ISSN: 0973-7510
E-ISSN: 2581-690X
Irula tribes in india live in rural areas under poor infrastructure, high poverty, low education, ignorant on the origin of diseases. Although, their lifestyle made them vulnerable to a variety of infectious diseases. Only few studies have been conducted on them, hence, there is inadequate data about these Irula tribal health. Serum samples were collected from 372 Participants of Irula tribes from 15 different locations of Tamil Nadu. Serum samples were tested for Hepatitis C Virus (Anti-HCV) by 3rd generation ELISA kit and data were subjected to analysis using SPSS (version 17.0) and Chi square test to determine the risk factors of Hepatitis C virus. HCV prevalence was 5.10 % and this percentage was high among females than males with the age group of 31 to 40. Among the various risk factors, were statistically analyzed (p<0.05) with HCV infection. The frequency of combination of risk factors were found in HJ+A+IDU+SI+M (100 %), A+IDU+SP (100 %) in Anti-HCV positive Irula tribes of Tamil Nadu. High prevalence of HCV infection was observed among the tribal population with the various risk factors such as series of injection, surgery with or without blood transfusion, tattooing sexual promiscuity, migration, jaundice in family and intravenous drug use.
Hepatitis C virus, Risk factors for HCV, Irula Tribes of Tamil Nadu
India is the second largest populated country in the world. India possess one of the largest tribal populations in the world and also India ranks number 2 in the list of countries based on the tribal population[1]. In India, the commonly accepted term for tribal indigenous people is “Adhivasi”, which refers to the people living in tribal communities are characterized by “distinct culture and dialect, geographical isolation and simple pre-literate people living in forests and hills, sharing a symbiotic relationship with nature” [2]. The tribal groups in India form a significant proportion (8.01 %) of the more than one billion population and they are believed to be the earliest settlers or inhabitants of the region. There are 427 tribal communities found in India which was known so far [3].
In Tamil Nadu state, Irulas are recognized as a Scheduled Tribe by the Government of India [4]. The Irula tribe is the most numerous and is confined to different locations [5]. Irulas live as a strongly knitted community; they lived originally in forests or in rural areas. They ate vegetables and meat food including fried rats. They are also identified as “rat and snake catchers” and “snake hunters” [6]. Many Irulas have been forced to leave the forest conservation reserves by the introduction of strict Forest Acts in 1976. These Irulas started moving to neighboring villages in search of new livelihood [7]. Tattooing, migrations and sexual promiscuity plays a major role in transmission of viral diseases[6,8,9,10]. Their life style made them vulnerable to a variety of infectious diseases. Only very few studies have been conducted exclusively on health status of Irula tribes though a few works analyze their health issues along with other tribes [11]. The present study analyzed the prevalence of hepatitis C virus with the associated risk factors in Irula tribal population of Tamil Nadu, India.
Population study
Tribal communities in 15 different habitants from Kanchipuram (Dist.), Cuddalore (Dist.) and Thiruvallur (Dist.) of Tamilnadu in India have been randomly selected for the study. The Irula tribal communities were persuaded by the tribal leaders to arrange a camp in the nearest primary health centers for sample collection. A proforma was formatted for this study and consent form was obtained from the participants (above 18 years of age) in their vernacular language.
Sample collection
From a total of 372 participants, 5 ml of blood was collected and the serum was separated by centrifugation and transported in dry ice. The serum samples were labeled and stored immediately at -20oC.
Serology
After bringing all the samples and reagents to the room temperature (20 to 25oC), the serum samples were screened for anti- HCV by using the commercial ELISA test kits (III generation ELISA Kit, J. Mitra & Co. Pvt. Ltd, India). The Absorbance OD value of a processed serum samples were taken in ELISA reader (Robonik – Absorbance microplate reader) at 450nm and the cut off value was calculated as per the instruction manual. Then all the positive cases were retested thrice for the confirmation of Anti-HCV (Hepatitis C virus).
Statistical Analysis
Statistical package for the social software SPSS (version 17.0) was used for analyzing the data. The chi-squared test was used to find out the significance of Hepatitis B virus with the risk factors. The p value <0.05 was used to indicate statistical significance.
Out of the 372 Irula tribes, 118 were males and 254 were females and the majority of them were in the age group of 21-30 years. The overall seroprevalence of Hepatitis C was 5.10% (19/372), which was more in females 14 (5.51%) than in males 5 (4.23%) in the age group of 21-60 years. The selected study indicates the high prevalence of HCV positivity were in 31 to 40 followed by 51 to 60 age groups of Irula tribes and also shows that females are mostly affected by Hepatitis C infection (Table 1).
