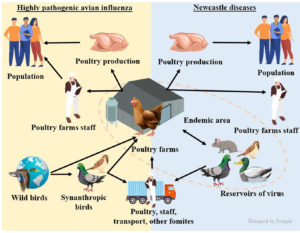
The paper highlights the impact of two cross-border poultry infections with zoonotic potential (avian flu and Newcastle disease) on the functioning of industrial poultry farms in the former Soviet Union counties (Ukraine, Russia, Belarus, Kazakhstan), where the poultry industry is fairly well-developed. Despite the permanent vaccination of poultry against Newcastle disease in industrial poultry farming, the disease still affects individual farms in Ukraine, the Russian Federation, and Kazakhstan. In case of outbreaks, the Russian Federation and Kazakhstan use inactivated influenza vaccines. In Ukraine, for almost 20 years, outbreaks of influenza have been confirmed mainly on individual farms, and one outbreak of highly pathogenic influenza was reported on an industrial poultry farm in 2020. In the Russian Federation, highly pathogenic influenza occurs on industrial poultry farms more often. In Russia, seven industrial poultry enterprises were affected by influenza in 2016-2017, and eight in 2018. Infection of poultry with influenza virus on poultry factory farms is an indication of shortcomings in compliance with biosecurity measures. Influenza and Newcastle disease are always likely to occur in the countries in question, as wild birds migrate through their territory, and they are a reservoir of pathogens, therefore outbreaks are often associated with spring and autumn migrations of wild birds. In all of said countries, a large number of poultry is kept by individual households, where basic biosecurity, sanitation and preventive vaccination measures are not applied. This component is often crucial in bringing viral infections such as influenza and Newcastle disease on large poultry farms. As a result, the virus is brought onto poultry farms by synanthropic birds, humans, transport, feed, etc.
Intensive Poultry Farming, Virus Zoonoses, Birds, HPAI, One Health
Poultry farming as an industry is the most viable, flexible and profitable component of animal husbandry, which can be developed on a limited area and on the basis of innovation and investment, using the experience of other countries. Broiler meat is predominantly produced at large broiler factory farms operating in a closed production cycle. High biological precocity and high reproductive cycle provide a regular supply of fresh high-calorie products to consumers in large cities, industrial centres and resort areas with a significant concentration of population, as well as help to successfully solve the food problem.1 Ukraine’s share in global poultry production currently stands at 1.1%, which has allowed the country to rank as the 21st world’s producer of such products.2 Ukraine ranks ninth in the world market for egg production (1.4%). The country’s share in the European egg production market is 7.6%.1 According to statistics, in Ukraine, there are currently 152 egg producers, 82 poultry producers, 102 producers of breeding products, as well as 338 inter-farm enterprises. Poultry is characterized by high productivity, intensive growth and the highest conversion of feed with good adaptation to industrial conditions. In reality, however, the efficiency of agricultural production in Ukraine is lower than desired, and the production of poultry products is insufficient to satisfy market needs.3
Meat is an important component of human nutrition and has high nutritional value. The content of nutrients in the meat of different animal species is basically similar. Although chicken meat is considered somewhat less caloric, it also contains less fat, which strengthens its dietary importance. In the production and processing of poultry meat, it is important to observe the standards of health and welfare of birds, which directly affects the quality of the final product. The meat of ducks, turkeys and other types of industrial poultry has its own specific features, remaining a desirable product in human nutrition.4
One of the advantages of technological processes of companies in Ukraine is the closed-loop character of all components of production. Such companies control the production and distribution units. The production process begins with the collection of grain from the company’s own arable land, from where the grain is then transported to their own elevators. In the next stage, raw materials are processed into feed at the company’s production facilities. At the same time, the incubation and brooding of young birds at incubators takes place. The breeding, slaughter and processing of poultry follows. As a next step, the companies use their own vehicles to transport the meat and eggs to supermarket chains, as well as their own outlets. The production process allows to minimize risks and transaction costs, and therefore reduce the cost of production. The main prerequisites for capturing the market by such enterprises are their large capacity and capital, the ability to use advanced equipment and operate in a closed production cycle.5,6 The authors provide evidence that introducing logistic management of production turnover of large poultry farming enterprises is necessary in order to facilitate transition to system planning and the organization, as well as to allow benefiting from the advantages of rationalization of production processes and the modern concept of management of material resources.
