Neelusree Prabhakaran, S.S.M. Umamageswaria*,
Muthumari and Kalyani Mohan
Department of Microbiology Saveetha Medical College and Hospital, Saveetha Nagar, Thandalam, Kancheepuram, Chennai – 602105, India.
ABSTRACT
Morbidity and mortality due to the rise in the incidence of fungal infections has increased significantly. The genus Candida is the most common cause of fungal infection worldwide. Recent evidence suggests that majority of infections produced by this pathogen are associated with biofilm growth. Biofilms are colonies of microbial cells encased in a self produced organic polymeric matrix. The biofilm production is more important for Non Albicans Candida (NAC); as C. albicans possess many other mechanisms to establish infections. Correct identification of Candida species has gained importance due to persistent rise in infections caused by NAC. The development of resistance in Candida is also raising due to the increase in immunocompromised status and critical patients. To study the prevalence of Candida species, biofilm formation for Candida isolates and their antifungal susceptibility pattern in a tertiary care centre.Sixty eight clinical isolates of Candida were collected from various clinical samples in a tertiary care centre, Chennai for a period of six months. Identification of Candida species was done by germ tube tests, test for chlamydoconidia production, sugar assimilation tests and CHROM agar isolation method. Antifungal susceptibility tests against fluconazole, Amphotericin B and Flucytosine were performed by disc diffusion method and for the resistant Candida strains Minimum inhibitory concentration (MIC) was performed using E-strip for fluconazole, Amphotericin B and Flucytosine. Out of 68 isolates C.albicans tops the first 67.6%, C. tropicalis ranks the next most predominant species 17.64% and the least common isolates C.parapsilosis 8.8% and C.glabrata 5.8%. Candidial infections were more commonly observed in female patients 57.3% and hospitalized in- patients 69.1% with increased number from non – catheterized urine samples. The Fluconazole susceptibility using disc diffusion showed 69% of C.albicans and 58.3% of C.tropicalis and 75.5% of C.parapsilosis were sensitive to fluconazole and sensitivity to Flucytosine were as follows C. albicans (86.60%) C. tropicalis (75%) C.parapsilosis and C.glabrata (100%).Though a higher degree of antifungal resistance noted among the various Candida species, still can be used as a treatment drug for critical care patients as the MIC of those anti fungal drugs were found to be in the susceptible range. Isolation of NAC from clinical samples, their biofilm formation and antifungal resistance are on a rise, they should be treated as a pathogen and not dismissed.
Keywords: CHROM agar, Minimum Inhibitory Concentration, Biofilms, Candida species.
INTRODUCTION
Candida spp are among the members of the normal flora of skin, mucous membrane and gastrointestinal tract. In the recent years due to advances in medical technologies there is an increase in number of immunocompromised individuals which has resulted in rise in the incidence of fungal infections especially those due to Candida species1. There are more than 200 spp, of which C.albicans is the most common, other commonly isolated species are C.glabrata, C. parapsilosis, C.tropicalis and C.krusei. Overgrowth of these fungus results in Candidiasis (Candidosis) which can cause superficial, subcutaneous and deep mycosis. The incidence of Candidemia in intensive care unit (ICU) patients is also on a rise. In addition, hospital acquired infections by C. albicans have become a major health concern2.The generous use of broad spectrum antibiotics and novel molecules to some extent have checked the infectious disease, but simultaneously have paved way for new advanced and modalities of opportunistic pathogen viz., Candida. Diabetes mellitus, the leading endocrine dysfunction and HIV infection, the new world pandemic provided a potent soil for Candidial infection.2