Table (1):
Age & sex distribution data from Irula tribal community for Hepatitis C virus infectivity.
| Age group | No. of participants | Male | Female | Total Percentage | ||
|---|---|---|---|---|---|---|
| No. tested | Anti-HCV +ve | No. tested | Anti-HCV +ve | |||
| 18-20 | 41 | 16 | 0 | 25 | 0 | 0 |
| 21-30 | 115 | 34 | 2 (5.88 %) |
81 | 4 (4.93%) |
6 (5.21 %) |
| 31-40 | 93 | 18 | 2 (11.11%) |
75 | 8 (10.66%) |
10 (10.75 %) |
| 41-50 | 64 | 22 | 0 | 42 | 1 (2.38 %) |
1 (1.56 %) |
| 51-60 | 42 | 17 | 1 (5.88%) | 25 | 1 (4.00 %) | 2 (4.76 %) |
| 61-70 | 14 | 8 | 0 | 6 | 0 | 0 |
| 71-80 | 3 | 3 | 0 | 0 | 0 | 0 |
| Total | 372 | 118 (31.72%) | 5 (4.23%) |
254 (68.27%) | 14 (5.51%) |
19 (5.10 %) |
All the 372 study subjects of Irula tribal population were evaluated with 10 different risk factors and behavioral aspects and correlated with Anti-HCV (i.e., Hepatitis C virus) as an evidence for Hepatitis C virus infection and obtained results were statistically analyzed for the significance.
This study shows that most of the Irula tribal peoples are addicted to alcoholism (191/372) and like to tattoo (236/372) their names in their hands and arms. And sexual promiscuity (108/372) shows high risk in transmission of sexually transmitted diseases like hepatitis and syphilis and HIV. Irula tribal peoples migrate (153/372) from one place to another for the occupation. Followed by the history of jaundice, jaundice in family, surgery with or without blood, series of injection, drug use are also showing the risk for transmission of hepatitis virus infection and these were statistically proved as significant risk factors for Hepatitis C Virus infection (Figure 1).
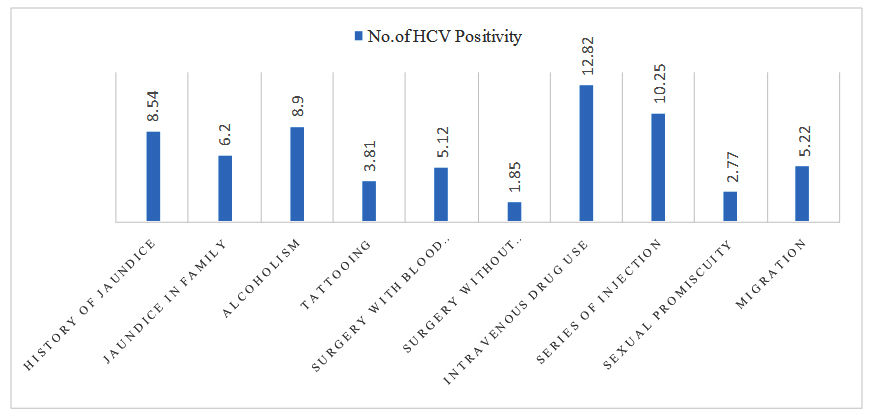
Fig. 1. Analysis of Risk factors with HCV positivity among the Irula tribes
Series of injection and surgery with blood transfusion plays a vital role in the transmission of Hepatitis C virus infection among Irula tribe people and alcoholism and history of jaundice shows a least infectivity for HCV infection (Table 2).
Table (2):
Pattern of Risk factors associated with the Irula tribal population and their positivity for HCV.