The authors conclude that poultry production to be optimized, an appropriate information infrastructure needs to be created to facilitate collecting, organizing and transmitting information using new technologies, modern software products, computers and computer networks.7 Poultry products are significantly cheaper than pork and beef, which is very important for countries where the majority of the population has low purchasing power.8,9 Today, a large part of the population prefers poultry meat due to availability, nutritional and price factors.10
For the production of broiler meat, the industrial poultry industry of Ukraine keeps highly-productive poultry of modern crosses supplied by the world’s leading companies. Upon reaching the age of 42 days, the body weight of chickens is 2.5–2.65 kg and the feed conversion ratio is 1.6–1.7 kg per kilogram of gain. In private (individual) household farms, broilers are kept for up to 3–4 months.11 In Ukraine, poultry other than chickens accounts for only 9% of all poultry. According to the State Statistics Service of Ukraine, 94% of geese, 96% of ducks and 65% of turkeys are kept in individual household farms.12 According to the Association of Poultry Farmers of Ukraine, in 2017, poultry meat produced in the country was obtained from: broilers – 94.3%; goose – 0.1%; duck – 0.18%; turkey – 2.3%; meat of other poultry species – 3.2%. In 2019, the poultry population in Ukraine increased by almost 10 million heads: from 222.6 million (as of December 1, 2018) to 232.2 million heads (as of December 1, 2019). From July 1, 2019 to July 1, 2020, the poultry population decreased by 1.9% – to 248.79 million heads: by 2.4% (to 121.35 million heads) in agricultural enterprises and by 1.4% (to 127.44 million heads) in small-scale farms. As of September 1, 2020, up to 254.7 million birds were kept in Ukraine. At the same time, according to FAO, Ukraine currently does not have objective indicators on the scale of threats to both food security and losses resulting from the diseases of animals, birds, bees, etc., due to the lack of primary data on livestock. However, as we can see, the number of poultry kept on individual farms (51.2% of the total number of 248.79 million) is slightly higher than in large industrial farms (48.8%).13
Today, industrial poultry farming in the Russian Federation is the main source of meat and meat products in the country’s food market. Poultry meat accounts for more than 40% of total meat resources. In the Russian Federation, a vast majority of poultry is produced by large industrial poultry enterprises. Of the total volume of poultry meat, 86% is produced by such companies and only 14% comes from small-scale and household farms.14,15
In the Republic of Kazakhstan, industrial poultry farming is represented by 62 powerful poultry farms, of which 36 are egg-laying enterprises, 23 produce broiler meat, and three are engaged in the production of waterfowl meat. An analysis of the state of poultry farming in this country showed that as of the beginning of 2019, the poultry population in all categories of farms was 44,452 thousand heads; in this case, large poultry farms accounted for 32,388.5 thousand heads, or 72.8%. The remaining birds are raised on small-scale and household farms.16
In the total meat production volume in the Republic of Belarus, the share of poultry is 42.8%, beef – 24.5%, pork – 25.7%, other species – 7%. The centralised governing body of the poultry industry is the republican association “Belarus Poultry”. The association consists of five poultry breeding enterprises,17 egg-direction poultry enterprises, 10 meat-direction poultry enterprises and two enterprises operating in the compound feed industry. In total, there are 56 state-held and private poultry enterprises operating in the industry of the Republic of Belarus. There are eight meat and 12 industrial poultry farms in the country, and they account for about 70% of the total production volume. Along with state-owned poultry farms, non-state-owned poultry enterprises are developing intensively.17,18 The share of total production of broiler meat was 93%, ducks – 0.6%, turkey meat – 0.5%, laying hens – 2%, other species (geese, ducks, ostriches) – 0, 02%.19
Poultry farming carried out on an industrial basis is the most intensively-developing branch of animal husbandry. It requires a high level material and technical base, breeding of special-breed linear hybrids, as well as uninterrupted and complete supply of high-quality feed with complete feeds and protein and vitamin supplements, trace elements, amino acids, antibiotics and vaccines for all species, ages and sex groups of birds.20
Biological features of a bird (meat crosses) can only be used in modern technological enterprises. Today, intensive technologies for the production of broilers of different weight categories allow for reasonable timing for breeding roosters and hens separately, facilitate the use of new parameters of stocking density, feeding and watering front, as well as other standards that ensure high productivity, and contribute to high economic efficiency of broiler production as a whole. All this suggests that further improvement of production through the use of resource-saving technologies and a fuller realization of technological and organizational potential are needed. Significant investments are possible only in large industrial poultry enterprises where the latest technologies are used.21
