ISSN: 0973-7510
E-ISSN: 2581-690X
Yeasts are found in almost all ecosystems, and soil is the typical habitat for storing and developing yeasts, even though they are associated with plants and animals. Their population in soil ranges from a few to several thousand based on the soil ecosystem. Soil edaphic factors determine the abundance and diversity of yeasts. Sugar-rich plant residues, such as fruit debris, root exudates, forest soil, etc., support yeast growth. The literature showed that soil-dwelling yeasts have an array of plant growth-promoting activities and mechanisms for soil structure maintenance. Soil yeasts own several plant growth-promoting properties like nitrogen mineralization, solubilization of phosphate, potassium releasing potential, sulfur oxidation, plant growth-promoting hormones production, and siderophore production. Certain soil yeasts were proven to possess the biocontrol potential against plant pathogens. Soil properties have an essential influence on plant growth, cycling of nutrients, and water-holding capacity. Soil yeasts significantly influence the soil’s physical (macro and micro aggregates formation), soil chemical (pH, Soil Organic Carbon, Soil Labile Carbon, Soil Protein Index), and soil biological properties (Dehydrogenase activity, Microbial Biomass Carbon, Extracellular Polymeric Substances (EPS) production). Application of yeasts resulted in a yield increase in the range of 20-30% in crops like wheat and sugarbeet. Soil incubation studies conducted with yeasts proved their potential to be used as bioinoculants for soil health enhancement. Studies conducted with yeasts recorded significant improvement in soil physical (macro and microaggregate formation), chemical (pH, increase in soil organic carbon, soil protein index, etc) and biological (dehydrogenase enzyme activity, microbial biomass carbon (MBC), soil colloidal polysaccharide) properties. Soil yeasts have a huge potential to be used as bio-inoculants for crop growth and soil health. The beneficial role of yeasts in agriculture remains unexplored, and finding suitable yeast candidates with plant growth promotion and soil health improvement traits will benefit the crops considerably.
Soil Yeasts, Plant Growth, Soil Health, Nutrient Mineralization
Plants and soil are closely interconnected to each other and so plant-microbe interactions are very important for plant health, biotic and abiotic stress mitigation, etc., In addition, these interactions also influence the soil parameters (physical, chemical, and biological properties) by biogeochemical cycles.1 In sustainable agriculture, an eco-friendly and efficient method to increase the growth of plants and soil health is the application of microbial inoculants. Organic farming is gaining momentum across the globe to safeguard the human health and protect the natural environment. Nowadays there is a growing trend towards shifting from chemical to biological inputs in agriculture. Exploiting the beneficial microbes in the form of microbial inoculants as an alternative to chemicals in organic farming is gaining importance due to its merits on the environment and agriculture. Microbial communities participate in nutrient and soil organic matter transformation from plant and animal residues. Nitrogen fixation, mineral solubilization, growth hormone production, pathogens suppression, and abiotic stress mitigation are some benefits that plants realize through plant-microbe interactions. Both plants and microorganisms gain an advantage by their mutual contribution. Plants have the potential to select their microbiome through the discharge of root exudates and in turn, microorganisms decide their host by its preferential selection process. Additionally, microbes are significant in stabilizing soil aggregates, reducing erosion, and maintaining water-holding capacity in soil. The contribution of fungi to soil microbial biomass and genetic diversity is notable.2 The unicellular fungi-yeasts which primarily proliferate through budding are having a notable impact on plant and soil health.
Yeasts are polyphyletic and reproduce through budding and cell fission. They produce meiosporangia which are not enclosed within fruiting bodies. Yeasts are able to multiply in liquid/submerged environments even within biofilms. Due to these advantages, yeasts are able to thrive in various habitats and play an important role in soil health maintenance. Yeasts have the ability to grow in a wide range of nutrient sources (carbon and nitrogen). Due to these reasons yeasts are able to grow in a wide range of soil conditions (nutrients, water, aeration, etc.). Yeasts with plant growth promoting traits are considered to be GRAS (Generally Recognized As Safe) as they are ecofriendly. In recent times, among all soil microorganisms, yeasts have gained some special attention towards biocontrol and plant growth promotions due to rapid growth, antibiotic production, cell wall degrading enzymes production, plant growth regulators, and induction of host system resistance.3 This review provides detailed insight into the role of yeasts plant growth promotion and soil health maintenance.
Occurrence of yeasts in soil ecosystems
The unicellular fungi significantly contribute to the microbial diversity and are found in all ecosystems. Soil is the typical habitat for yeasts, even though it is associated with plants and animals. The population of yeasts ranges from a few number of cells to several thousands in one gram of soil. Yeasts are rich in topsoil up to 10 cm. When compared to the bacteria, population of yeasts in soil are comparatively lower. But as the cytoplasm volume of yeasts is higher when compared to bacteria,4 their contribution to the ecological processes is considerable. The presence and abundance of yeasts in soil are influenced by factors such as soil type, rainfall, and climate. Yeasts are essential in all ecosystems and play a vital role in biodiversity. Soil is the habitat structure for the repository and development of yeast sp. Soil yeast population size positively correlates with the soil’s organic carbon and organic nitrogen content. The population of yeasts is higher in rhizosphere soil when compared to bulk soil.5
Slavikova and Vadkertiova6 reported that the average population of yeasts in agricultural soils reached approximately 1.12 × 103 Colony Forming Units/g (CFU/g) of soil and 1.4 × 104 CFU/g soil in forest soils. Due to tillage operation, the soil’s yeast population was found to be reduced by Slavikova and Vadkertiova.7 In the soil, the population of yeasts is low compared to bacteria and fungi.3 Slavikova and Vadkertiova7 reported that the predominant yeast species found in forest soils were Candida maltosa, Cryptococcus laurentii, Metschnikowia pulcherrima, and Sporobolomyces salmonicolor. They also stated that Trichosporon cutaneum was present in sugarbeet field soils. Trichosporon pullulans were high in maize and potato fields whereas the population of Candida valida, Cryptococcus albidus, Debaryomyces occidentalis var. occidentalis, and Williopsis saturnus var. saturnus were higher in forest soils when compared to agricultural soils.
Diversity of soil yeasts
Terrestrial yeasts occur abundantly in plants, animals, and soil. The diversity among soil yeasts may be attributed to various microsite structures in soils similar to other soil microbes. Each yeast community is defined by its habitat, wherein an assemblage of yeast thrives in groups. Some of these are allochthonous and are mostly transient or accidentally isolated from soil. Their original habitat may be animals or vegetative debris. Several yeast strains were predominantly found in forest soils. Yeast isolates such as Trichosporon cutaneum, Rhodotorula aurantiaca, Leucosporidium scottii, Cystofilobasidium capitatum, and Cryptococcus laurentii were frequently found in soils of deciduous forest, coniferous forest and the deciduous forest park. Yeast species like Cryptococcus laurentii and Cryptococcus albidus were primarily found in forest soils.6 Occurrence of specific yeasts in a particular habitat is attributed by several factors like soil type, climatic conditions and vegetation. These yeasts isolates were characterized by their ability to assimilate various carbon sources like xylose, cellobiose, trehalose, L-arabinose, etc. Slavikova and Vadkertiova6 concluded that decaying wood of forests supported the growth of soil yeasts with ability to utilize pentoses as their carbon source. These yeasts were also found to utilize nitrate as their nitrogen source. These yeasts were found to possess aerobic metabolism instead of fermentative metabolism during their growth. Slavikova and Vadkertiova8 also stated that some of the isolated yeasts possess the potential to degrade phenolic and chlorophenolic compounds present in decaying wood. Saccharomyces cerevisiae involved in beverage fermentation are phylogenetically diverse and create a portion of the soil microbe community and become ubiquitous.17
The occurrence and abundance of yeasts are higher in soil very close to fruit-bearing trees. This is because the spoiled fruit from the tree gets deposited on the top soil and serves as a source of nutrients for yeast species in the soil.18 Likewise, the root exudates act as a readily available food source for soil yeasts because they seem to source various simple organic carbon compounds easily assimilated by yeasts.19 According to Cloete et al.,5 the population of yeasts in rhizosphere soil is higher than the bulk soil. Pandi et al.,20 isolated yeasts with plant growth-promoting traits from garden land soil and reported that the population of yeasts predominates in orchard soils (Table 1).
