C, respectively. The tubes were observed every day upto 7 days for growth. LPCB mount was done for culture positive samples to identify the fungal isolates. The antifungal susceptibility testing was done for the Mucorales group of isolates based on CLSI M27 guidelines for Amphotericin B, and Posaconazole.
Table (1):
Total samples and culture outcome (N=347).
Total Samples |
Culture Positive |
Culture Negative |
|---|---|---|
347 |
87(25%) |
262(75%) |
Table (2):
Koh and culture correlation (N=347).
Culture Positive |
Culture Negative |
|
|---|---|---|
KOH Positive |
64(18%) |
5(2%) |
KOH Negative |
23(7%) |
255(73%) |
Table (3):
Age-Sex distribution oF COVID-19 Associated Mucormycosis (n=27).
Age |
Male |
% |
Female |
% |
|---|---|---|---|---|
21-30 |
3 |
11 |
– |
|
31-40 |
4 |
15 |
– |
|
41-50 |
4 |
15 |
2 |
7.5 |
51-60 |
6 |
22 |
2 |
7.5 |
>60 |
3 |
11 |
3 |
11 |
Total |
20 |
74 |
7 |
26 |
Table (4):
Correlation of COVID-19 associated mucormycosis with comorbidities/risk factors (n=27).
Conditions |
Percentage |
|---|---|
Diabetes mellitus |
12(44%) |
Hypertension |
2(7.4%) |
Diabetes mellitus & Hypertension |
6(22%) |
Cerebrovascular accident |
2(7.4%) |
Sepsis |
1(3.7%) |
Respiratory failure |
1(3.7%) |
None |
3(11%) |
Table (5):
The diverse clinical manifestation in descending order (n=27).
Clinical Manifestation |
F |
M |
Total |
|---|---|---|---|
Acute fungal sinusitis |
6 |
12 |
18 |
Acute rhino sinusitis |
1 |
2 |
3 |
Orbital cellulitis |
2 |
0 |
2 |
Preseptal cellulitis |
1 |
0 |
1 |
Lacrimation |
2 |
0 |
2 |
Blood stained nasal discharge |
0 |
1 |
1 |
Table (6):
Susceptibility of fungal isolates to amphotericin b and posaconazole (N=27) by microbroth dilution method as determined by CLSI M27.
| Fungal Isolate | Drug | MIC(µg/ml) of the isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ||
| Rhizopus sp(20) | Amphotericin B | 5 | 5 | 3 | – | 4 | 1 | – | 1 | 1 | – |
| Posaconazole | 4 | 7 | 6 | 3 | – | – | – | – | – | – | |
| Mucor sp(5) | Amphotericin B | 2 | 1 | 1 | – | – | 1 | – | – | – | – |
| Posaconazole | 2 | 1 | – | 1 | – | – | – | – | – | – | |
| Rhizomucor sp(2) | Amphotericin B | 1 | 1 | – | – | – | – | – | – | – | – |
| Posaconazole | – | 2 | – | – | – | – | – | – | – | – | |
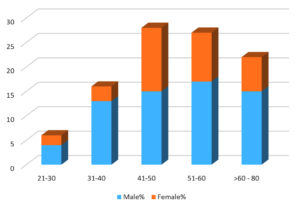
The current study was conducted in the Department of Microbiology, Stanley Medical College, Chennai, for a period of six months from May 2021 to October 2021. A total of 347 samples received during the study period were processed. Among the total 347 patients suspected with Mucormycosis, majority of the patients were male (65%) and the predominant age group was 51-60 years [Figure 1]. In a study conducted by Martin Hoenigl et al.,24 it was shown that the majority of patients were male (62 [78%] of 80).
Among the total 347 samples processed, 87(25%) were positive for fungal culture and 262(75%) were negative for fungal culture [Table 1]. Among The processed samples, both KOH and culture positive were 18%, KOH negative and culture positive were 7% and KOH positive and culture negative were 2% [Table 2]. On comparison of KOH and culture, Sensitivity of KOH is 74%, specificity 98%, positive predictive value 93%, negative predictive value 92% and the accuracy was 92% in this study.
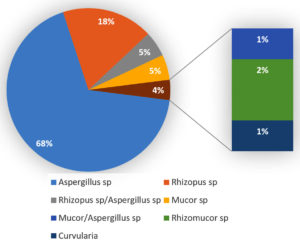
Among the total 87 fungal isolates, the majority of organism isolated was Aspergillus species(68%), followed by Rhizopus sp(18%).Rhizopus/Aspergillus species(5%), Mucor species (5%), Rhizomucor sp (2%), Mucor/Aspergillus sp(1%), Curvularia sp (1%) were the other fungi isolated. Five patients showed co-infection with Mucor species and Aspergillus species [Figure 2]. In a study done by Muhammed Niyas et al., among the total 15 patients, 11 patients had rhino orbital Mucormycosis, 2 patients had invasive Aspergillosis and 2 patients had co-infection with Mucor and Aspergillus.25
Among the total 27 cases of COVID-19 Associated Mucormycosis, the predominant age group was 51 to 60 years with males (22%) and females (7.5%) and the least affected age group was between 21-30 years. The male to female ratio among the most commonly affected age group of 51-60 years is 3:1. [Table 3]. Kumar A et al. suggested that the total number of COVID-19 Associated Mucormycosis cases in a 2 months period were 55 and male to female ratio was 1.89:1.26 Sudhir Bhandari et al. suggested, among the total 231 cases reported with mucormycosis, the most affected age group was 41 – 50 years (28%).