S. No |
Risk factors (Study subject n:523) |
Anti HCV (+ve) cases |
P.value |
|---|---|---|---|
1 |
History of Jaundice (HJ) (n:199) |
17 (8.54%) |
0.00 |
2 |
Jaundice in Family(JF) (n:258) |
16 (6.20%) |
0.06 |
3 |
Alcoholism(A) (n:191) |
17 (8.90%) |
0.00 |
4 |
Tattooing(T) (n:236) |
9 (3.81%) |
0.51 |
5 |
Surgery with Blood transfusion (SBT) (n:39) |
2 (5.12%) |
0.72 |
6 |
Surgery without Blood transfusion(S) (n:54) |
1 (1.85%) |
0.32 |
7 |
Intravenous drug use(IDU) (n:39) |
5 (12.82%) |
0.01 |
8 |
Series of Injection(SI) (n:79) |
4 (10.25%) |
0.76 |
9 |
Sexual Promiscuity(SP) (n:108) |
3 (2.77%) |
0.37 |
10. |
Migration(M) (n:153) |
8 (5.22%) |
0.38 |
(A:Alcoholism, HJ: History of Jaundice, IDU: Intravenous Drug Use, JF: Jaundice in Family, SI: Series of Injec-tion, SP:Sexual Promiscuity, S:Surgery without Blood transfusion, SBT: Surgery with Blood transfusion, T: Tat-tooing, M: Migration)
Predominant risk factor combination among the study population were evaluated as the high occurrence of HCV positivity with these risk factor patterns such as, HJ+A+IDU+SI+M, A+IDU+SP, HJ+T+S+SP+M and A+IDU+SP+M (Table 3).
Table (3):
Frequency of combination of Risk factors in Anti-HCV positivity among the Irula Tribes.
Groups |
Risk Factor Combinations |
Total no. of study subjects (n= 372) |
Anti-HCV (+) (n=19) |
|---|---|---|---|
1 |
HJ + JF +A+T+SBT+S+M |
3 (0.57%) |
– |
2 |
HJ+ JF+A+T+SBT+M |
32 (6.11%) |
2 (6.25%) |
3 |
HJ+ JF +A+T+M |
22 (4.20%) |
2 (9.09 %) |
4 |
HJ + JF +A+M |
97(18.54%) |
3 (3.09 %) |
5 |
HJ + JF+M |
21(4.01%) |
2 (9.52 %) |
6 |
JF+T+S |
12 (2.29%) |
1(8.33%) |
7 |
A+IDU+SP+M |
16 (3.05%) |
2(12.5 %) |
8 |
JF+T |
12 (2.29%) |
– |
9 |
T+S+SI |
24 (4.58%) |
– |
10 |
A+IDU+SP |
13(2.48%) |
1(7.69%) |
11 |
HJ+T+S+SP+M |
2(0.38%) |
1 (50 %) |
12 |
HJ+A+IDU+SI+M |
2(0.38%) |
2(100%) |
13 |
A+IDU+SP |
1(0.19%) |
1(100%) |
14 |
T+SI |
48 (9.17%) |
2 (4.16%) |
15 |
A+IDU |
6 (1.14%) |
– |
16 |
HJ |
11 (2.10%) |
– |
17 |
JF+T+SP |
71(13.57%) |
– |
18 |
S |
14(2.67%) |
|
19 |
HJ+T |
9(1.72%) |
– |
20 |
SBT+SI+SP |
4 (0.76%) |
– |
21 |
A+T+S+SI+SP+IDU |
1 (0.19%) |
– |
22 |
No Risk factor |
54 (10.32%) |
– |
Prevalence of HCV among the Irula tribes
Hepatitis C infection is a major cause of chronic liver disease, with >185 million infection worldwide. HCV infection is chronic in 75% to 85% of infected individuals [12]. An estimate of approximately a quarter of a million deaths occur per annum due to chronic liver disease associated with HCV [13]. Epidemiological burden of HCV infection varies throughout the world, with country-specific prevalence ranging from1% to 10% and population based anti-HCV prevalence is important for the surveillance purposes as it provides an insight in to the burden of diseases [14].
Of the 372 samples included in this study, 19 samples are positive for anti-HCV antibodies with a prevalence of 5.10%, which is high, compared to the other reports. None of them (0.00%) are positive in Kolli hill tribes (9), 2.97% and 2.25%is observed in East and Northern region of India [15,16], none of them are found positive in tribal population in southern region of India and 0.3 % is positive in general population of Southern region of India [17].
Age wise prevalence of HCV among Irula tribes
There are both geographic and temporal differences in the patterns of HCV infection found in all over the world [18]. United States, Turkey, Spain, Italy and Japan has the similar and over all prevalence of HCV infection (1.0% – 1.9%) but have different patterns of age-specific prevalence will varies. In the United States, prevalence of HCV infection is highest among the persons who are 30-49 years old and lower than average among the persons less than 20 and greater than 50 years old [19]. In contrast, some countries like Egypt, Japan, China and most of the African countries, there is a high prevalence of HCV infection predominantly appeared in younger population [20]. And also in some states of India, high prevalence of HCV infection found on the age group of 20 to 30 years old [21].