Table (1):
Diversity of yeasts in different ecosystems
No. |
Yeast species |
Population Range |
Habitat |
Ref. |
|---|---|---|---|---|
1. |
Cystofilobasidium capitatum, Cryptococcus laurentii, Leucosporidium scottii, Rhodotorula aurantiaca and Trichosporon cutaneum |
1.5 x 103 CFU/g to 1.1 x 104 CFU/g soil |
Deciduous and coniferous forests |
6 |
2. |
Naganishia uzbekistanensis (35.71%), Candida (25%), Rhodotorula toruloides (10.71%), Trichosporon coremiiforme (17.85%) New species for mycobiota of Iran: Rhodotorula toruloides, T. coremiiforme, C. catenulata, C. boidinii and Lecythophora sp. |
NR |
Uninhabited soils of Kermanshah province, Iran |
8 |
3. |
Apiotrichum dulcitum, Apiotrichum porosum, Cutaneotrichosporon moniliiforme, Fellozyma inositophila Saitozyma podzolica & Solicoccozyma terricola |
The number of yeast Operational Taxonomic Units (OTU) – ranged on average from 10 to 44 |
Training Forest Enterprise Masaryk Forest Krtiny of Mendel University in Brno (Krtiny Forest) |
9 |
4. |
Debaromyces subglobosus, Guehomyces pullulans, Rhodotorula graminis, Sporobolomyces roseus, Cryptococcus terreus, Cryptococcus terricola |
NR |
Aerable land under agricultural rotation |
10 |
5. |
Cryptococcus terricola, Trichosporon spp. |
NR |
Forest land |
|
6. |
Schwanniomyces accidentalis, Cryptococcus terreus, Rhodosporidium azoricum, Rhodotorula graminis, Hannaellazeae, Cryptococcus adeliensis, Williopsis saturnus, Cryptococcus terricola, Guehomyces pullulans, Aureobasidium pullulans |
NR |
Permanent
grassland |
|
7. |
Holtermaniella watticus, Cryptococcus terricola, Cryptococcus aerius |
NR |
Vineyard |
|
8. |
Williopsis saturnus |
NR |
Rapeseed field |
|
9. |
Holtermaniella takashimae |
NR |
Wheat field |
|
10. |
Guehomyces pullulans, Cryptococcus terricola |
NR |
Hardwood forest |
|
11. |
Crystofilobasidium macerans |
NR |
Alfalfa field |
|
12. |
Saitozyma podzolica – in areas with aluminium |
NR |
Quadrilatero Ferrifero in Minas Gerais (Iron mining areas) |
11 |
13. |
Saitozyma podzolica, Filobasidium chernovii |
NR |
Alluvial soil in the inland area on the bank of Dong Nai river, South Vietnam |
12 |
14. |
Saitozyma podzolica, Aureobasidium pullulans, Readerielliopsis fuscoporiae, Candida akabanensis |
NR |
The watershed area of Slate Ridge, South Vietnam |
|
15. |
Candida battle |
Yeast colony count 1. Lemon: 7.71 × 102 CFU/g in fruits 2. Mango: 4.10 × 102 CFU/g 3. Guava: 4.25 × 102 CFU/g 4. Sugarcane: 3.86 × 102 CFU/g |
Tree barks and fruit samples |
13 |
16. |
Saccharomyces |
Yeast colony count in fruits 1. Harbuu bark: 1.15 × 105 CFU/g 2. Qilxuu barks: 1.14 × 105 CFU/g 3. Hadaamii barks: 1.06 × 104 CFU/g |
Harbuu and Qilxuu barks |
|
17. |
Candida humilis, Hansenia sporauvarum, Meyerozyma guilliermondii, Lachancea thermotolerans and Pichia kudriavzevii |
(1.29 × 105 CFU/g rhizosphere) |
Both bark and rhizosphere |
|
18. |
Non-Saccharomyces-98 Saccharomyces sp.-41 |
NR |
Vineyards and forest oak soils in Douro region, Portuguese |
14 |
19. |
Zygosaccharomyces, Filobasidium, Cyniclomyces, and Papiliotrema-alkaline environments |
NR |
Peach orchard |
15 |
20. |
Tausonia, Solicoccozyma, Trigonopsis, and Goffeauzyma-nutrient-rich environments |
NR |
NR: Not reported
According to Bates et al.,21 the pyro-sequencing results showed that free-living fungal yeasts such as Aureobasidium pullulans and Sarcinomyces spp. dominate in the crusts, which is also dominated by cyanobacteria. Yeast-like communities detected in various crusts mostly dominated by cyanobacteria show that this type of yeast can tolerate the exudates released by cyanobacteria. Exophiala crusticola, which is a black-colored yeast isolated from crusts exhibited better growth in cyanobacterial exudates.21
Plant growth-promoting characteristics of soil yeasts
Yeasts remain underexploited as biofertilizer organisms when compared to filamentous fungi and bacteria. In India, soil yeast research is still in its initial stage, though it possesses plant growth-promoting and soil structure maintenance traits. Very few attempts have been made to adopt yeast as a biofertilizer. The plant growth-promoting characteristics of various yeasts like production of Indole acetic acid, siderophores production, polyamines, ammonia, and enzymes like ACC deaminase production were reported by Shih et al.22 Amprayn et al.23 reported that, Candida tropicalis CtHY a soil yeast recorded favorable to a certain number of familiar traits for plant growth-promotion like production of IAA, ACC deaminase, phytase, polyamine and solubilization of tricalcium phosphate.
Nutrient transformation
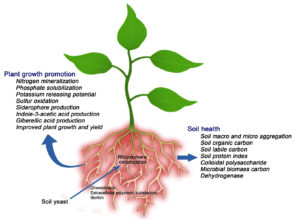
In soil, yeasts play a principal role in the solubilization of nutrients. Pandi et al.,20 reported that soil yeasts harbor plant growth-promoting features viz., phosphate solubilization, Zn solubilization, and K-releasing properties (Figure). Many yeast species synthesize specific antimicrobial compounds and serve as potential antagonists against plant pathogens. Yeasts can produce various growth regulators like indole-3-pyruvic acid, indole-3-acetic acid, and gibberellins under in vitro conditions. Yeasts produce polyamines and they are known to promote plant growth.
Nitrogen
Plants benefit through ammonia (NH3) production by plant-associated microorganisms because nitrogen provided by ammonia supports plant growth. Biological Nitrogen Fixation is a process of converting N to ammonia by some bacteria and archaea with the use of Mo nitrogenase.24 It is a well-known fact that eukaryotes lack this particular enzyme and nowadays efforts were made to engineer Saccharomyces cerevisiae for the expression of nif genes from various diazotrophic bacteria and archaea.25
Gori et al.26 reported that, in yeasts ammonia production is quite rare and Debaryomyces hansenii is an exception as it can produce NH3 in cheese agar. Yeast species belonging to Geotrichum, Rhodotorula, Candida, Saccharomyces, and Williopsis could nitrify ammonium to nitrite and nitrate under lab conditions.27 Falih and Wainwright28 reported that the yeast isolates, Williopsis californica and Saccharomyces cerevisiae, can hydrolyze urea to ammonia and oxidize ammonia to nitrate, forming trace amounts of nitrite. Rezende et al.29 reported that the increased nitrate concentration in soil amended with yeast is due to yeast mineralization. They also confirmed that N mineralization increased with incubation time, production of CO2, and reduced microbial biomass. Increased mineralization of N negatively correlates with the C/N ratio and it has a positive correlation with the time of incubation.
Phosphorous
Phosphate solubilizing microorganisms employ various mechanisms to solubilize phosphates and secretion of organic acid is one among them. Through this mechanism, these organisms acidify the environment organic acid production or H+ secretion during P solubilization. Hence inorganic phosphates will be released by substitution of protons for Ca2+.30 The phosphate solubilizing ability of bacteria and filamentous fungi was extensively studied when compared to yeasts. Cy et al.,31 studied the calcium phosphate solubilizing potential of yeasts and reported that the pH of the medium gets reduced due to inoculation of calcium phosphate solubilizing yeasts. This might be attributed to the excretion of H+ or due to the production of organic acids by yeasts. Vogel and Hinnen32 reported that for phosphate metabolism yeast cells secrete a cluster of enzymes which includes acid phosphatase, alkaline phosphatase, and permeases.