Poultry complexes are modern intensive poultry enterprises. They are equipped with complex technological systems for the preparation and distribution of forages, automatic ventilation and climate control. A large number of poultry is kept in a limited space. The optimal microclimate in poultry houses contributes to the full realization of the birds’ genetic potential, disease prevention, increasing natural resistance, as well as extending the service life of the facilities and equipment. An optimal microclimate in the premises is achieved through compliance with scientifically-sound values of the environmental parameters (temperature, humidity, speed, etc.) that comprise it.22,23
Thus, industrial poultry farming is the branch of animal husbandry that allows to obtain a significant amount of high-quality food for the population in the shortest possible time. Modern intensive poultry farming can pose a potential risk to the health of birds as well as people working in such facilities.24 Researchers point out that the functioning of livestock and poultry complexes entails a threat to the environment, as harmful gases, dust and bioaerosols with a high content of pathogenic bacteria, viruses, fungal spores and endotoxins are released into the atmosphere on a daily basis. In most cases, the concentrations of these pollutants are higher than the maximum allowable level, which poses a potential risk to the health of animals, poultry, poultry workers (farms), as well as the population of nearby areas. The high population density of birds and animals creates conditions for outbreaks of mass infections, including zoonoses, which spread rapidly to all livestock.24
Further intensive development of poultry farming in a market economy requires, first and foremost, the veterinary welfare of flocks & herds. Among the various responsible veterinary measures, the main ones are preventing major diseases among poultry and preventing acute infectious diseases from being possibly introduced onto farms. Currently, viral infections are especially widespread in poultry. Zoonoses such as bird flu and Newcastle disease continue to be the most common and dangerous diseases in the world. Wild migratory birds play quite an important role in originating and spreading these diseases.25,26 There is always a threat of bringing bird flu and Newcastle disease into the territory of Ukraine during seasonal migrations of birds, and thus the arising emergencies leading to significant economic losses, especially in the regions that are geographically at risk because of the main migration routes of wild migratory birds that run through them.26-28 The concept of “One Health” defines the joint efforts in several disciplines undertaken at the local, national and global levels to ensure the health of humans, animals and plants in the environment. Nowadays, in the context of new zoonoses whose causative agents originate from wild animals, scientists emphasise that it is vital that different disciplines (medicine, veterinary medicine and others) work together to solve these problems. Transboundary zoonoses (which include pathogens of highly-pathogenic avian influenza and Newcastle disease) are frequent, and they also require joint interdisciplinary collaboration to develop strategies for the control, monitoring, and response measures.29 Over the past 30 years, there has been a significant increase in new infectious human diseases, of which more than 70% are zoonotic.30,31
Sources and data
The authors used the official data from the OIE, Rosselkhoznadzor, the State Service of Ukraine on Food Safety and Consumer Protection on the epizootic situation of highly pathogenic avian influenza and Newcastle disease in Ukraine, the Russian Federation, Kazakhstan, and Belarus.
This paper draws on specialist literature from CIS countries (states of the former USSR) – Russian-language and Ukrainian-language publications, as well as a small number of English-language sources. Information processing was performed by retrospective analysis.
As a standard choice, the selection of scientific articles on the matters at hand mainly comprises experimental and review articles on the technological aspects of poultry keeping in high-end enterprises, outbreaks of highly pathogenic influenza and Newcastle disease, cross-border diseases, zoonoses, farm biosafety, control and prevention of the diseases in Ukraine, Russian Federation, Kazakhstan, and Belarus, the potential role of intensive poultry farming technologies in spreading said diseases on poultry farms. The specialist literature mostly belongs to peer-reviewed publications. Methodical approaches meet the criteria for authoritative scientific publications. The specialist literature (85-90%) was published no later than in 2005.