Infection caused by NAC may be indistinguishable but the importance in their detection lies in the fact that many non albicans species are inherently resistant or likely to acquire resistance to the routinely used anti-fungal drugs. One of the reasons would be biofilm which may form on the surface of implantable medical devices. Biofilms are colonies of microbial cells encased in a self-produced organic polymeric matrix and represent a common mode of microbial growth. Recently, microbial biofilms have gained prominence because of the increase in infections related to indwelling medical devices.(3) The advantages of forming a biofilm for the organism include protection from the environment, nutrient availability, metabolic cooperation and acquisition of new genetic traits.4 Biofilms may help maintain the role of fungi as commensal and pathogen, by evading host immune mechanisms, resisting antifungal treatment and withstanding the competitive pressure from other organisms. Consequently, biofilm related infections are difficult to treat.5 The biofilm production is also associated with high level of antimicrobial resistance of the associated organisms.6 The proportion of infections due to NAC is persistently increasing the need for a correct identification of Candida isolates at the species level. Although C. albicans remains the most common fungal isolate recovered from blood, recent reports indicate a trend toward an increasing prevalence of infections caused by NAC.7-10 The proportion of biofilm production is much higher among isolates of NAC species recovered from blood than other sites.11
MATERIALS AND METHODS
A descriptive study was undertaken in a tertiary care centre, Chennai for a period of six months. Candida strains were collected from various clinical samples like urine, exudates, blood and sputum (confirmed as Candida by observing the characteristic budding yeast cells with pseudohyphae in gram stain and 10% KOH mounts).Only pure growth of Candida spp (no other aerobic pathogens) excluding normal flora were included in this study. The confirmed Candida isolates were further speciated by germ tube test, chlamydoconidia production test on corn meal agar, sugar assimilation tests for Dextrose, Lactose, Sucrose, Trehalose and Maltose and by the colour of growth on HiChrom Candida agar isolation method, Hi media laboratories Pvt. Ltd, India.
Biofilm formation: Presterilized polystyrene, flat bottom 96 well microtitre plate were used.
Procedure:
Step 1
Adhesion phase
100 ml of standardized cell suspension (10-7 cells/ml) was transferred into each well of plate with a sterile pipette and Incubated for 1.5 hours at 37°C in a shaker at 75 rpm. (Yeast adheres to the surface of the wells).
Step 2
Cell suspension was aspirated and wells washed twice with 150 ml of Phosphate – Buffered Saline (PBS) to remove closely adherent cells.
Step 3
100 ml of yeast Nitrogen base medium was transferred to the wells and Incubated at 37°C (75 rpm)
Step 4
Biofilm was allowed to develop for up to 66 hours. Yeasts were quantified by crystal violet staining methods. The medium was replenished daily by aspiration of fresh medium.
Crystal violet staining
Biofilm formation was quantified by a modification of crystal violet assay. Biofilm plates were washed twice with 200 ml of PBS, air dried for 45 minutes. Wells were stained with 110 ml of 0.4% aqueous crystal violet solution for 45 minutes. Candida albicans ATCC 90028 and Candida parapsilosis ATCC 96142 were used as controls.
Antifungal susceptibility test
Anti-fungal susceptibility testing was performed on Muller Hinton agar with 0.5 µg/ml methylene blue and 20% glucose. The turbidity of the inoculums was compared with 0.5 McFarland standard. Tests were interpreted after 24 hours incubation at 37°c by visual method with reading mirror and compared with controls (C. albicans ATCC 90028 and C. parapsilosis ATCC 95142). The zone size was compared with NCCLSI guidelines.
RESULTS
A descriptive study was carried out in a tertiary care centre, Chennai for a period of six months. Both in- patients and out patients samples were included in this study. A total of 68 Candida species were isolated and included in this study. In our study we observed Candidial infections were more among the in-patients 69.1% ( n=47) than out patients 30.8%(n=21) with an increased number of female predominance n= 39 (57.3%) than male patients n= 29(42.6%).The maximum number of isolates were obtained from patients in the age group 31-50 years. The demographic distribution of the patients included in the study is shown in table 1; fig 1
Table 1: Demographic distribution of patients
Age |
10-20 |
21-30 |
31-40 |
41-50 |
>50 |
Male |
3 |
6 |
9 |
6 |
5 |
Female |
2 |
2 |
11 |
18 |
6 |
TOTAL |
5(7.3%) |
8(11.7%) |
20(29.4%) |
24(32.3%) |
11(16.1%) |
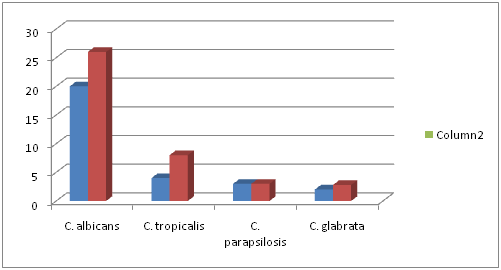
Fig. 1. Distribution of Candida species in male and female
The mentioned table 2 shows the commonest source of isolation of Candida species in the decreasing order of frequency with increase number in urine samples 70.5% (among which non –catheterised sample were 68.7%) followed by exudates 20.5%, respiratory 5.8% and blood 2.9%.