Alonso et al.33 states that yeasts like Rhodotorula and Cryptococcus can solubilize phosphate. They reported that Rhodotorula was able to solubilize 8 µg P mL-1 calcium phosphate and 4 µg P mL-1 iron phosphate when grown at 30 °C for 72 hours with a cell concentration of 106 CFU mL-1. In the same growth condition Cryptococcus solubilized 5 µg P mL-1 calcium phosphate and 10 µg P mL-1 iron phosphate. Alonso et al.,33 and Vassilev et al.,34 characterized several yeast strains with the capability to solubilize insoluble inorganic phosphates, including calcium, iron, and rock phosphates. Vassileva et al.,34 reported that Yarrowia lipolytica had the rock phosphate solubilization potential.
They studied the phosphate solubilization potential of freely suspended and agar-encapsulated Yarrowia lipolytica cells by growing them in phosphate amended broth (@3.5 g/l & 7.0 g/l) and found that the soluble phosphate available in culture broth amended with 3.5 g/l phosphate was 13.5% and 18.5% higher for freely suspended and agar-encapsulated Yarrowia lipolytica cells when compared to 7.0 g/l.
Hesham and Mohamed35 also reported the P solubilization potential of yeast isolates. Amprayn et al.,23 also reported that Candida tropicalis HY has a better P solubilization efficiency of 119 ± 10 µg mL-1. The yeast Yarrowia lipolytica possesses plant growth-promoting activities such as plant phosphorus acquisition and growth promotion of Dorycnium pentaphyllum.36 Hesham and Mohamed35 reported that forty yeast strains with PGP traits were isolated from different regions of Egypt and screened to know their phosphate solubilization efficiency based on precise zone formation around the colonies when grown on tricalcium phosphate medium. Among forty, nine isolates showed positive results for phosphate solubilization with a solubilization index ranging from 1.19 to 2.76. The higher P solubilization index was observed in PSY-4, which was documented as Saccharomyces cerevisiae. They also found that inoculation of Saccharomyces cerevisiae to corn improved the uptake of P, and root/shoot dry weight compared to uninoculated control. Falih and Wainwright28 reported that Williopsis californica and Saccharomyces cerevisiae had the potential to solubilize insoluble phosphate under in vitro conditions.
According to Nakayan et al.37 the yeast isolates Pichia sp. exhibited phosphate solubilizing activities when grown in a tri-calcium phosphate medium and increased phosphorus availability in the soil. They also reported that the dry weight of lettuce was increased due to the application of yeast strain along with 50% chemical fertilizer in comparison to the application of 50% chemical fertilizer alone. A diversified range of yeasts exhibit nutrient uptake and phyto-hormone production.38 They also reported that ACC deaminase and phosphate solubilization activity were significantly higher in Candida tropicalis CtHY when compared to the corresponding literature reference strains. Cy et al.23 reported that inoculation of Cryptococcus laurentii promoted the growth of Arabidopsis thaliana in calcium phosphate dibasic dehydrate supplemented inorganic phosphate-deficient medium and also observed the inorganic phosphate levels were higher in plants inoculated with yeast. Mineral solubilization including phosphorous by microorganisms which is essentially required for plant growth is mainly affected by the secretion of various organic acids. Naturally, yeasts produce a wide range of organic acids. In phosphate solubilization gluconic acid plays a significant role. The presence of gluconic acid in yeast-like fungi-Aureobasidium pullulans is reported by Ramachandran et al.39
Potassium and zinc
Yeast possesses the ability to release potassium from silicate minerals through production of organic acids. Organic acids play a profound role in mineral solubilization. Yeasts of genera Candida, Hansenula, Pichia, Rhodotorula, Saccharomyces, Zygosaccharomyces, Torula, etc are known to produce citric acid through the Krebs cycle.40 Oleaginous yeasts like Yarrowia lipolytica are known to produce succinic acid. Torulaspora globosa was found to solubilize the alkaline ultramafic rock and set free approximately 38% of total potassium.41 A study on muscovite mica solubilization by soil yeasts in maize crops was conducted by Mohamed et al.42 and they found that Rhodotorula glutinis and Pichia anomala have the potential to solubilize potassium minerals in addition to other plant growth-promoting traits (~14% & ~23% increment in roots and shoots respectively). Pandi et al.,20 reported the first evidence for potassium releasing and zinc solubilization potential of soil yeasts. Among 54 yeast isolates 43% of isolates were found to be positive for releasing potassium and 81% of isolates exhibited zinc solubilization.
Sulfur
Falih and Wainwright28 reported that the soil yeast, Williopsis california and Saccharomyces cerevisiae oxidized elemental sulfur to thiosulphate, tetrathionate, and sulfate. Williopsis californica when oxidizing elemental sulfur in vitro was found to form a huge quantity of tetrathionate and thiosulphate. Williopsis and Saccharomyces under Ascomycetes could oxidize elemental S into thiosulphate, tetrathionate, and sulfate under in vitro conditions.28
Growth hormones
Indole-3-acetic acid an auxin derivative, have an important role in plant cell elongation, division, and differentiation.43 Spaepen et al.44 reported the production of IAA by certain endophytic yeasts. Xin et al.45 experimented to observe the potential of yeast strains to produce IAA. They isolated three yeast strains, Populus trichocarpa from wild cottonwood stems, Populus trichocarpa and Populus deltoids from hybrid poplar stems. Rhodotorula glutinis ATCC 2527 and a Baker’s yeast Saccharomyces cerevisiae were used as reference strains. They evidenced that if the medium was not supplemented with L-tryptophan, they could not produce IAA. Upon incubation with 0.1% L-tryptophan, all yeast strains were observed to produce IAA except Baker’s yeast. The total IAA production by Populus trichocarpa was higher when compared to others. Candida tropicalis CtHY is the yeast that can produce indole acetic acid (IAA) and possesses high ACC deaminase activity. The overall IAA production by Candida tropicalis was found to increase with time.23 Shih et al.,22 stated that plant growth-promoting yeasts could produce IAA. Among the eight IAA-producing yeast isolates screened by Nassar et al.,46 Williopsis saturnus a potential plant growth-promoting yeast registered the higher IAA production with or without L-TRP (9.67 µg mL-1) in the medium.
Nassar et al.,46 stated that the yeast Williopsis saturnus as an endophyte in the roots of maize improved the plant growth by production of indole-3-acetic acid (IAA) and indole-3-pyruvic acid (IPYA). They also indicated that through the pruned-root dip method, Williopsis saturnus can be introduced into the maize plants. Due to the inoculation of Williopsis saturnus the length and dry weight of roots and shoots were found to be increased. Production of IAA and IPYA also increased when compared with control plants. At the same time, another endophytic yeast isolate – Rhodotorula glutinis did not produce detectable levels of IAA or IPYA in vitro compared to W. saturnus. The colonization potential of R. glutinis was comparable to that of W. saturnus in maize root tissues. Both endophytic yeasts were not capable of releasing gibberellic acid, isopentenyl adenine, isopentenyl adenosine, or zeatin under in vitro conditions at detectable levels in their culture filtrates.