Characteristics of the most significant pathogens of infectious poultry diseases with zoonotic potential
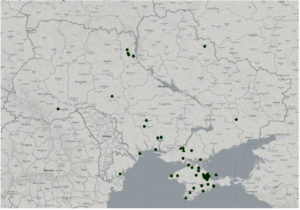
Both infectious diseases in question (highly pathogenic influenza and Newcastle disease) have a high zoonotic potential and are considered cross-border infectious diseases.32 These infections can be introduced into the territory of Ukraine with imported livestock products or poultry, or spread through wildlife.33,34 Regarding Ukraine, considerable risks exist of cross-border entry and spread of disease from the territory of its neighbours or countries with which Ukraine maintains close trade and economic relations.35 According to the State Service of Ukraine on Food Safety and Consumer Protection and World Organization for Animal Health (WOAH) data, over the last 20 years, bird flu was confirmed in the country in 2002 (Kyiv), in 2004 (Kyiv), in 2005 (16 contaminated areas in Autonomous Republic of Crimea, in 2006 (Sumy Oblast, Odesa Oblast – two contaminated areas, Autonomous Republic of Crimea – eight contaminated areas), in 2008 (Autonomous Republic of Crimea – two contaminated areas), in 2010 (Kyiv), in 2016 (Kherson Oblast – three contaminated areas), in 2017 (Mykolaiv Oblast, Odesa Oblast, Ternopil Oblast, Chernivtsi Oblast – two contaminated areas), in 2020 (Vinnytsia Oblast, Kherson Oblast, Mykolaiv Oblast, Odesa Oblast – two contaminated areas) (Figure 1), Newcastle disease was officially documented in 2006 (Kharkiv Oblast, Rivne Oblast – four contaminated areas), in 2007 (Zhytomyr Oblast) (Figure 2b).
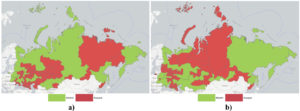
According to the World Organization for Animal Health (WOAH) and the Federal Service for Veterinary and Phytosanitary Supervision of the Russian Federation, (over the last 10 years) in the territory of the Russian Federation bird flu was documented in 2010 – one contaminated area, in 2014 – two, in 2015 – six, in 2016 – eight, in 2017 – 35, in 2018 – 89, in 2019 – two, in 2020 – 80 contaminated areas, in 2121-2022 also present in limited zones (Figure 2a). Newcastle disease in 2020 – 11 contaminated areas, in 2121-2022 also present in limited zones (Figure 2b). In Kazakhstan, according to the same sources and over the same period, the flu was registered in 2015 – one contaminated area, in 2017 – one, in 2020 – 11 contaminated areas in 2121-2022 present in limited zones; Newcastle disease was confirmed in 2013 and 2018 (Table 1).
Table (1):
Presence of HPAI and Newcastle desease in Ukraine, Russia, Belarus and Kazahstan 2005-2022 by report of WOAH (https://wahis.woah.org).
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Highly Pathogenic Avian Influenza | UA | Domestic | PR | PR | AB | PR | AB | AB | AB | AB | AB | AB | AB | PL | PL | AB | AB | PL | PL | n/a |
| Wild | PR | PL | AB | PR | AB | AB | AB | AB | AB | AB | AB | PL | PL | n/a | n/a | n/a | n/a | n/a | ||
| RU | Domestic | PR | PR | PR | PR | AB | AB | AB | AB | AB | PL | AB | PL | PL | PL | PL | PL | PL | PL | |
| Wild | PR | PL | AB | AB | PR | PR | AB | PL | AB | PL | PL | Inf | n/i | n/a | n/a | n/a | n/a | n/a | ||
| BY | Domestic | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | n/a | n/a | n/a | |
| Wild | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | n/i | n/a | n/a | n/a | n/a | n/a | ||
| KZ | Domestic | PR | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | PL | PL | PL | |
| Wild | AB | Inf | AB | AB | AB | AB | AB | AB | AB | AB | PL | AB | n/i | n/a | n/a | n/a | n/a | n/a | ||
| Newcastl deseaese | UA | Domestic | AB | PR | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | n/a |
| Wild | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||
| RU | Domestic | PR | PR | PR | PR | PR | PR | PL | PR | PR | PL | AB | PL | AB | AB | PL | PL | PL | PL | |
| Wild | AB | AB | AB | AB | n/i | n/i | n/i | PR | ni | PR | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||
| BY | Domestic | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | AB | n/a | n/a | n/a | |
| Wild | AB | AB | AB | AB | n/i | n/i | n/i | n/i | n/i | n/i | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ||
| KZ | Domestic | AB | AB | AB | AB | AB | AB | AB | AB | PL | AB | AB | AB | PL | PL | n/a | n/a | n/a | n/a | |
| Wild | AB | AB | AB | AB | AB | n/i | n/i | n/i | AB | AB | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Notes: PR- Present, PL – Present limited zones, Inf – Inf./infes., NR – Never reported, AB – Absent, n/i – No information, n/a – No data available.