Table 2: Isolation of C. albicans and Non – Albicans Candida from various clinical samples
Sample |
Total number |
C.albicans |
Non Albicans Candida |
Urine (voided and catheter) |
48(70.5%) |
29(42.6%) |
17(27.9%) |
Exudate |
14(20.5%) |
11(16.1%) |
5. (4.4%) |
Respiratory |
4 (5.8%) |
4 (5.8%) |
– |
Blood |
2 (2.9%) |
2 (2.9%) |
– |
Identification of Candida species
On performing germ tube test we found that C.albicans were n=46 (67.6%) and Non-albicans Candida n= 22 (32.3%). Based on germ tube test and chlamydoconidia production tests, CHROM agar test and sugar assimilation tests were performed. In our study among the NAC,C. tropicalis ranks the first n= 12(54.5%), n= 6 (27.2%) were C. parapsilosis and only n= 4 (18.1%) of C. glabrata; fig 3, fig 4, fig 5
Fig 3: CHROM agar identification of Candida species:

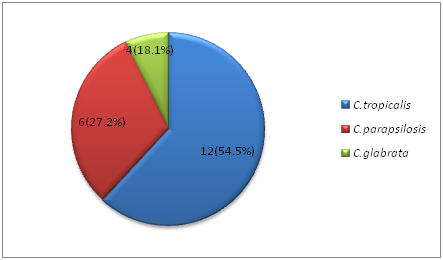
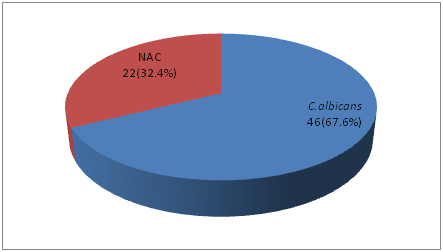
Fig. 4: Break up of Candida isolates

Fig. 5: Break up of Non Albicans Candida species
Biofilm formation was done by using 96 microtitre plate method and quantified using 0.4% crystal violet. C. albicans showed heavy biofilm formation, C. tropicalis and C. glabrata showed moderate biofilm formation and other Candida species showed weak biofilm formation.Table 3; fig 6, fig 7
Table 3: Biofilm formation by Candida species
Biofilm formation |
C. albicans |
C .tropicalis |
C. glabrata |
C. parapsilosis |
Heavy |
26 |
1 |
3 |
2 |
Moderate |
10 |
11 |
3 |
2 |
Antifungal susceptibility pattern of the Candida isolates is shown in below table 4,
table 5.
Table 4: Fluconazole susceptibility testing of Candida isolates
Fluconazole |
C.albicans
n =46 |
C.tropicalis
n=12 |
C.glabrata
n=6 |
C.parapsilosis
n=4 |
Sensitive |
32(69.5%) |
7(58.3%) |
– |
3(75%) |
Resistant |
14(30.4%) |
5(41.6%) |
6(100%) |
1(25%) |
Table 5: Flucytosine susceptibility testing of Candida isolates
Flucytosine |
C.albicans
n =46 |
C.tropicalis
n=12 |
C.glabrata
n=6 |
C.parapsilosisn=4 |
Sensitive |
38(82.6%) |
9(75%) |
6(100%) |
4(100%) |
Resistant |
8(17.3%) |
3(25%) |
– |
– |
- parapsilosis showed maximum sensitivity to fluconazole (75%) followed by C. albicans (69.5%) and C.tropicalis (58.3%). All flucytosine resistant Candida strains were found to fluconazole resistant. Keeping in mind the intrinsic resistance to azoles by C.glabrata. MIC for the resistant strains n=20 shown in the below table 6
Table 6: Minimum inhibitory concentration of resistant strains
SPECIES |
ANTIFUNGAL AGENT |
50s |
90s |
RANGE |
C.albicans |
Flu
Fe Amp |
0.50
0.19 0.016 |
64.0
0.75 0.012 |
0.064-<256
0.023-2 >0.002-0.064 |
C.tropicalis |
Flu
Fe Amp |
1.00
0.047 0.002 |
2.00
0.125 0.016 |
0.5-2
0.023-0.19 >0.002-0.047 |
C.parapsilosis |
Flu
Fe Amp |
0.50
0.032 >0.002 |
1.00
0.047 >0.002 |
>0.25-1
0.003-0.5 >0.002-0.002 |
Flu- Fluconazole , Fe- Flucytosine , Amp- Amphotericin B
Fluconazole exhibited MIC 50 at 0.50µg/ml for C. albicans, 1.00 µg/ ml for C. tropicalis and C. parapsilosis0.50 µg/ ml. Flucytosine showed MIC 50 at 0.19µg/ ml for C. albicans and C. tropicalisat 0.047µg/ml for C. parapsilosisat 0.032µg/ ml. Amphotericin B exhibited MIC 50 at 0.016µg/ml for C. albicans, 0.002µg/ml for C. tropicalis and C. parapsilosis>0.002µg/ml.