In this study, among the various comorbidities associated with COVID-19 Associated Mucormycosis, patients with diabetes mellitus was 44%, hypertension was 7.4%, both diabetes mellitus and hypertension was 22% [Table 4]. Sudhir et al.27 discussed the most common comorbidity associated with Mucormycosis in COVID-19 patients was Diabetes mellitus. It is being speculated, that this may be due to the exceptional damage to the pancreas caused by “ Delta” or B.1.617.2 and the most acknowledged speculation was combination of diabetes and high dose steroids in which both are considered as the risk factors of mucormycosis.
Acute fungal sinusitis was the most common clinical presentation in 18 patients of culture confirmed cases of COVID-19 associated mucormycosis, followed by acute rhinosinusitis which was presented in 3 patients. Orbital cellulitis and lacrimation were presented in 2 patients. Blood stained nasal discharge and Preseptal cellulitis were reported in 1 patient separately which were the least common clinical presentation in this study [Table 5]. Sangitha kamath et all discussed that 73.3% of Mucormycosis patients were presented with periorbital swelling followed by facial pain and retro-orbital pain (10%) and the least common presentation were cellulitis of cheek, diplopia, facial deviation, dysarthria and tinnitus which all constitute 1%.28 All the strains of Mucorales were sensitive to Posaconazole and one strain showed resistance to amphotericin B with MIC 8 µg/ml [Table 6].
The overall prevalence rate of Mucormycosis in this study is 7.8%. The cases were in peak during the second wave of COVID-19 which could be due to the sudden spurt in the number of COVID-19 cases, prolonged hospital stay, management with O2 and steroids, diabetes mellitus both newly diagnosed and old cases. Cases other than Mucormycosis constitute 17.2% which includes Aspergillus species and Curvularia species. Acute fungal sinusitis is the most common clinical presentation among the culture confirmed cases of Mucormycosis. All the isolates of Mucorales were sensitive to Posaconazole and one isolate of Rhizopus species showed resistance to Amphotericin B with MIC of 8 µg/ml. The prevalence of Mucormycosis in this study is significantly higher when compared to the prevalence in normal population thereby proving that Mucormycosis is one of the major complications of COVID-19.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
VD conceptualized the study and designed the work. RM, VI performed data collection. VD, RM, VI performed data analysis and interpretation.VD, RM, VI wrote the manuscript. VD, PB critically revised the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Chander J. Text book Medical Mycology, 4th Editon, Jaypee Publisher;2018.
- Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and Diagnosis of Mucormycosis: An Update. J Fungi. 2020;6(4):265.
Crossref - Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in Human Disease. Clin Microbiol Rev. 2000;13(2):236-301.
Crossref - Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009;15(Suppl 5):2-9.
Crossref - Prakash H, Ghosh AK, Rudramurthy SM, et al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med Mycol. 2018;57(4):395-402.
Crossref - Lin E, Moua T, Limper AH. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45(4):443-448.
Crossref - Peng M, Meng H, Sun Y, et al. Clinical features of pulmonary mucormycosis in patients with diferent immune status. J Thorac Dis. 2019;11(12):5042-5052.
Crossref - Chakrabarti A, Kaur H, Savio J, et al. Epidemiology and clinical outcomes of invasive mould infections in Indian intensive care units (FISF study). J Crit Care. 2019;51:64-70.
Crossref - Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
Crossref - Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242.
Crossref - Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733.
Crossref - WHO. Listings of WHO’s response to COVID-19. https://www. who.int/news/item/29-06-2020-covidtimeline. Published 2020. Accessed May 16, 2021.
- CCfSSa. Coronovirus COVID-19 global cases. https://gisanddata. maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740 fd40299423467b48e9ecf6. 2021. Accessed May 16, 2021.
- Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9):e10726.
Crossref - Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395-1399.
Crossref - Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442-447.
Crossref - Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186(2):289-298.
Crossref - Rudramurthy SM, Singh G, Hallur V, Verma S, Chakrabarti A. High fungal spore burden with predominance of Aspergillus in hospital air of a tertiary care hospital in Chandigarh. Indian J Med Microbiol. 2016;34(4):529-532.
Crossref - WHO COVID-19 Weekly Epidemiological Update.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20210525_weekly_epi_update_41.pdf?sfvrsn=d602902c_6&download=true. 2021. Accessed on May 28, 2021.
- Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245-e253.
Crossref - Song G, Liang G and Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185(4):599-606.
Crossref - Black fungus: here is a list of states with highest number of mucormycosis cases. Hindustan Times. May 21, 2021. https://www.hindustantimes.com/india-news/black-fungus-states-with-highest-number-of-mucormycosis-cases-101621559394002.html. Date Accessed: May 28, 2021.
- Chander J, Kaur M, Singla N, et al. Mucormycosis: Battle with the Deadly Enemy over a Five-Year Period in India. J Fungi (Basel). 2018;4(2):46.
Crossref - Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022;3(7):543-52.
Crossref - Niyas VKM, Arjun R, Felix V, Kumar MAS, Lalitha S. COVID-19 associated Invasive Fungal Rhinosinusitis: A Retrospective Analysis of 15 Cases. Int J Infect Dis. 2022;116(Suppl):S56.
Crossref - Kumar A, Verma M, Hakim A, Sharma S, Meena R, Bhansali S. Epidemiology of Mucormycosis Cases During the Second Wave of COVID-19 in a Tertiary Care Institute in Western Rajasthan, India. Cureus. 2008;14(3):e22973.
Crossref - Bhandari S, Kakkar S, Dube A, et al. Mucormycosis Infections during the Second Wave of COVID-19: Experience from a Tertiary Care Centre in India. Modern Medicine. 2021;28(4):425-431.
- Kamath S, Kumar M, Sarkar N, Ahmed T, Sunder A. Study of Profile of Mucormycosis During the Second Wave of COVID-19 in a Tertiary Care Hospital. Cureus. 2022;14(1):e21054.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.