Likewise, in the study, it is observed that the Irula tribal people had infection more prevalent in the middle age group 31 to 40 and followed by 21 to 30. There is a sharp peak of HCV infection is found on the age group of 31 to 40 (10.75 %) years and a substantial prevalence till 60 years.
Gender wise prevalence of HCV among Irula tribes
Other than being a population based prevalence study, there are some special characteristics from the study which should be noted. The study compose of more number of females than the males, whereas many other previous studies have accounted almost exclusively on males, this is particularly tribals, general populations, blood donors and hospital based studies where the female population has always been much lesser than males [15,22,23]. High prevalence of HCV among males has also been reported in a study conducted among the tribals of North East India [24]. In contrast, the prevalence of Hepatitis C infection higher among Females (5.51 %) than males (4.23 %) of Irula tribal peoples.
Risk factors
The factors associated with HCV infection have been identified in this study; very few studies only in India have studied these factors. It is assumed that temporal association between the risk factors such as history of jaundice, jaundice in family, alcoholism, tattooing, and surgery with blood transfusion, surgery without blood transfusion, intravenous drug use, and series of injection, sexual promiscuity and migration.
Because of more variety of human activities that involve the potential for percutaneous exposure to blood or blood derived body fluids, there are numerous other biologically plausible modes of transmissions besides those with clearly demonstrated epidemiologic associations with infection. These include cosmetic procedures (tattooing, body piercing), intravenous drug use and religious or cultural practices such as ritual scarification, circumcision. In worldwide, there are insufficient data to determine whether these risk factors make any measurable contribution to the overall HCV transmission [21].
In the study, it is found that in Irula tribal population tattooing, surgery with blood transfusion and without transfusion, sexual promiscuity, series of injection, migration, jaundice in family and Intravenous drug use are the leading risk factors for HCV and these are statistically significant. History of jaundice and Alcoholism are not statistically associated with HCV but there is a high prevalence in getting infection among the Irula tribes (Table 2).
In the study from Kolli tribal population, none of them are positive for HCV and they could not predict the HCV risk factors and these combinations [8]. But in the present study, among the Irula tribes, A+IDU+SP, (100 %) HJ+A+IDU+SI+M (100%) and HJ+T+S+SP+M (50%) combinations are the highly associated risk patterns for Hepatitis C infection. It shows that every factor has a risk of getting a HCV infection but with combinations of some factors lead to high risk for getting an infection of Hepatitis C (Table 3).
Tattooing
Due to the accidental inoculation of contaminated blood during certain cultural practices like tattooing and piercing can transmit HCV virus. HCV transmission could occur at different stages of tattooing and piercing, from the reuse of non-disposable needles, inappropriate sterilization of equipment, or reuse of ink contaminated with blood from an infected person [25,26]. Risk of HCV infection is significant among the high risk groups when nonsterile tattooing equipment is used, especially in the unregulated settings, such as homes of prison [27,28]. Minor routes of HCV transmission includes multiple uses of unsafe injections and procedures by private practitioners and dental surgeons respectively, sharing of shaving kits and visiting roadside barbers have played an vital role of transmission [21,23].
Surgery with or without blood transfusion
Blood transfusion is an effective mode of transmission of blood-borne infections including Hepatitis C virus. Prior to the introduction of effective screening of blood donors, blood transfusions were recognized as the leading source of HCV infection [29,30]. In 147 Chilean patients with chronic hepatitis C, the most common risk factor was blood transfusion in 54% [31]. In India, transfusion of blood or blood product is considered as the most common route for HCV transmission [32]. In a study from Kolkata confirms that the blood transfusion has the most significant route of HCV transmission [33,34]. Blood transfusion was an important factor for transmission of HCV in hemodialysis patients.
Risk factors such as surgery, with or without blood transfusion were involved in HCV transmission and re-confirms the surgery with blood transfusion around 5.12% and surgery without blood transfusion was 1.85% as the most significant route of HCV transmission among the Irula tribes.