Gibberellin production by plant growth-promoting rhizobacteria (PGPR) promotes the growth and yield of many crop plants.47 Twfiq et al.48 reported that baker’s yeast have the ability to produce GA3. The yeast isolates Trichosporon asahii, Candida valida, and Rhodotorula sp. produced GA3 at 6, 5, and 8 µg ml-1 respectively.49 Pandi et al.,20 reported that among the yeasts isolated from garden land soil, the isolate Pichia sp. produced the maximum amount of gibberellic acid (GA3). Cytokinins promote plant growth directly through cellular division. The most common cytokinin-Zeatin stimulates plant cell proliferation. Aureobasidium pullulans a rhizosphere yeast, Metschnikowia pulcherrima, and Sporobolomyces roseus were found to produce zeatin and promotes plant growth.50 Yeasts like Kluyveromyces lactis, Schizosaccharomyces pombe, and Saccharomyces cerevisiae were found to synthesize cytokinins.51 Zeatin production during the exponential phase was found in yeasts like Metschnikowia pulcherrima and Rodotorula mucilaginosa.52,53 Moesziomyces antarcticus produces zeatin in addition to the production of enzymes and glycolipid surfactants.54
Siderophore production
Verma et al.55 deliberated that siderophores due to their iron-transporting abilities suppress plant pathogens and play a vital role in promoting the plant growth. Rhodotorula strains produce 60% of siderophore as rhodotorulic acid and during Fe-stress conditions they secrete hydroxamate-type of siderophores (iron-binding compounds) which plays an essential role in controlling apple and pears post harvest diseases.56 These findings serve as an evidence to prove the biocontrol potential of yeasts through Fe3+ sequestration in the root region. Siderophores play a major role in crop production by growth promotion and yield increase through increased iron uptake in many of the commercially important plant species. Fu et al.57 reported that out of 13 yeast species taken for study, Pseudozyma aphidis exhibited a higher amount of siderophore production. Endophytic yeast-R. graminis and Cryptococcus sp. produce siderophores through sugar and amino acids assimilation from plants.58 El-Maraghy et al.59 evidenced the higher siderophore production and sidD gene expression due to Cd2+ and Pb2+ toxicity stress in Trichosporon ovoides and Saccharomyces cerevisiae.
Crop responses to yeast inoculation
Edi et al.60 reported that applying Sporobolomyces roseus to wheat increased the yield by 16 to 30%. Similarly, Abd El-Hafez61 stated that the application of Rhodotorula sp. increased the fruit weight of tomato. Rhodotorula sp. can promote growth and fruit yield in tomatoes. Growth of sugar beet is promoted by the application of Candida valida, Rhodotorula glutinis, and Trichosporon asahi as soil inoculum.19 Agamy et al.62 reported that the sugar content of sugar beet increased by 43% due yeast inoculation and the treated plants exhibited increased growth. They also reported that yeast application positively affects sugar beet leaf anatomical structure.
Mekki and Ahmed63 stated that plant dry weight indicated the physiological status of the plants. When the soybean plants were applied with the combination of biofertilizer, yeast (Candida tropicalis), and organic manure; the number of branches, plant growth, and yield were found to be increased positively. Gaballah and Gomaa64 conducted an experimental trial on a pot with certain varieties of fava beans grown in sandy soil to investigate the effect of soil yeast Rhodotorula glutinis. When compared with uninoculated ones, in all tested varieties, plants inoculated with Rhodotorula showed a positive increment in dry weight ranging from 17.78 to 8.18 g/plant. Cloete et al.5 reported that certain soil yeasts through direct and indirect mechanisms enhanced the plant root growth. El Tarabily49 carried out the root colonization plate assay experiment in sugar beet. They tested the yeasts potential in colonizing plant roots effectively by sand tube method. In this assay, Candida valida and Trichosporon asahii colonized ninety five percent of roots after six days of inoculation. Rhodotorula glutinis colonized ninety percent of sugar beet root after eight days of inoculation. Marques et al.65 reported that due to inoculation-Rhodosporidium diabovatum in the seedlings of Vriese aminarum the photosynthesis rate was found to be increased due to the IAA production, siderophores production, and solubilization of phosphate. Co-culturing of yeast Sporidiobolus ruineniae with Nicotiana benthamiana seedlings increased the lateral root and root hair growth (Table 2).57
Table (2):
Summary of yeast isolates and their corresponding plant growth-promoting activities
| Organism | PGPR activities | Ref. |
|---|---|---|
| Influence on plant growth (germination, root & shoot growth) and yield | ||
| Torulapsis sp. | Stimulates the germination of cabbage seeds | 16 |
| Rhodotorula sp. | Promotes plant growth and increases fruit yield of tomato | 61 |
| Candida valida, Rhodotorula glutinis, Trichosporon asahii | Improves growth in sugar beet plant. Production of gibberellic acid | 3 |
| Rhodotorula glutinis | Improved the dry weight of fava beans | 64 |
| Yarrowia lipolytica | Promoted the growth of Dorycnium pentaphyllum | 36 |
| Candida tropicalis | Improved growth and yield in soybean | 63 |
| Pichia sp. | Increased the dry weight of lettuce and exhibited tri-calcium phosphate solubilization potential | 37 |
| Saccharomyces cerevisiae | Inoculation in corn plants increased the phosphorous uptake and dry weight of the shoot/root | 35 |
| Sporidiobolus ruineniae | Improved growth of lateral roots and root hair in Nicotiana benthamiana by co-cultivation | 57 |
| Cryptococcus laurentii | Promoted growth of Arabidopsis thaliana | 31 |
| Sporobolomyces roseus KBP Y-5472 and KBP Y-5432, Metschnikowia pulcherrima KBP Y-6020 Aureobasidium pullulans KBP Y-5404 | Production of zeatin and plant growth promotion | 54 |
| Rhodosporidium diabovatum | Improved photosynthesis in Vriese aminarum | 65 |
| Influence of soil yeasts on nutrient mineralization and growth hormone production | ||
| Debaromyces and Saccharomyces | Oxidize sulfur | 68 |
| Rhodotorula | Oxidation of sulfite and thiosulphate | 69 |
| Williopsis californica and Saccharomyces cerevisiae
|
Oxidizes elemental sulfur to thiosulphate, tetrathionate, and sulfate Hydrolyse urea to ammonia and oxidized ammonia to nitrate forming trace amounts of nitrite Solubilize insoluble phosphate | 28 |
| Yarrowia lipolytica | Solubilizes rock phosphate | 34 |
| Rhodotorula | Produces siderophore | 70 |
| Candida, Geotrichum, Rhodotorula, Saccharomyces and Williopsis | Converts ammonium to nitrate through nitrite under in vitro conditions | 27 |
| Williopsis saturnus | Production of Indole-3-acetic acid (IAA) and indole-3-pyruvic acid (IPYA) Increased the lengths, and dry weight of shoots/roots in maize | 46 |
| Rhodotorula, Cryptococcus | Phosphate solubilization | 33 |
| Populus trichocarpa | Production of Indole-3-acetic acid (IAA) | 45 |
| Candida tropicalis CtHY | IAA, ACC deaminase, phytase, and polyamine production | 23 |
| Torulaspora globosa | Mobilize potassium from silicate minerals Solubilization of alkaline ultramafic rock and release of 38% potassium | 41 |
| Metschnikowia pulcherrima and Rodotorula mucilaginosa | During the exponential growth phase synthesizes zeatin | 52, 71 |
| Pseudozyma aphidis | Siderophore production | 57 |
| Moesziomyces antarcticus | Synthesize zeatin | 54 |
| Pichia anomala and Rhodotorula glutinis | Solubilization of muscovite mica | 42 |
| R. graminis and Cryptococcus sp. Saccharomyces cerevisiae Pichia sp. | Siderophore production through the assimilation of sugars and amino acids from plants Production of gibberellic acid Production of gibberellic acid (GA3) | 58, 48, 20 |
| Trichosporon ovoides and Saccharomyces cerevisiae | Siderophore production | 59 |
A study using Candida tropicalis SSm-39 as a soil inoculant improved maize growth by 85% by doubling soil nutrient status.66 Foliar yeast application (5 g/L) enhanced sugarbeet root, shoot and sugar yields by 38.43%, 17.87%, and 67.56%, respectively as compared to soil application (15 g/L). Three foliar yeast sprays, along with 100 kg N/fed, produced the maximum sugar yields and sucrose percentage.67 Saccharomyces cerevisiae (10 g/L) enhanced cucumber fresh and dry weights by 30-40% while doubling fruit yield (100% increase, from 2.07 kg to 4.14 kg per plot) compared to controls. The yield was also 70% higher than in Ethoprophos-treated plants (2.416 kg per plot). Yeast-treated plants showed higher phenolic content, which enhanced resistance and demonstrated its efficiency as a sustainable biocontrol agent.72 A study on the Mexican maize landrace “Raza conico” (red and blue variations) found 87 yeast strains with plant growth-promoting properties, including auxin synthesis (11.9-52 µg/mL from L-Trp). Key strains (Solicoccozyma sp. RY31, C. lusitaniae Y11, R. glutinis Y23, and Naganishia sp. Y52) promoted Arabidopsis thaliana root growth. In maize, inoculation with auxin-producing yeasts caused 1.5 fold increase in plant height, fresh weight, and root length compared to controls.73 Vazquez et al.74 isolated 95 phylloplane yeasts from legume leaves, with Candida tropicalis KPS2219 standing out for producing 54.10 mg/g IAA, high ammonia (1.16 mg/mL), and siderophore activity. Greenhouse trials revealed that seed priming and foliar spraying with C. tropicalis KPS2219 increased chilli seedling development i.e., root length by 17.72%, shoot length by 29.15%, root dry weight by 60% and stem dry weight by 46.15%, respectively, as compared to controls.