Fluconazole exhibited MIC 90 at 64.0µg/ml for C. albicans, 2.00 µg/ml for C. tropicalis and C. parapsilosi1.00 µg/ml. Flucytosine showed MIC 90 at 0.75µg/ml for C. albicansand C. tropicalisat 0.125µg/ml for C. parapsilosis at 0.047µg/ ml. Amphotericin B exhibited MIC 90 at >0.002µg/ml for C. albicans, >0.002µg/ml for C. tropicalis and C. parapsilosis>0.002µg/ml.In this study candida isolates showed 83% susceptiblity to Fluconazole. For Flucytosine, read at almost complete inhibition of growth (98%) and for amphotericin B, at the point of complete inhibition (100%).
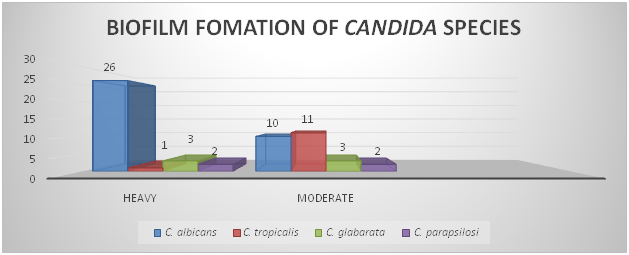
Fig 6: Biofilm formation of Candida species
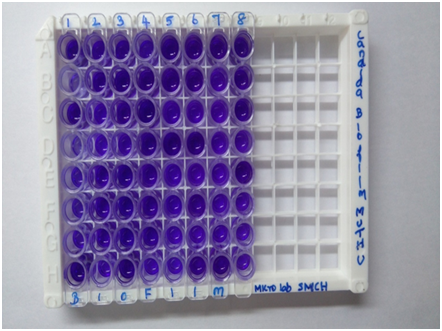
Fig 7: Biofilm production of Candida species
Discussion
The last few decades have seen an increase in Candidemia.(12) Of all the known species of Candida only 10% are pathogenic to humans. Unlike the previous reports we are now universally noticing an increase in the incidence of NAC.
A total of 68 Candida species were isolated over a period of six months from various clinical samples, in a tertiary care centre, Chennai. Out of 68 Candida species, 46(67.6%) were C.albicans, the rest were NAC of which 12(17.6%) C.tropicalis, 6(8.8%) C. parapsilosis and 4(5.8%) C.glabrata. Among the isolates, C.albicans predominated i.e (67.6%). Predominance of C.albicans were also observed in other studies done by L.Sumithradevi etal(13) which showed 31 ( 52%) C.albicans isolated out of 60 samples, and in another study done by Sri Janani B et al(14) 27 (39%) C.albicans were isolated out of 70 samples.
However higher incidence of NAC were also observed in various studies. In this study among the non albicans species, C.tropicalis is found to be the most predominant species similar to the study done by Kothavade RJ et al(15) out of 429 samples 64% were C.tropicalis and only 14% were C.albicans. The reason for predominance or rise in infections of C.tropicalis or NAC is unknown.