Sexual Promiscuity
HCV infection is transmitted predominantly by the parenteral route. Multiple sexual partners were one of the risk factors for the transmission of HCV and they observed 18.68% of patients had a history of multiple sexual partners. Female partners were highly infected with HIV & HCV controlled infection from their respective spouses [34]. The role of sexual activity in the transmission of HCV remains unclear and NHANESIII (National Health and Nutrition Examination Survey III) study showed that number of sexual partners (OR 2.54 for 249 partners) and age of the first sexual intercourse (OR 2.94) had a significant correlation with HCV Antibody and this has been confirmed in other studies [35]. Likewise, in a study among spouses in Egypt, it was wife to husband transmission was 34% and 10% among women with and without detectable HCV RNA. HCV transmission was estimated from Husband to wife as 3 %. In moreover, 6% was estimated to have contracted HCV from their spouse [36]. The transmission between spouses can only be assumed to be sexual in nature and there was lack of evidence found for sexual transmission of HCV among men who have sex with men [37]. These new evidences supports that sexual transmission of HCV is still rare, but for some reason is higher among those with high risk sexual activity [30]. HCV is mostly transmitted by sexual intercourse when the infected partner is in the early phase of acute infection [21].
In the present study, Irula tribal people are more traditional and religious and most of the participants did not accept to disclose such personal information and very poor response on these questions. From the obtained data, sexual transmission of HCV is observed as 2.77% among these Irula study population.
Intravenous drug abuse
Transmission of Hepatitis C virus has been strongly associated with the intravenous and percutaneous drug and needle use. These injection drug users are at high risk for blood borne infections including HCV virus [38]. In India very few studies are available on the intravenous drug abuse induced HCV infection and the prevalence of HCV antibody was ranging from 5 to 93 % among the intravenous drug users [39,40], 3.85 % of patients had a history of intravenous drug abuse in tertiary care hospital study in Kolkata [34]. Only few data exist regarding the prevalence of injection drug use and its contribution to HCV transmission among Irula tribal people. With the obtained data, it is found that there is high risk of HCV transmission is 12.82 % and occurs in intravenous drug abusers.
Series of Injection
The risk factors revealed that the needle prick or multiple injection, dental procedure and surgery etc., as cause of infection with Hepatitis C virus, among patients under study. This study has found that needle prick (33.3 %) as major risk factor for transmission of HCV. The reuse of disposable syringe and needles were soaking in the boiler or bowl with tepid water is common. The sharing of syringes, reuse of injection accessories, blood transfusions and drug abuse are strongly associated with HCV positivity among injecting and non-injecting drug users [41]. Present study found that series of injection as a major risk factor about 10.27% with HCV positivity among Irula tribal participants.
Migration
Among the many factors contributing to the changing epidemiology of viral hepatitis, the movement of people within and between countries is potentially important one. Migration and disease in today’s context of rapid global migration, there is a potential for any disease to be moved further and faster than previously possible. Despite the fact that viral hepatitis has become a global public health threat, both HBV and HCV have remained neglected and relative to the attention given to HIV and other diseases [42,43]. So is in the case of HCV, millions of migrant workers act as biological transporters. Likewise, from the obtained data about migration is 5.22% and the risk of getting HCV infection via migration is significant route of transmission.
Jaundice in family
The transmission routes of HCV is most common via mucous membrane exposure i.e., sexual or perinatal exposure to high risk body fluids like semen, saliva or cervical secretions. The most infections occur by exposure to infected body fluids through high risk sexual behaviors or injecting drug use [43]. Common modes of transmission in developing countries are perinatal, early childhood infections through close interpersonal contact with the infected household contacts, infected mother from child and infected wife from husband or infected husband to wife [44-46]. The seroprevalence of HCV was higher in the patients 6/25 (24%) who were contact with jaundiced person and the data are statistically significant [47]. Similarly, a study on the frequency of HCV among Irula tribes reported a high prevalence and the exposure of jaundice in family lead to the transmission of Hepatitis C virus in 16/258 (6.20 %) participants got infected from their family members of Irula tribals.