Soil health
In sustainable agriculture soil health is considered as a critical component. The inherent assignment of soil health and quality is “the capacity of soil functions”, which can be assessed by physical, chemical, and biological properties of soil as the indicators. Soil health and quality determines agricultural sustainability and environmental quality, which ultimately results in improved plant, animal, and human health.75 Efficient and potential soil microbiota is a significant factor that decides soil health. The microbial communities’ composition influences i) conversion of biodegradable residues into organic matter of soil and influencing the plant nutrients availability, ii) soil aggregate stabilization, iii) erosion reduction, and iv) maintenance of water-holding capacity. In agriculture the application of microbial inoculants has gained importance due to their merits, such as plant growth promotion, soil health improvement, residue-free environment, etc. Filamentous fungi and bacteria have been explored and exploited as potential microbial inoculants to a maximum level. The unicellular fungi-yeasts remain unexplored as microbial inoculants.
Soil structure maintenance is essential to sustain crop productivity. Structural properties of soil influence the soil’s potential to improve plant growth, cycling of nutrients, and water-holding properties. The microbial inoculants used in agriculture improve the structure of soil through production of extracellular polymeric substances and plant growth promotion. Soil aggregate formation plays an important role in the maintenance of soil structure and also serves as a measure for soil structure evaluation. The occurrence and stability of these aggregates positively influence crop growth. Formation of soil macro-aggregates and micro-aggregates are profoundly influenced by soil microbiome and this is due to the secretion of Extracellular Polymeric Substances (EPS) which serve as binding agents for the formation of aggregates (Figure). Lehmann et al.77 reported that most of the earlier research about soil aggregation focused on earthworms, Arbuscular Mycorrhizal fungi, bacteria, and very few reports are available about fungi.
Amellal et al.78 reported that inoculation of extracellular polymeric substances-producing organisms improved the macro porosity of the soil, improved soil aggregation, and hence regulated the moisture content of soil positively. Botha19 reported that yeasts belonging to the genera like Cryptococcus and Lipomyces influence the soil texture by secreting EPS which serves as connective bridges of soil/sand grains and hence formation of aggregates. Cho et al.79 reported that genera such as Cryptococcus, Lipomyces, and Rhodotorula were well known EPS producers. These EPS can generate a capsule-like structure that envelops the cells of yeast and leads to biofilm formation, thereby providing the capability to resist desiccation.80
The yeast genera Lipomyces, Cryptococcus, and Rhodotorula were found to survive better in soil with poor nutrients and this might be due to the high production of EPS, which leads to soil aggregate formation that could help the yeast to adopt such harsh habitats. Furthermore, the extracellular polymeric substances of microbial biofilms act as an adhesive between soil particles and contribute a lot to the soil aggregate formation and stability.81,82 Cho et al79 isolated Rhodotorula glutinis from soil and examined the culture conditions for improving the exo-polysaccharides production by the yeast isolate. The presence of uronic acid/mannose-rich acidic heteropolysaccharide composed of 85% neutral sugars and 15% uronic acid in the EPS produced by Rhodotorula glutinis was recorded. Gientka et al.83 reported that the EPS produced by soil yeasts was composed of a linear arrangement of mannans, gluco-oligosaccharides, galacto oligosaccharides, and other hetero-polysaccharides linked by a-(1,2;1,3;1,6), b-(1,3;1,4) bonds.
Biofilm is formed by many microbial communities living together; it may be a surface associated with or attached to a self-produced protective extracellular matrix (ECM). Broad genera of bacteria and fungi colonize together and attach to the surface to form the multicellular community called biofilms.84 Biofilm quantification is a measure to assess the EPS production by microorganisms. Several reports on biofilms with potential applications in agriculture are available.1 In 2002, Ramage et al.85 reported the Candida albicans biofilm’s antifungal activity using the 96-well microtitre plate model. Similarly, the microtiter plate biofilm assay for Cryptococcus neoformans was developed by Martinez and Casadevall.86 While the biofilm assay helps identify the antimicrobial activity of the drug on a sessile population compared with untreated control, the metabolic variability among different isolates makes it worthwhile to quantify biofilm formation.87
The phytopathogenic activity of cyanobacteria-based fungal and bacterial biofilms has been extensively studied.88 Swarnalakshmi et al.89 reported the positive impact of cyanobacterial-based biofilms in wheat crops over the soil chemical and biological properties. Prasanna et al.90 reported that application of microbial biofilms had differential effects on growth of plants and nutrient dynamics of soil in flooded and SRI (System of Rice Intensification) rice. Ramya et al.76 reported that biofilms are produced by certain yeast species possessing the plant growth-promoting potential. Among the sixteen plant growth-promoting yeast isolates tested, Pichia kudriavzevii was found to produce more B/P (Biofilm producing cells/Planktonic cells) ratio with an increase in time. Soil health is one of the major goals of sustainable agriculture and hence developing new bioinoculants with biofilm-forming potential is a potential area to enhance soil health.
Yeast inoculation on soil health
Crop management practices like fertilizer application, intercultural operations, cropping patterns, microbial inoculants, etc., majorly influence soil physiochemical properties.91 Balancing soil properties is essential for the soil health maintenance and improving the plant productivity. Microbial partners of soil influence its properties to a large extent when compared to other factors (Table 3).
Table (3):
Summary of yeast isolates and their corresponding properties in enhancing soil health
No. |
Organism name |
Properties |
Ref. |
|---|---|---|---|
1. |
Pichia kudriavzevii (OT3 8), Candida tropicalis (OT3 12), Pichia kudriavzevii (OT3 2), Candida tropicalis (OT3 5), Pichia kudriavzevii (OT3 2), Pichia kudriavzevii (RT2 4) |
The formation of soil aggregates increases the organic carbon content and protein index of soil |
76 |
2. |
C. tropicalis SSm-3 |
Increases organic carbon content in soil |
66 |
3. |
Yarrowia lipolytica |
Increases in soil dehydrogenase activity and lowering of soil pH |
36 |
Soil physical properties
The application of organic manures affects the microbial abundance, properties, and nutrient cycling in soil.92 Soil stability was found to be improved by formation of micro aggregates within macro aggregates.93 The various functions of yeasts isolated from the soil positively influences the soil aggregate formation. By production of extracellular polymeric substances at higher levels, some yeasts like Lipomyces and Cryptococcus increase the aggregate formation and hence influence the physical properties of soil positively. Soil amendment with Yarrowia lipolytica significantly influenced the soil’s nutritional status and its properties (physical, chemical, and biological).36
Soil chemical properties
The changes in various abiotic factors like soil organic matter content, pH, temperature, soil moisture, and macronutrients like nitrogen, potassium, sodium and magnesium influences the soil yeasts population.19,4 In assessing the soil quality index Soil labile carbon (SLC) serves as an important biological indicator. A change in soil pH from 8.90 to 8.75 was observed upon inoculation with Yarrowia lipolytica.36 Ramya et al.,76 studied the effect of soil yeasts on chemical properties of soil. They reported that pH of soil remained neutral throughout time following the introduction of soil yeast isolates. Inoculation of Pichia kudriavzevii exhibited an increase in soil organic carbon content, with observed values of 7.72 and 8.36 milligram per gram of soil at fifteen and thirty days after inoculation, respectively. Inoculation of soil yeasts positively influenced the soil’s labile carbon status. Due to inoculation of soil yeasts the soil protein index ranged from 3.78 to 8.29 microgram per gram of soil on the fifteenth day after inoculation.