Figure 2. Geographical distribution of (a) HPAI and (b) Newcastle desease on the territory of Ukraine, Russia, Belarus and Kazakhstan 2005-2022 by report of OIE WOAH (https://wahis.woah.org)
Humans can be affected by avian influenza A (H5N1), A (H7N9), A (H9N2) and swine flu subtypes A (H1N1), A (H1N2), (H3N2), reassortments (shift variants) of avian influenza and swine flu may also occur. Humans can become infected mainly through direct contact with sick birds and animals, or through pathogen transmission factors (virus-contaminated environmental objects). It should be noted that the effective transmission of avian virus between humans has still not been confirmed. Avian and swine flu viruses and their reassortants cause a disease with a mild upper respiratory tract infection, in which case fever and cough are frequent symptoms, and patients have a significant sputum secretion (especially in the early stages). In some cases, the infection can progress to severe pneumonia and even death. Depending on the subtype of the virus, intestinal symptoms, conjunctivitis, encephalitis and encephalopathy can develop. As the waterfowl is the leading reservoir of these pathogens (almost all subtypes), eradication of these infections is almost impossible, therefore such outbreaks will occur in the future. Therefore, constant epidemic surveillance, risk assessment and epidemic investigation of each case of such infections, etc. is crucial.
Confirmed outbreaks of avian influenza in large industrial enterprises lead to substantial economic losses. Companies incur considerable costs in eliminating the consequences of outbreaks, and do not sell young birds obtained from parent herds (compliance with quarantine zones requirements). As a result, meat and eggs production drops significantly and discrepancy between the population’s demand and the supply by livestock producers may occur. Influenza outbreaks in 2004–2005 in Europe, the Middle East, Central and Southeast Asia, Africa, and North America resulted in significant economic losses. Mankind will never be protected from bird flu outbreaks, because wild birds know no boundaries. The risk of infection can only be reduced to a minimum through constant compliance with biosafety requirements.36,32
Geographically, the territory from Russian Murmansk to Kamchatka, as well as the marshy plains of northern Canada are a giant “melting pot” in which migratory birds of the Northern and partly Southern hemispheres nest every summer. In this melting pot, various combinations of influenza virus subtypes, reassortants and new strains are formed. Young migratory birds are bred and fed there, infected by these viruses in the nest and then carrying them to new territories (migration routes). Such migratory flows of birds also pose a threat to local natural populations and poultry. In winter, the “exchange” of viruses continues in their wintering areas (coastal areas of the seas of Africa, Asia, Europe, etc.). At the end of winter, the reverse (spring), northward migration begins. This is the so-called circular motion. In countries such as China, Egypt and others, avian influenza is an endemic infection, and the disease is now being confirmed regardless of the time of seasonal flight of birds (the virus is reserved by local birds).36
According to WHO, in 2016, more than 600 people died of bird flu in China alone. In Europe, the number of deaths was 150. Another factor in increasing the virulence of influenza viruses in Southeast Asia and North Africa is the constant interaction of poultry with wild birds, different species of animals, and human populations. This is another type of “melting pot”, where reassortment between influenza viruses of birds, humans and animals (mostly pigs) occurs.36
According to the results of serological tests performed by the Institute of Experimental and Clinical Veterinary Medicine (Kharkiv) in 2003, antibodies to avian influenza virus subtype H5 were detected in the serum of 4.93% of the wild birds under study. In the same year, antibodies to avian influenza virus subtype H5 were found in egg yolks of 16.6% of the studied birds. In 2007, 2008 and 2011, antibodies to avian influenza virus subtype H5 were detected in the blood serum of mallards. The vast majority of highly pathogenic variants belonged to A H5 and H7 influenza viruses, so given the threat to birds, special attention should be paid to these viruses and studies of their circulation should be undertaken. Avian influenza viruses of these subtypes began to infect humans. In this case, the mortality rate was about 60%.37 Serological studies of B. Stehniy et al.38 showed that antibodies to influenza virus subtype H10 were detected in 33.3% of the samples, to subtype H13 – in 10% and to subtype H14 – in 90% of the samples taken from pied avocets. From the samples of yolk extracts taken from the slender-billed gull, 20% were positive for influenza virus subtypes H10 and H14, and 80% tested positive for subtype H13. In a study of Newcastle disease, antibodies were detected only in the serum of the Eurasian oystercatcher.