History of Jaundice
One of the main symptom of Hepatitis C is jaundice, a yellowing of the skin and whites of the eyes occur, they usually appear within two weeks to six months after the exposure. It is possible to have HCV for years or even decades without symptoms [48]. The rate of chronic HCV infection is lower in the patients who develop jaundice or symptoms during the acute onset of HCV infection as compared to those who are without jaundice. In a study of 142 HCV-infected subjects with a history of illicit drug use, subjects with viral clearance were more likely to have the symptoms of jaundice (p=0.03) [49]. In Germany the long-term follow-up study of women infected with contaminated Rh immune globulin exhibited a rate of chronicity in 43% and with the history of jaundice, as compared to 60% among those who remained anicteric (p<0.001). Many have speculated that the jaundice may be associated with a more robust immune Th1 lymphocyte and cytokine response to the HCV [50,51]. In some study conducted in normal population of Tamilnadu state about HCV is not statistically significant with the history of jaundice [17,52]. Furthermore, in the present study explains about the history of jaundice may lead to Hepatitis C infection with the competency of immune response and 8.52% (17/199) had a history of jaundice with the Hepatitis C positivity among Irula tribes.
Alcoholism
The prevalence of anti-HCV antibodies among the alcoholic patients is higher when compared to the general population and the rate seems to vary according to the presence or absence of co-existing liver disease [52]. Regarding the epidemiology of HCV among the alcoholic patients, several studies found a strong association between serum markers for the HCV infection and the presence of parenteral risk factors [17,47,53,54]. High prevalence (15%) of anti-HCV antibodies among the unselected alcoholics consecutively admitted to a detoxification unit and 71% of patients had a serum HCV-RNA detectable by PCR, especially those with the elevated aminotransferases (92%) [55]. In the present study, among Irula tribes 8.90% (17/191) were positive for HCV infection but lacking of the statistical association with HCV (Table 2).
The burden of Hepatitis C infection among the Irula tribes has reached really at a significant level compared to other studies. It has high prevalence in the females than males with the age group of 31 to 40. Strong associations between HCV infection were observed related to the gender, age group, jaundice in family, intravenous drug use, series of injection, sexual promiscuity, surgery with or without blood transfusion, migration and tattooing but less association with the history of jaundice, alcoholism among the Irula tribes. An understanding of the natural history of Hepatitis C is essential to effectively manage, treat, and counsel the individuals with HCV infection. There is lack of awareness, hygiene and shortage of health facilities. To prevent the spread of HCV, there is an urgent need to organize public awareness and health education campaigns targeting healthcare practices, proper counseling and treatment to reduce the transmission of the virus.
ACKNOWLEDGMENTS
The authors are thankful to the Karpagam Academy of Higher Education, Coimbatore. I greatly acknowledge the guidance, encouragement, support of (Late) Dr.P.Rajendran, Professor of Microbiology, Madha Medical College, Chennai, in all the ways to continue my research work. and P.V.Geetha, Senior Research fellow in Sri Ramachandra Medical College, Chennai for the great support for this Study and help in organizing the camps. Authors would like to thank all participants to conduct this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- India Population (2018) – Worldometers [Internet]. [cited 2018 Apr 3]. Available from: http://www.worldometers.info/world-population/india-population/.
- Stephens C, Porter J, Nettleton C, Willis R. Disappearing, displaced, and undervalued: a call to action for Indigenous health worldwide. The Lancet. 2006; 367(9527):2019–2028.
- Singh KS. People of India: the profile of a national project (1985–92). Curr Sci. 1993; 64(1):5–10.
- Jeyadev CJ, Ragupathy M. Ancient culture and tribal culture. Chennai Publ Gov Mus. 1962;.
- Watkins W.S., Prasad B. V. R., Naidu J. M., et al. Diversity and Divergence Among the Tribal Populations of India. Ann Hum Genet. 2005; 69(6):680–692.
- Gnanasekaran A, Paramasivam R, Mohan K, et al. Seroprevalence of certain bacterial and viral infections among the Irula tribal population of Marakkanam, Tamil Nadu state, India. Prim Health Care Res Amp Dev. 2013; 14(2):185–191.
- Sharma AVN. Adivasis of Kodiakkarai Chennai. Publ Gov Mus. 1996; .
- Kalaivani V, Rajendran P, Thyagarajan SP, Rajesh PK, Hari R, Selvakumar C, et al. The seroprevalence of hepatitis B and C viruses and the associated risk factors in the Kolli hills tribal population of Tamil Nadu. Biomedicine 2001; 21(1):7-13.
- Geethavani B, Sangamithra V, Balamuruganvelu S, Rajendran P. Seroprevalence of syphilis and leptospirosis among tribal population of Tamil Nadu and qualitative analysis of the risk factors associated with the diseases. Int J Pharm 2014; 4(4):190-4.