Soil biological properties
The biological properties of soil like dehydrogenase enzyme activity, microbial biomass carbon (MBC), soil colloidal polysaccharide, chemical properties like pH, soil organic carbon, soil labile carbon, soil protein index, and other physical properties used to determine the quality of soil are known as soil quality indicators.94-96
As a potential indicator of soil health, the Microbial Biomass Carbon (MBC) responds to different soil management strategies.97 Soil management practices through organic means increase the soil microbial activity and hence the microbial biomass carbon. Finally, this increases the nutrient availability which are essential for crop growth.98 Application of organic manures increases the availability of essential nutrients in the soil for microbial growth and hence the Microbial Biomass Carbon also increases. Ramya et al.,76 reported that soil microbial biomass carbon increased in yeast-inoculated soil. Among the isolates used for the study, MBC value of 0.98 mg/g of soil and 0.90 mg/g of soil at fifteen and thirty days after inoculation was observed due to inoculation of Candida tropicalis Soil dehydrogenase activity acts as the function of oxidative activity and microbial count and serves as a good unit for measurement of microbial activity.
The dehydrogenase activity of Yarrowia lipolytica amended soil was found to be increased.36 Ramya et al.,76 stated that due to the application of Pichia kudriavzevii higher soil dehydrogenase activity of 0.64 µg TDF.g-1.day-1 was observed. Intra and extracellular polymeric substances synthesized by microbes benefit the soil microbial community and have wide practical applications. The properties of the EPS were documented by many researchers after intensive research. The specific properties of colloidal polysaccharides secreted by certain microbes remain unrevealed so far. A detailed investigation of the colloidal polysaccharide produced by soil yeasts may reveal its exact chemical nature and role in maintaining soil structure.
As a result of the global demand for food, it is essential to increase the production of agricultural commodities. However, it is abundantly evident that the solution does not lay in excessive synthetic fertilizers and pesticides, as these practices degrade soil health, which is the foundation of sustainable agriculture. Global adoption of sustainable agricultural practices is crucial for assuring food security and a healthy environment. The efficient and robust soil microbiota is one of the most critical determinants of soil health, as it substantially affects soil properties and promotes optimal plant growth. Recognizing the limitations of conventional agricultural methods, the use of microbial inoculants in agriculture has gained popularity due to their numerous benefits, which include the promotion of plant growth, improvement of soil health, and creation of residue-free environments. At the same time, filamentous fungi and bacteria have been exhaustively studied and utilized as potential microbial inoculants whereas yeast-unicellular fungi still need to be explored. Notably, yeasts contribute to the microbial diversity of ecosystems, although to a lesser degree than other microbial groups.
Scientists are currently assiduously investigating the soil microbiome in search of novel microbial inoculants that can sustain and enhance crop production to meet the requirements of a constantly expanding global population. The current limitation in utilizing yeasts as microbial inoculants is that most of the earlier research has primarily focused on the taxonomic diversity of soil yeasts, but there is an urgent need to investigate their functional diversity. By doing so, we can unleash the untapped potential of soil yeasts as valuable plant growth promoters and soil quality enhancers.
Although our knowledge of soil yeast is growing exponentially, there is much to learn about soil yeast for exploring them for sustainable growth in agriculture.
- What is the extent of soil yeasts’ diversity present in agricultural soils? Did the diversity vary among the soil types? Which soil attributes contribute to soil yeast’s diversity? Did the present agronomical features and environmental factors affect the soil yeast’s diversity? For this, high throughput assays need to be employed as that of soil and plant-associated bacterial diversity.
- What are the key roles soil yeasts perform in the soil food web and nutrient cycling? This ecological approach is essential to unlock the functions of soil yeast in soil health and quality.
- Do the soil yeast have special mechanisms to colonize the plants or arbitrarily colonize the root in search of food? What is the interaction effect of soil yeast with other commensals, symbionts, and parasites? How do host plants choose their yeast species for their interactions? This will understand the plant-yeast interactions to enhance their beneficial role in agriculture.
- Is it possible to engineer the plant-yeast interactions to enhance their beneficial role in plant health and fitness?
- Are yeast inoculants comparable with bacterial inoculants for production, product formulation, shelf life, inoculation, persistence in the host plant, and beneficial role in crops? What are the strategies for the effective delivery of yeast inoculants to different crop niches?
- Is it possible to utilize soil yeast as potential inoculant for drought mitigation and to improve crop growth in problem soils?
- Do the soil yeast’s metabolites could be a potential resource for biostimulants for agriculture?
- Can yeast’s population and diversity be an indicator of soil health? How can the yeast’s physiology and nutritional behavior in the soil ecosystem be improved to contribute more towards soil health?
Exploring and using these often-overlooked microorganisms gives us hope for a better and more sustainable future while protecting the environment. Accepting the variety of our soil microbiota, including yeasts, is vital in finding the right balance between farming output and caring for the environment. A better understanding of various roles of soil yeasts will pave way to attain sustainability in agriculture.
ACKNOWLEDGMENTS
The authors are thankful to Tamil Nadu Agricultural University, Coimbatore, for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Velmourougane K, Prasanna R, Saxena AK. Agriculturally important microbial biofilms: present status and future prospects. J Basic Microbiol. 2017;57(7):548-573.
Crossref - Ekelund F, Ronn R, Christensen S. Distribution with depth of protozoa, bacteria and fungi in soil profiles from three Danish forest sites. Soil Biol Biochem. 2001;33(4-5):475-481.
Crossref - El-Tarabily KA, Sivasithamparam K. Potential of yeasts as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Mycoscience. 2006;47(1):25-35.
Crossref - Botha A. The importance and ecology of yeasts in soil. Soil Biol Biochem. 2011;43(1):1-8.
Crossref - Cloete KJ, Valentine AJ, Stander MA, Blomerus LM, Botha A. Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a sclerophyllous medicinal shrub, Agathosma betulina (Berg.) Pillans. Microb Ecology. 2009;57(4):624-632.
Crossref - Slavikova E, Vadkertiova R. The occurrence of yeasts in the forest soils. J Basic Microbiol. 2000;40(3):207-212.
Crossref - Slavikova E, Vadkertiova R. The diversity of yeasts in the agricultural soil. J Basic Microbiol. 2003;43(5):430-436.
Crossref - Jamali S, Gharaei M, Abbasi S. Identification of yeast species from uncultivated soils by sequence analysis of the hypervariable D1/D2 domain of LSU-rDNA gene in Kermanshah province, Iran. Mycologia Iranica. 2016;3(2):87-98.
Crossref - Masinova T, Bahnmann BD, Vetrovsky T, Tomsovsky M. Drivers of yeast community composition in the litter and soil of a temperate forest. FEMS Microbiol Ecol. 2017;93(2):fiw223.
Crossref - Lipsa F-D, Ulea E. Assessment of yeast diversity in different soil types under various management regimes in Moldavia region, Romania. Lucrari Stiintifice USAMV Iasi Seria Agronomie, 2017;60(2):11-14.
- Moreira GAM, Vale H. Occurrence of yeast species in soils under native and modified vegetation in an iron mining area. Revista Brasileira de Ciencia do Solo. 2018;42:e0170375.
Crossref - Glushakova A, Maksimova I, Morozova A, Kachalkin A. Distribution features of yeasts in soils of South Vietnam (case study of the biogeocenoses of the National Park Cat Tien). IOP Publishing:012012.
Crossref - Koricha AD, Han D-Y, Bacha K, Bai F-Y. Occurrence and molecular identification of wild yeasts from Jimma Zone, South West Ethiopia. Microorganisms. 2019;7(12):633.
Crossref - Chandra M, Mota M, Silva AC, Malfeito-Ferreira M. Forest oak woodlands and fruit tree soils are reservoirs of wine-related yeast species. Am J Enol Vitic. 2020;71(3):191-197.
Crossref - Zhu SS, YanLi C, Yang L, Jie X, YoungHui L, YanFei S. Effects of temporal and spatial scales on soil yeast communities in the peach orchard. Front Microbiol. 2023;14:1226142.