The epizootic situation of highly-pathogenic influenza in the Russian Federation is also quite strained.39,40 During the monitoring of the influenza virus in the Saratov Oblast, influenza pathogen A/H5N6 of clade 2.3.4.4 was confirmed to circulate for the first time in Russia. In the spring of 2018, two different genetic lines of A/H9N2 influenza virus, which were isolated during outbreaks in several poultry farms, were confirmed to circulate for the first time in Primorsky Krai and Amur Oblast. Subsequently, this subtype of the virus continued to spread in Russia, which was confirmed when influenza A/H9N2 virus was identified in wild birds in the Khabarovsk Krai and Tomsk Oblast.41
Infection with the influenza virus in wild birds and domestic ducks causes almost no harm to them.42 In infected birds, a slight decrease in body weight is observed,43,44 and a mild, short-term fever,45 as well as minor changes in immunological parameters.46 In wild ducks a decrease in the number of eggs laid during one week after becoming infected was observed. Infected birds do not lose the ability to fly long distances and thus can spread the virus during seasonal migrations.47-49 Waterfowl and shorebirds are natural reservoir species of influenza virus. However, poultry are not natural reservoirs; they are sensitive to infection and they develop the disease.50,44 Thus, the wild bird-carrier of influenza virus (which in most cases is not clinically ill) secretes the pathogen with saliva, nasal leaks and manure, pollutes the environment and infects other species of birds with which it comes into close contact. Avian influenza viruses spread globally thanks to such bird reservoirs. The ecology of bird flu is in many respects determined by the state of the immune system, annual life cycles, the nature of migration and nutrition of wild birds as a leading reservoir of the pathogen. Biodiversity loss, as well as a radical change in wetland ecosystems affects the activity of bird flu.51,52 The causative agent of avian influenza easily adapts to different species of animals and birds. Moreover, such bird species as ducks, swans or pelicans can pose a real threat to humans.53 Human and avian influenza viruses easily and rapidly exchange amino acid residues to form reassortants, which are fairly dangerous to humans.54
Thus, wild waterfowl and shorebirds are the main natural reservoir of influenza viruses and play a major role in maintaining the circulation of this pathogen. Influenza viruses of all known subtypes of hemagglutinin (H1–H16) and neuraminidase (N1–N9) have been isolated from wild birds belonging to more than 100 species of 12 orders. However, most of these viruses were isolated from the representatives of Anseriformes and Charadriiformes. Viruses are constantly isolated in Anatinae, Laridae and Sternidae, the great crested grebe (Podiceps cristatus), domestic ducks (Anasplatyrhynchos domesticus), mute swans (Cygnusolor) and others. In line with the World Organization for Animal Health classification according to the structure of the hemagglutinin cleavage site, as well as the ability to cause disease in birds, influenza viruses are divided into low pathogenic (LPAI) and highly pathogenic (HPAI). The international scientific community constantly monitors and studies the disease, its epizootiology and epidemiology, as well as the features of the biological and genetic structure of the pathogen. Information on the potential of some influenza viruses (especially the highly pathogenic ones) to overcome the interspecies barrier has prompted a new wave of research on the ecology of influenza virus, the discovery of new types of influenza virus (influenza D virus in ruminants) and new hosts of influenza A (two new subtypes of hemagglutinin H17, H18 and neuraminidase N10–N11 have been found in bats in South America). However, globally, the most attention is paid to influenza A viruses, because especially dangerous highly pathogenic influenza viruses belong to this type. The winter season in the Northern Hemisphere is usually associated with an increased risk of spread of avian influenza. For example, in the winter season of 2016–2017, a significant spread was observed of highly pathogenic avian influenza subtype H5N8 in Europe and influenza virus subtype H5N6 in Asia. Although the epizootic situation shows encouraging signs of stabilization with the onset of spring, there is an increased risk of further outbreaks associated with spring migration of birds, and therefore constant monitoring of the situation is essential. Ukraine is no exception – nine outbreaks of the new highly pathogenic H5N8 bird flu were registered between November 2016 and March 2017. Zoonotic strains of avian influenza virus that have become endemic in China (H7N9) and parts of Africa and Asia (H5N1) pose the most significant risks to human health, so their biological properties are constantly being studied and the veterinary community is making every effort to control these viruses and prevent their further spread. There is no scientific evidence to suggest that any restriction and attempts to regulate the number of free-living wild birds (destruction of nesting and resting places, shooting, etc.) can be effective in controlling highly pathogenic avian