Given that females are more susceptible to Candida infection due to physiological factors (age, pregnancy, use of oral contraceptives) could be the reason why our study shows Candida species isolated were more in the female patients 39 (57.3%) similar to the study done by Dr.Jayalakshmi L et al (2) which showed that out of 100 samples 57 Candida species were isolated from female patients.
The sample gaining importance is urine due to increased incidence of biofilm production in the Catheterised patients. Biofilm as a virulence factor thus appears to contribute most in pathogenesis of urinary tract infection and other luminal infections, compared to other clinical conditions. In this study increased number of Candida were isolated from urine samples ; non – catheterised samples 33(68.7%%) which is similar to a study done by N Pahwa et al (16) were of 237 samples the maximum isolates were from urine samples 19% were from voided sample and (13.9%) from urine of patients with in-dwelling urinary catheter .A study by Tumbarello et al (17) showed .C albicans was predominant in catheterized patients than non albicans out of 294 patients 136 were positive for C.albicans and 90 were positive for non albicans unlike our study were we found that out of the 15 (23%) samples from catheterized patients 6 were positive for C. albicans and 9 were positive for non albicans.
In our study CHROM agar was helpful for presumptive identification of NAC and can be used in case of multi species opportunistic infection. In our study out of 68 isolates, culture based on CHROM agar showed 100% growth accuracy to C.albicans, C.tropicalis,C. parapsilosis and C. glabrata, similar to the studies done by Barry AL, Brown SD et al(18) and Beighton.D et.al (19). Another study done by Jain N et al(20) reported that the performance of a commercially available chromogenic Candida speciation as highly accurate out of 429 samples processed on CHROM agar showed over 82% accuracy to all the isolates.
The biofilm growth protects the microorganism from the host defense and antimicrobial agents. In this line, biofilm formation is a risk factor that increases the mortality rate in Candidiasis in critically ill patients or immunocompromised individuals.(17) Recent studies have documented a shift toward NAC species from C. albicans.(21) Some studies have reported increasing trend of incidences of infections caused by NACs, gradually surpassing C. albicans as cause of Candidemia in some regions.(22) Factors such as increased use of antifungal drugs and broad spectrum antibiotics, long-term use of catheters and increase in the number of immunocompromised patients have contributed to the emergence of NAC species in increasing numbers.(15,23-24) In this study C.albicans isolates showed strong biofilm formation similar to the study done by Ana siladias et al(25) which showed out of 184 isolates 49 C.albicans showed heavy biofilm formation. Although in our study the percentage of strong biofilm producers of NAC is not very high, this finding could be incidental and cannot be generalized as the sample size is very small and needs to be studied in higher number of isolates.
Biofilms represent the most prevalent type of microbial growth in nature and are crucial to the development of clinical infections.(26) The ability to form extensive biofilms on the surface of catheters, and other prosthetic devices also contributes to the high prevalence of the organism as etiologic agent of intravascular nosocomial infections.(27) Biofilm formation should be considered as an important virulence determinant during candidiasis.(5)
Diabetes mellitus is reported to be a general risk factor for Candida infections, as the glucose is thought to serve as the carbohydrate energy source required by Candida for biofilm formation, perhaps necessary to produce the polysaccharide matrix. Hence, it is plausible that a hyperglycaemic condition may favour adaptation of Candida organisms to a biofilm lifestyle. In this study out of 68 patients 33 (48.5%) were diabetic related to the study done by Tumbarello et.al(17)
In this study antifungal susceptibility test for fluconazole showed 69.5% of C. albicans as sensitive and 30.4% as resistant to the drug which is similar to the study done by Dr.Jayalakshmi et.al(2) which showed (n=25) 84.8% sensitivity and (n=4) 12.1% resistance. The same study shows (n=15) 28.6% sensitivity to C.tropicalis and (n=18) 64.3% resistance. In our study C. tropicalis showed 58.3% sensitivity (n=7) and 41.6% resistance (n=5).C. Parapsilosis showed (n=3) 75.5% sensitivity and (n=1) 25% resistance related to the above mentioned study which showed (n=7) 63.6% sensitive and (n=3) 27.3% resistance. In our study antifungal susceptibility test for flucytosine showed C.albicans n=8 (17.3%), C.tropicalis n=3 (10.86%). No resistance observed among C.glabrata and C.parapsilosis similar to study done by N Pahwa et al (16). Antifungal susceptibility test for Amphotericin B showed 100% sensitivity.