Crossref - Bab’eva I, Belyanin A. Yeasts of the rhizosphere. Mikrobiologiya. 1966;35:712-720.
- Sniegowski PD, Dombrowski PG, Fingerman E. Saccharomyces cerevisiae and Saccharomyces paradoxus coexist in a natural woodland site in North America and display different levels of reproductive isolation from European conspecifics. FEMS Yeast Res. 2002;1(4):299-306.
Crossref - Phaff HJ, Miller MW, Mrak EM. The Life of yeasts: Their nature, activity, ecology, and relation to mankind. Cambridge, Mass., Harvard Univ. Press. 1966.
- Botha A. Yeasts in soil. Biodiversity and ecophysiology of yeasts. 2006:221-240.
Crossref - Pandi R, Velu G, Devi P, Dananjeyan B. Isolation and screening of soil yeasts for plant growth promoting traits. Madras Agricultural Journal. 2019;106(7-9):439-443.
Crossref - Bates ST, Garcia-Pichel F, Nash Iii TH. Fungal components of biological soil crusts: insights from culture-dependent and culture-independent studies. Biology of Lichens-Symbiosis, Ecology, Environmental Monitoring, Systematics, Cyber Applications. 2010;105:197-210.
- Shih PM, Vuu K, Mansoori N, et al. A robust gene-stacking method utilizing yeast assembly for plant synthetic biology. Nat Commun. 2016;7:13215.
Crossref - Amprayn K-o, Rose MT, Kecskes M, Pereg L, Nguyen HT, Kennedy IR. Plant growth promoting characteristics of soil yeast (Candida tropicalis HY) and its effectiveness for promoting rice growth. Appl Soil Ecol. 2012;61:295-299.
Crossref - Santos PCD, Fang Z, Mason SW, Setubal JC, Dixon R. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genomics. 2012;13:1-12.
Crossref - Wang M, Shang Y, Liu X, Chen S. Assembly of nitrogenase biosynthetic pathway in Saccharomyces cerevisiae by using polyprotein strategy. Front Microbiol. 2023;14:1137355.
Crossref - Gori K, Mortensen HD, Arneborg N, Jespersen L. Ammonia production and its possible role as a mediator of communication for Debaryomyces hansenii and other cheese-relevant yeast species. J Dairy Sci. 2007;90(11):5032-5041.
Crossref - Al-Falih AM. Phosphate solubilization in vitro by some soil yeasts. Qatar Univ. Sci. J. 2005;25:119-125.
- Falih AM, Wainwright M. Nitrification, S-oxidation and P-solubilization by the soil yeast Williopsis californica and by Saccharomyces cerevisiae. Mycol Res. 1995;99(2):200-204.
Crossref - Rezende LA, Assis LC, Nahas E. Carbon, nitrogen and phosphorus mineralization in two soils amended with distillery yeast. Bioresour Technol. 2004;94(2):159-167.
Crossref - Meena VS, Meena SK, Verma JP, et al. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: a review. Ecol Eng. 2017;107:8-32.
Crossref - Cy K, Sf F, Chou FC, Chen RY, Chou JY. Phosphate-solubilizing characteristics of yeasts. Mycosphere. 2018;9(6):1117-1131.
Crossref - Vogel K, Hinnen A. The yeast phosphatase system. Mol Microbiol. 1990;4(12):2013-2017.
Crossref - Alonso LM, Kleiner D, Ortega E. Spores of the mycorrhizal fungus Glomus mosseae host yeasts that solubilize phosphate and accumulate polyphosphates. Mycorrhiza. 2008;18(4):197-204.
Crossref - Vassileva M, Azcon R, Barea J-M, Vassilev N. Rock phosphate solubilization by free and encapsulated cells of Yarowia lipolytica. Process Biochem. 2000;35(7):693-697.
Crossref - Hesham A-L, Mohamed HJJMB. Molecular genetic identification of yeast strains isolated from Egyptian soils for solubilization of inorganic phosphates and growth promotion of corn plants. J Microbiol Biotechnol. 2011;21(1):55-61.
Crossref - Medina A, Vassileva M, Caravaca F, Roldn A, Azcon R. Improvement of soil characteristics and growth of Dorycnium pentaphyllum by amendment with agrowastes and inoculation with AM fungi and/or the yeast Yarowia lipolytica. Chemosphere. 2004;56(5):449-456.
Crossref - Nakayan P, Shen F-T, Hung M-H, Chiu-Chung Y. Effectiveness of Pichia sp. CC1 in decreasing chemical fertilization requirements of garden lettuce in pot experiments. Asian Journal of Food and Agro-Industry. 2009:66-68.
- Nutaratat P, Srisuk N, Arunrattiyakorn P, Limtong S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biol. 2014;118(8):683-694.
Crossref - Ramachandran S, Fontanille P, Pandey A, Larroche C. Gluconic acid: properties, applications and microbial production. Food Technol Biotechnol. 2006;44(2).
- Borekci BS, Kaban G, Kaya M. Citric acid production of yeasts: an overview. The EuroBiotech Journal. 2021;5(2):79-91.
Crossref - Rosa-Magri MM, Avansini SH, Lopes-Assad ML, Tauk-Tornisielo SM, Ceccato-Antonini SR. Release of potassium from rock powder by the yeast Torulaspora globosa. Braz Arch Biol Technol. 2012;55(4):577-582.
Crossref - Mohamed HM, El-Homosy RF, Abd-Ellatef A-EH, Salh FM, Hussein MY. Identification of yeast strains isolated from agricultural soils for releasing potassium-bearing minerals. Geomicrobiol J. 2017;34(3):261-266.
Crossref - Halliday KJ, Martinez-Garcia JF, Josse E-M. Integration of light and auxin signaling. Cold Spring Harb Perspect Biol. 2009;1(6):a001586.
Crossref - Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31(4):425-448.
Crossref - Xin G, Glawe D, Doty SL. Characterization of three endophytic, indole-3-acetic acid-producing yeasts occurring in Populus trees. Mycol Res. 2009;113(9):973-980.
Crossref - Nassar AH, El-Tarabily KA, Sivasithamparam K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol Fertil Soils. 2005;42(2):97-108.
- Pandya ND, Desai PV. Screening and characterization of GA3 producing Pseudomonas monteilii and its impact on plant growth promotion. Int J Curr Microbiol App Sci. 2014;3(5):110-115.
- Twfiq AA, Al-Shaheen MR, Farhan MA, Al-Shaheen MR. The possibility of cytokinins production from regular dry bakery yeast (Saccharomyces cerevisiae). International Journal of Recent Innovation in Food Science & Nutrition. 2018;1(1).
- El-Tarabily KA. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth promoting yeasts. J Appl Microbiol. 2004;96(1):69-75.
Crossref - Streletskii RA, Kachalkin AV, Glushakova AM, Yurkov AM, Demin VV. Yeasts producing zeatin. Peer J. 2019;7:e6474.
Crossref - Laten HM, Zahareas-Doktor S. Presence and source of free isopentenyladenosine in yeasts. Proc Natl Acad Sci U S A. 1985;82(4):1113-1115.
Crossref - Hussain A, Hasnain S. Cytokinin production by some bacteria: its impact on cell division in cucumber cotyledons. Afr J Microbiol Res. 2009;3(11):704-712.
- Kudoyarova GR, Korobova AV, Akhiyarova GR, et al. Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). J Exp Bot. 2014;65(9):2287-2294.
Crossref - Streletskii RA, Kachalkin AV, Glushakova AM, Demin VV, Chernov IY. Quantitative determination of indole-3-acetic acid in yeasts using high performance liquid chromatography-tandem mass spectrometry. Microbiology. 2016;85:727-736.
Crossref - Verma VC, Singh SK, Prakash S. Bio control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss. J Basic Microbiol. 2011;51(5):550-556.
Crossref - Calvente V, de Orellano ME, Sansone G, Benuzzi D, de Tosetti MIS. A simple agar plate assay for screening siderophore producer yeasts. J Microbiol Methods. 2001;47(3):273-279.
Crossref - Fu S-F, Sun P-F, Lu H-Y, et al. Plant growth-promoting traits of yeasts isolated from the phyllosphere and rhizosphere of Drosera spatulata Lab. Fungal Biol. 2016;120(3):433-448.