- Ramya Dinesh E, Ramalakshmi S. Prevalence of Hepatitis B virus and associated risk factors in Irula tribal Population. Asian J Pham Clin Res. 2017; 10(8):100-102
- Sinha A.K. , Banerjee B.G. , Edwin C.J. , Pathak R.K. , Vasishat R.N. 2008. Bio-Social Issues in Health. Northen Book centre
- Re VL, Kostman JR. Management of chronic hepatitis C. Postgrad Med J. 2005; 81(956):376–382.
- Ray Kim W. Global epidemiology and burden of hepatitis C. Microbes Infect. 2002; 4(12):1219–1225.
- Vriend H J, Coul O de, M EL,et al. Hepatitis C virus seroprevalance in the netherlands. Eur J Public Health. 2012;22(6):819-821.
- Krishnasamy N, Chezhian A, Senthilkumar R, Sathishkumar E. Study of Hepatitis B and C Virus Infection in Urban and rural Population of Tamil Nadu, India. Int.J.Curr.Microbiol.App.Sci. (2015); 4(6): 443-451.
- Chowdhury Abhijit, Santra Amal, Chakravorty Runu, et al. Community based epidemiology of hepatitis B virus infection in West Bengal, India: Prevalence of hepatitis B e antigen negative infection and associated viral variants. J Gastroenterol Hepatol. 2005; 20(11):1712–1720.
- Krishnasamy N, Rajendran K, Radhakrishnan P, Annasamy C, Ramalingam S. Seroprevalence and factors associated with surface antigen of Hepatitis B virus and anti Hepatitis C virus antibody among southern region of India, Tamil Nadu. Int J Infect Control. 2015; 11(1):1-10.
- Missiha SB, Ostrowski M, Heathcote EJ. Disease Progression in Chronic Hepatitis C: Modifiable and Nonmodifiable Factors. Gastroenterology. 2008; 134(6):1699–1714.
- Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000; 132(4):296–305.
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006; 45(4):529–538.
- Malhotra P, Malhotra V, Malhotra N, Singh I, Chugh A, Chaturvedi A. Epidemiological Profile of Hepatitis C Patients at India’s New Hub–Haryana. Adv Res Gastroenterol Hepatol. 2015; 1(1):001-006
- Bhattacharya S. Seroprevalence of hepatitis C virus in a hospital based general population in South India. Indian J Med Microbiol. 2003; 21(1):43-45.
- Khan MSA, Khalid M, Ayub N, Javed M. Seroprevalence and risk factors of hepatitis C virus (HCV) in Mardan, NWFP: a hospital based study.-. Rawal Med J. 2004; 29(2):57–60.
- Medhi S, Goswami B, Das AK, et al. New insights into hepatitis C virus infection in the tribal-dominant part of Northeast India. Arch Virol. 2012; 157(11):2083–2093.
- Long GE, Rickman LS. Infectious complications of tattoos. Clin Infect Dis. 1994; 18(4):610–619.
- Tohme RA, Holmberg SD. Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clin Infect Dis. 2012; 54(8):1167–1178.
- Hellard ME, Aitken CK, Hocking JS. Tattooing in prisons—not such a pretty picture. Am J Infect Control. 2007; 35(7):477–480.
- Teutsch S, Luciani F, Scheuer N, et al. Incidence of primary hepatitis C infection and risk factors for transmission in an Australian prisoner cohort. BMC Public Health. 2010; 10(1):633.
- Alashek WA, McIntyre CW, Taal MW. Hepatitis B and C infection in haemodialysis patients in Libya: prevalence, incidence and risk factors. BMC Infect Dis. 2012; 12(1):265.
- Sy T, Jamal MM. Epidemiology of hepatitis C virus (HCV) infection. Int J Med Sci. 2006; 3(2):41.
- Soza A, Arrese M, González R, et al. Clinical and epidemiological features of 147 Chilean patients with chronic hepatitis C. Ann Hepatol. 2004; 3(4):146–51.
- Pérez CM, Suárez E, Torres EA, Román K, Colón V. Seroprevalence of hepatitis C virus and associated risk behaviours: a population-based study in San Juan, Puerto Rico. Int J Epidemiol. 2005; 34(3):593–599.
- Neogi DK, Bhattacharya N, Chakrabarti T, Mukherjee KK. HCV activity in Calcutta—a serological study. J Commun Dis. 1997; 29(1):1–6.