In our study resistance strains n=20 were selected and MIC was done using E-STRIP for fluconazole, Amphotericin B, Flucytosin. In present study Candidial isolates showed 69.5% susceptible to Fluconazole similar to the study done by R Adhikaryet al(28) which showed 75% susceptibility to Fluconazole. In this study for flucytosine, read at almost complete inhibition of growth (98%) and for amphotericin B, at the point of complete inhibition (100%) similar to the study done by Sharon C.A.Chen et.al(29)which showed (90- 94%) susceptible to flucytosin and (100%) susceptible to Amphotericin B.
Conclusion
Due to the increasing incidence of Candida infections, there is great interest in Candida virulence factors, which are in turn important in the establishment of the strategies for control and prevention of Candidiasis.(30) NAC species cannot be overlooked as mere contaminant or nonpathogenic commensals. Research on prevalent Candida species along with their virulence factors in a given set up would be an important tool to prove the relation between the infective species of Candida and infection. The changing patterns of the Candida isolation from various clinical samples have made identification of Candida species producing virulence factors compulsory for diagnostic Microbiology service.(31) This changing trend of causative role of Candida in different studies from different parts of the world and from India and the emergence of NAC species and their association with virulence factors cannot be overlooked.(23) Increased isolation and complete identification of Candida species in more Microbiology laboratories might be instrumental in reports of rising NAC emergence. More multicenteric studies on larger sample size will definitely go a long way in revealing epidemiology, emergence and spread of NAC.
Acknowledgments
We would like to Acknowledge and thank our professors for their help, support, suggestions and guidance for completion of this study.
References
- Rizvi MW, Malik A ,Shahid M , Singhal S . Candida albicans infections in a north Indian tertiary care hospital: antifungal resistance pattern and role of SDS- PAGE for characterization ,Biology and Medicine, 2011; 3 (2) Special issue 176-181.
- Dr.Jayalakshmi L, Dr. G. Ratnakumari, Dr. S.H. Samson. Isolation, speciation and antifungal susceptibility testing of Candida from clinical specimens at a tertiary care hospital- Sch. J. App. Med. Sci., 2014: 2(6E): 3193-3198.
- Mukherjee PK, Zhou G, Munyon R, Ghannoum MA. Candida biofilm: A well designed protected environment. Med Mycol 2005;43:191 208.
- De Bernardis F, Agatensi L, Ross IK, Emerson GW, Lorenzini R, Sullivan PA, et al. Evidence for a role for secreted aspartate proteinase of Candida albicans in vulvovaginal candidiasis. J Infect Dis 1990; 161:1276 83.
- Baillie GS, Douglas LJ. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol 1999;310: 644 56.
- Ozkan S, Kaynak F, Kalkanci A, Abbasoglu U, Kustimur S. Slime production and proteinase activity of Candida species isolated from blood samples and the comparison of these activities with minimum inhibitory concentration values of antifungal agents. MemInstOswaldo Cruz 2005; 100:319 23.
- Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non immunosuppressed patients. LancetInfect Dis 2003; 3: 685 702.
- Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: Frequency of occurrence and antifungal susceptibility in the SCOPE Program. SCOPE Participant Group. Surveillance and Control of Pathogens of Epidemiologic.Diagn Microbiol Infect Dis 1998; 30:121 9.
- Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, et al.Risk factors for candidal bloodstream infections in surgical intensive care unit patients: The NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey.ClinInfect Dis 2001; 33:177 86.
- Miller LG, Hajjeh RA, Edwards JE Jr. Estimating the cost of nosocomial candidemia in The United States. Clin Infect Dis 2001; 32:1110.
- Hawser SP, Douglas LJ. Biofilm formation by Candida species on the surface of catheter Materials in vitro. Infect Immun 1994; 62:915 21
- Xess I, Jain N, Hassan F, Mandal P, Banerjee U. Epidemiology of Candidemia at a tertiary care centre of North India: 5 year study. Infection 2007; 35:256-9.