Crossref - Joubert PM, Doty SL. Endophytic yeasts: Biology, ecology and applications. Endophytes of Forest Trees. 2018:3-14.
Crossref - El-Maraghy SS, Tohamy TA, Hussein KA. Expression of SidD gene and physiological characterization of the rhizosphere plant growth-promoting yeasts. Heliyon. 2020;6(7):e04384.
Crossref - Edi Premono M, Moawad AM, Vlek PLG. Effect of phosphate-solublizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indonesian Journal of crop science. 1996;11(1):13-23.
- Abdel-Hafez A, Shehata S. Field evaluation of yeasts as a biofertilizer for some vegetable crops. Arab Univ J Agric Sci. 2001;9:169-182.
- Agamy R, Hashem M, Alamri S. Effect of soil amendment with yeasts as bio-fertilizers on the growth and productivity of sugar beet. Afr J Agric Res. 2013;8(1):46-56.
- Mekki B, Ahmed AG. Growth, yield and seed quality of soybean (Glycine max L.) as affected by organic, biofertilizer and yeast application. Res J Agric Biol Sci. 2005;1(4):320-324.
- Gaballah M, Gomaa A. Performance of Faba Bean Varieties Grown under Salinity. J Appl Sci. 2004;4(1):93-99.
Crossref - Marques AR, Resende AA, Gomes FCO, et al. Plant growth-promoting traits of yeasts isolated from the tank bromeliad Vriesea minarum LB Smith and the effectiveness of Carlosrosaea vrieseae for promoting bromeliad growth. Braz J Microbiol. 2021;52(3):1417-1429.
Crossref - Mukherjee S, Sen SK. Exploration of novel rhizospheric yeast isolate as fertilizing soil inoculant for improvement of maize cultivation. J Sci Food Agric. 2015;95(7):1491-1499.
Crossref - Nemeat Alla HEA, El-Geddawy DIH, Makhlouf BSI. Effect of yeast application method and number on yield and quality of sugar beet under different levels of nitrogen. Journal of Plant Production. 2015;6(9):1475-1490.
Crossref - Vitolins MI, Swaby RJ. Activity of sulphur-oxidizing microorganisms in some Australian soils. Soil Research. 1969;7(2):171-183.
Crossref - Kurek EJ. An enzymatic complex active in sulphite and thiosulphate oxidation by Rhodotorula sp. Arch Microbiol. 1983;134(2):143-147.
Crossref - Calvente V, De Orellano ME, Sansone G, Benuzzi D, Sanz de Tosetti MI. Effect of nitrogen source and pH on siderophore production by Rhodotorula strains and their application to biocontrol of phytopathogenic moulds. Journal of Industrial Microbiology and Biotechnology. 2001;26(4):226-9.
- Kudoyarova GR, Korobova AV, Akhiyarova GR, et al. Accumulation of cytokinins in roots and their export to the shoots of durum wheat plants treated with the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP). Journal of Experimental Botany. 2014;65(9):2287-94.
- Karajeh MR. Efficacy of Saccharomyces cerevisiae on controlling the root-knot nematode (Meloidogyne javanica) infection and promoting cucumber growth and yield under laboratory and field conditions. Archives of Phytopathology and Plant Protection. 2013;46(20):2492-2500.
Crossref - Ramos-Garza J, Aguirre-Noyola JL, Bustamante-Brito R, et al. Mycobiota of Mexican Maize Landraces with Auxin-Producing Yeasts That Improve Plant Growth and Root Development. Plants. 2023;12(6):1328.
Crossref - Vazquez MM, Quintana S, Medici S, Gende LB. Evaluate the effectiveness of brewer’s yeast by-product of the brewing industry as a biostimulant in hydroponics. Innotec;2022:e622.
Crossref - Doran JW. Soil health and global sustainability: translating science into practice. Agric Ecosyst Environ. 2002;88(2):119-127.
Crossref - Ramya P, Gomathi V, Devi RP, Balachandar D. Pichia kudriavzevii-a potential soil yeast candidate for improving soil physical, chemical and biological properties. Arch Microbiol. 2021;203(7):4619-4628.
Crossref - Lehmann J, Bossio DA, Kogel-Knabner I, Rillig MC. The concept and future prospects of soil health. Nat Rev Earth Environ. 2020;1(10):544-553.
Crossref - Amellal N, Burtin G, Bartoli F, Heulin T. Colonization of wheat roots by an exopolysaccharide-producing Pantoea agglomerans strain and its effect on rhizosphere soil aggregation. Appl Environ Microbiol. 1998;64(10):3740-3747.
Crossref - Cho DH, Chae HJ, Kim EY. Synthesis and characterization of a novel extracellular polysaccharide by Rhodotorula glutinis. Appl Biochem Biotechnol. 2001;95(3):183-193.
Crossref - Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect Immun. 2003;71(9):4862-4872.
Crossref - Baldock J. Interactions of organic materials and microorganisms with minerals in the stabilization of soil structure. John Wiley and Sons, Ltd: Chichester, West Sussex, UK. 2002;85-131.
- Ashman MR, Hallett PD, Brookes PC, Allen J. Evaluating soil stabilisation by biological processes using step-wise aggregate fractionation. Soil and Tillage Research. 2009;102(2):209-215.
Crossref - Gientka I, Blazejak S, Stasiak-Rozanska L, Chelbowska-Smigiel A. Exopolysaccharides from yeast: insight into optimal conditions for biosynthesis, chemical composition and functional properties? review. Acta Sci Pol Technol Aliment. 2015;14(4):283-292.
Crossref - Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35(4):340-355.
Crossref - Ramage G, Saville SP, Wickes BL, Lopez-Ribot J. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68(11):5459-5463.
Crossref - Martinez LR, Casadevall A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 2006;50(3):1021-1033.
Crossref - Kuhn DM, Balkis M, Chandra J, Mukherjee PK, Ghannoum MA. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin Microbiol. 2003;41(1):506-508.
Crossref - Prasanna R, Kumar A, Babu S, et al. Deciphering the biochemical spectrum of novel cyanobacterium-based biofilms for use as inoculants. Biol Agric Hortic. 2013;29(3):145-158.
Crossref - Swarnalakshmi K, Prasanna R, Kumar A, et al. Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur J Soil Biol. 2013;55:107-116.
Crossref - Prasanna R, Adak A, Verma S, et al. Cyanobacterial inoculation in rice grown under flooded and SRI modes of cultivation elicits differential effects on plant growth and nutrient dynamics. Ecol Eng. 2015;84:532-541.
Crossref - Pernes-Debuyser A, Tessier D. Soil physical properties affected by long term fertilization. Eur J Soil Sci. 2004;55(3):505-512.
Crossref - Bastida F, Zsolnay A, Hernandez T, Garcia C. Past, present and future of soil quality indices: a biological perspective. Geoderma. 2008;147(3-4):159-171.
Crossref - Garcia-Franco N, Martinez-Mena M, Goberna M, Albaladejo J. Changes in soil aggregation and microbial community structure control carbon sequestration after afforestation of semiarid shrublands. Soil Biol Biochem. 2015;87:110-121.
Crossref - Balachandar D, Chinnadurai C, Tamilselvi SM, Ilamurugu K, Arulmozhiselvan K. Lessons from long-term nutrient management adoptions in semi-arid tropical alfisol. Int J Plant Soil Sci. 2016;10(2):1-14.
Crossref - Chinnadurai C, Gopalaswamy G, Balachandar D. Long term effects of nutrient management regimes on abundance of bacterial genes and soil biochemical processes for fertility sustainability in a semi-arid tropical Alfisol. Geoderma. 2014;232-234:563-572.
Crossref - Tamilselvi SM, Chinnadurai C, Ilamurugu K, Arulmozhiselvan K, Balachandar D. Effect of long-term nutrient managements on biological and biochemical properties of semi-arid tropical Alfisol during maize crop development stages. Ecological Indicators. 2015;48:76-87.
Crossref - Marschner P, Kandeler E, Marschner B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem. 2003;35(3):453-461.
Crossref - Wang Y, Shi J, Wang H, Chen XC, Chen YX. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol Environ Safety. 2007;67(1):75-81.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.