influenza. Detecting avian influenza, including HPAI, in wild birds does not affect the country’s sanitary status and does not lead to the loss of disease-free status, and therefore there is no reason to apply restrictive measures when trading poultry or poultry products with other countries.55-61 HPAI viruses spread rather rapidly and become endemic to poultry, especially in free-range domestic ducks (as was the case in 2003–2007 in Asia). In this case, spread of the virus from poultry to wild birds is inevitable.62 In China, influenza virus H5N8 of clade 2.3.4.4 was first isolated in 2010.63,64 The pathogen has become widespread and has caused outbreaks among wild and domestic birds in various parts of the world. In 2014, a subtype of H5N8 influenza virus circulated in Southeast Asia and then entered Europe and North America with wild birds traveling along migration routes through the Russian Federation.65,66 The latter was confirmed by a study of strain A/wigeon/Sakha/1/2014 (H5N8) isolated in the Far East of Russia.67 Study of the biological properties of the virus showed a high degree of identity with the strains circulating in Europe and Southeast Asia. After a wide spread in 2014, the H5N8 flu virus continued to circulate, and – according to OIE – H5N8 outbreaks continued to be reported in Korea and Taiwan until early 2016.68 In May 2016, H5N8 appeared in the Republic of Tuva in the Russian Federation. In 2016–2017, this virus spread to the European part of the Russian Federation.69,70
Influenza A variants adapted to birds have a2′-3′-specificity. In birds, the terminal a2′-3′-sialosides are found mostly on the surface of the epithelial cells of the intestinal mucosa, so in birds the flu occurs in the form of enteritis. Aerogenic transmission of the virus is possible by combining a2′-6′- and a2′-3′- specificities with salivary epitopes. This combined specificity is characteristic of the pandemic influenza A (H1N1) pdm09 virus, which, as a consequence, has the ability to spread by airborne droplets and cause severe pneumonia in birds and other animals. The ability of the influenza virus to adapt to different hosts depends on its ability to rapidly change its receptor specificity.71 Interspecific transmission of influenza A virus is a multifactorial process and occurs infrequently. Examples of overcoming the interspecific barrier are cases of human infection with influenza A viruses in birds and pigs of subtypes H5N1, H9N2, H7N7, H7N3, H7N2, H1N2, H1N1v, H3N2v in Southeast Asia and some countries in Europe and the United States.32,72,73
All subtypes of influenza virus hemagglutinin are found in wild birds, but only some of them are characteristic of mammalian influenza viruses (H1, H2, H3 in humans, H1 and H3 in pigs, H3 and H7 in horses).74,75 The incidence of influenza has been reported in many species of domestic and wild birds: chickens, ducks, turkeys, Japanese quails, partridges, pigeons, gulls, terns, guineafowls, penguins and others.72,74 In the study of influenza, one of the most important issues is identifying the sources and reservoirs of infection. As already mentioned, in this respect wild birds are of key importance. They usually have influenza with little or no clinical symptoms, often in the form of enteritis, which shows, first of all, a high degree of adaptation and suggests that birds are natural hosts for influenza virus A. However, in some cases the disease is manifested by the mass death of wild birds.72 High resistance of viruses in the environment, especially in water, and the fecal-oral route of infection contribute to the constant persistence of influenza pathogens among wild birds.72,74
During the surveillance of wild birds for avian influenza virus (2001–2012) in the Azov-Black Sea region of Ukraine, which is part of the transcontinental migration routes of wild birds from North Asia and Europe to the Mediterranean, Africa and South-West Asia, researchers identified 27 combinations of HA and NA antigens. All isolates were low pathogenic (LP) AIV, with the exception of eight highly pathogenic (HP) AIV, which were obtained during H5N1 HPAI outbreaks in 2006–2008.76 Low-pathogenic strains of influenza virus do not cause clinical disease, but almost always replicate in the trachea and spleen, as well as cause local and systemic cellular and humoral immune responses.77 Sequencing and phylogenetic analysis of hemagglutinin (HA) genes have revealed epidemiological links between the Azov-Black Sea region and Europe, the Russian Federation, Mongolia, and Southeast Asia. Subtypes AIV H1, H2, H3, H7, H8, H6, H9 and H13 were closely related to European, Russian, Mongolian and Georgian isolates. Subtypes H10, H11 and H12 AIV have been epidemiologically associated with viruses originating in Europe and Southeast Asia. Ukrainian researchers also identified a new variant of influenza virus H15 AIV in 2010; the latter has a unique HA-NA subtype combination H15N7. The new virus, along with the H15 virus isolated in Siberia in 2008, constituted a new clade of H15 AIV isolates.78