- L. Sumitra Devi, MeghaMaheshwari. Speciation of Candida Species Isolated From Clinical Specimens by Using Chrom Agar and Conventional Methods –International Journal of Scientific and Research Publications, 4(3), March 20141 ISSN 2250-3153 www.ijsrp.org
- Sri Janani B., Premamalini T., Rajyoganandhan S.V., Anupma J., Kindo. Pattern of susceptibility to azoles by E- test method in candidema patients- International journal of research in medical science / August 2015/ vol 3/ issue 8.
- 15. Kothavade RJ, Kura MM, Valand AG, Panthaki MH.Candida tropicalis: Its prevalence, pathogenicity and increasing resistance to fluconazole. J Med Microbiol 2010; 59:873 80.
- N Pahwa, R Kumar, S Nirkhiwale, A Bandi. Species distribution and drug susceptibility of candidain clinical isolates from a tertiary care centre at Indore- IJMM Year : 2014; 32(1): 44-48.
- Mario Tumbarello,BrunellaPosteraro, Enrico Maria Trecarichi,Barbara Fiori,MariannaRossi,RosariaPorta, Katleen de Gaetano Donati, Marilena La Sorda, Teresa Spanu, GivanniFadda, Roberto Cauda, and Maurizio Sanguineti. Biofilm Production by Candida species and Inadequate Antifungal Therapy as Predictors of Mortality for Patients with Candidemia- Journal of Clinical microbiology, 2007: 1843-1850.
- Barry AL, Brown SD. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J ClinMicrobiol.1996; 34:2154-57.
- Beighton. D.; Ludford, R.; Clark, D.T. et al. Use of CHROMagar Candida medium for isolation of yeasts from dental samples. J Clin Microbiol.1995; 33: 3025-7.
- Jain N1, Mathur P, Misra MC, Behera B, Xess I, Sharma SP. Rapid identification of yeast isolates from clinical specimens in critically ill trauma ICU patients.-J Lab Physicians. 2012 Jan; 4(1):30-4. doi: 10.4103/0974-2727.98667
- Dan M, Poch F, Levin D. High rate of vaginal infections caused by non C.albicansCandida species among asymptomatic women. Med Mycol 2002; 40:383 6.
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. ClinMicrobiol Rev 2007; 20:133 63.
- Kumari V, Banerjee T, Kumar P, Pandey S, Tilak R. Emergence of non albicans Candida among candidalvulvovaginitis cases and study of their potential virulence factors, from a tertiary care center, North India. Indian J PatholMicrobiol 2013; 56:144 7.
- Shivaprakasha S, Radhakrishnan K, Karim PM. Candida spp. other than Candida albicans: A major cause of fungaemia in a tertiary care centre. Indian J Med Microbiol 2007; 25:405 7.
- Ana Silva –Dias, Isabel M. Miranda, Joana Branco, Matildemonterio- soares, Cidaliapina- Vaz and Acacio G. Rodrigues .Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: relationship among candida spp- Microbiol., 12. March 2015.
- Chander J. Textbook of Medical Mycology. 3rd ed. New Delhi: Mehta Publishers; 2008. p.278 83.
- Matsumoto FE, Gandra RF, Ruiz LS, Auler ME, Marques SA, Pires MF,et al. Yeasts isolated from blood and catheter in children from a public hospital of São Paulo, Brazil. Mycopathologia 2002; 154:63 9.
- R Adhikary, S Joshi-Species distribution and anti-fungal susceptibility of Candidaemia at a multi super-specialty center in Southern India- IJMM 2011; 29(3) : 309-311
- Sharon C. A. Chen, Maryann L. O’Donnell, Suzannah Gordon and Gwendolyn L. Gilbert- Antifungal susceptibility testing using the E test: comparison with the broth macro dilution technique-Journal of Antimicrobial Chemotherapy 1996: 37, 265-273.
- Salyers A, Witt D, editors. Virulence factors that promote colonization.In: Bacterial Pathogenesis: A Molecular Approach. Washington, D.C.:ASM Press; 1994. p.30 46.
- Deorukhkar SC, Saini S. Non albicansCandida species: Its isolation pattern, species distribution, virulence factors and antifungalsusceptibility profile. Int J Med SciPublic Health 2013; 2:533 8.