- Chakraborty A, Pramanik SB, Roy DS, Sarkar S, Chakraborty M, Nandi A. A Retrospective study on the sero-prevalence of hepatitis c infection in a tertiary care hospital in Kolkata, India. Int J Curr Microbiol App Sci. 2015; 4(3):115–123.
- Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999; 341(8):556–562.
- Magder LS, Fix AD, Mikhail NN, et al. Estimation of the risk of transmission of hepatitis C between spouses in Egypt based on seroprevalence data. Int J Epidemiol. 2005; 34(1):160–165.
- Alary M, Joly JR, Vincelette J, Lavoie R, Turmel B, Remis RS. Lack of evidence of sexual transmission of hepatitis C virus in a prospective cohort study of men who have sex with men. Am J Public Health. 2005; 95(3):502–505.
- Mishra S, Chayani N, Sarangi G, Mallick B, Pati SB. Seroprevalence of anti HCV antibody in and around Cuttack, Orissa. Indian J Med Microbiol. 2002; 20(1):40.
- Mehta SH, Vogt SL, Srikrishnan AK, et al. Epidemiology of hepatitis C virus infection & liver disease among injection drug users (IDUs) in Chennai, India. Indian J Med Res. 2010; 132(6):706.
- Kumar MS, Mudaliar S, Thyagarajan SP, Kumar S, Selvanayagam A, Daniels D. Rapid assessment and response to injecting drug use in Madras, south India. Int J Drug Policy. 2000; 11(1):83–98.
- Basu D, Sharma AK, Gupta S, Nebhinani N, Kumar V. Hepatitis C virus (HCV) infection & risk factors for HCV positivity in injecting & non-injecting drug users attending a de-addiction centre in northern India. Indian J Med Res. 2015; 142(3):311.
- Carballo M, Maclean EC, Gudumac I, Van Damme P. Hepatitis C and migration: a public health challenge. J Fam Med. 2016; 3(4):1065.
- Ahmed A, Reintjes R. Current Hepatitis B & C Screening practices for Migrants & Barriers to screening. European Conference on Migrant & Ethnic Minority Health. Granada, Spain. 2014. [Internet]. [cited 2018 Apr 3]. Available from: http://hepscreen.eu/wp-content/uploads/2014/09/Ahmad-A_Screening_EUPHA-Mig-Health_2014.pdf
- Nepal A, Kunwar B. Evidence of Hepatitis C Virus Infection and Associated Treatment in Nepal. J Mol Biomark Diagn. 2016; 7(270):2.
- Stockholm. Technical Report:Surveillance & Prevention of hepatitis B & C in Europe. 2010:1-145 [Internet]. [cited 2018 Apr 3]. Available from: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/101012_TER_HepBandC_survey.pdf
- Organization WH. WHO fact sheet 164-hepatitis C. Updat July. 2013.
- Ayele AG, Gebre-Selassie S. Prevalence and risk factors of hepatitis B and hepatitis C virus infections among patients with chronic liver diseases in public hospitals in Addis Ababa, Ethiopia. ISRN Trop Med. 2013; 2013:1-9
- Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997; 26(3S1):15-20.
- Villano SA, Vlahov D, Nelson KE, Cohn S, Thomas DL. Persistence of viremia and the importance of long term follow up after acute hepatitis C infection. Hepatology. 1999; 29(3):908–914.
- Grüner NH, Gerlach TJ, Jung M-C, et al. Association of Hepatitis C Virus—Specific CD8+ T Cells with Viral Clearance in Acute Hepatitis C. J Infect Dis. 2000; 181(5):1528–1536.
- Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000; 191(9):1499–1512.
- Schiff ER. Hepatitis C and alcohol. Hepatology. 1997; 26(3S1):39-42.
- Befrits R, Hedman M, Blomquist L, et al. Chronic hepatitis C in alcoholic patients: prevalence, genotypes, and correlation to liver disease. Scand J Gastroenterol. 1995; 30(11):1113–1118.
- Coelho Little ME, Jeffers LJ, Bernstein DE, et al. Hepatitis C virus in alcoholic patients with and without clinically apparent liver disease. Alcohol Clin Exp Res. 1995; 19(5):1173–1176.
- Galperim B, Cheinquer H, Stein A, Fonseca A, Lunge V, Ikuta N. Prevalence of hepatitis C virus in alcoholic patients: role of parenteral risk factors. Arq Gastroenterol. 2006; 43(2):81–84.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