In total, from 1959 to 2013, 29 epizootics of highly pathogenic avian influenza caused by influenza viruses of subtypes H5 and H7 were confirmed worldwide. The epizootic of highly pathogenic avian influenza H5N1 began in 1996 and resulted in the death of more than 250 million domestic and wild birds in 63 countries in 16 years. More than 25 years ago, an epizootic caused by the highly virulent influenza A/H5N1 virus broke out in the southern Chinese province of Guangdong, marking the beginning of a major epizootic of the 21st century. Hemagglutinin of the prototype strain A/goose/Guangdong/1/1996 (H5N1) has changed many times and evolved into new genetic subgroups, participating in various reassortments, and has survived to this day. Its evolution took place in Eurasia, Africa and America. The global nature of the movement of these viruses has been confirmed by many scientists.79 In 2018, the H7N9 subtype virus, which caused poultry disease in China, showed a high zoonotic potential and killed significantly more people than the H5N1 virus.80 In H5N2 and H7N8 isolates isolated in the Crimea, phylogenetic analysis revealed ecological links between these viruses and isolates from Siberia and Europe.81 A study conducted by scientists in the Russian Federation in 2015 showed that influenza viruses of type A subtypes H5, H7 and H9 circulated among wild birds in their country. The researchers noted that the risk of introduction and occurrence of highly pathogenic avian influenza is the highest in the regions bordering China and Mongolia. They noted that outbreaks of highly pathogenic influenza among wild birds may occur in the future, and that this may also lead to influenza outbreaks on farms with low biosecurity.82 There has been a recent widespread distribution of H5 subtypes with neuraminidases of various subtypes in different countries of Asia, Europe and Africa.83-87
According to monitoring studies conducted in the Russian Federation in 2017–2018, antibodies to influenza A virus were detected in private, small-scale farms owned by the residents of Smolensk oblast and the Republic of Crimea, influenza A/5 – in the Altai Territory, Rostov and Kaliningrad oblasts, to influenza virus A/H9 – in the Primorsky Krai. In 2018, antibodies to influenza A/H9 virus were also detected in unvaccinated chickens on two poultry farms in the Primorye Territory.88 Epidemiological monitoring conducted in the Russian Federation in 2019 did not confirm the presence of antibodies to the bird flu virus in unvaccinated birds, while studies conducted in 2017–2018 revealed the presence of post-infection antibodies to influenza A virus. H5 virus was detected in serum samples of birds in the Altai Territory, Rostov and Kaliningrad oblasts. Influenza A/H9 virus was also found in serum samples of birds in private, small-scale farms and at two poultry farms in the Primorsky Krai.89 Influenza viruses also cause significant damage to poultry farms where there is still an appropriate level of biological protection. In the period 2016–2017, seven large poultry farms in the Russian Federation were affected by influenza.80 Since 2005, mainly subtype H5N1 of clade 2.2 was isolated on poultry farms in Russia. Until 2015, H5N1 was detected in Russia, but of a slightly different line. High virulence for chickens remains a characteristic feature of influenza A/H5N1 viruses of the Asian genetic line A/Gs/Gd/96.90-93 In 2014, H5N8 was detected. Further, H5N8 was detected in 2016–2017. In 2017, H5N2 was first isolated on a poultry farm in Kostroma.69,88
Apart from humans and birds, many species of animals are also susceptible to influenza A virus. Not only does the virus easily overcome the interspecific barrier in birds, but it also infects mammals. Humans as well as domestic (pigs, horses) and wild animals (seals, minks, cetaceans, etc.) are sensitive to influenza pathogens. However, in the ecology of influenza A virus, wild birds are of particular importance as they are its natural reservoir. As already mentioned, almost all combinations of hemagglutinin subtypes were isolated from wild birds94-96 and bats (except one – H17).97 As subtypes of the virus circulate among wild birds, 15 subtypes of influenza A virus that infect birds are referred to as avian influenza viruses (Avian Influenza Viruses, AIV).98 These influenza viruses are found mainly in populations of migratory waterfowl of certain species. The pathogen is isolated from saliva, nasal secretions and manure. The virus spreads in birds with weakened immunity, through contact with contaminated nasal, respiratory or fecal material from infected birds. Infection occurs mainly by the fecal-oral (alimentary) route.94 Thus, poultry and wild birds are the main source of the virus for interspecific transmission of various taxa to mammals, including whales, seals, pigs, horses and humans.99-103 Influenza A viruses, which often cross the interspecific barrier due to the reassortment of influenza virus genes and subsequently acquire the potential for direct human-to-human transmission, entail the highest risk of epidemics among humans caused by animal and avian influenza strains.104
Given the fact that the influenza virus can cause annual epidemics worldwide, it can be argued that influenza is a problem of global importance.105 According to phylogenetic analysis, some genes of pandemic virus strains still circulate in infected wild birds. It is likely that the triple reassortant virus that caused the 2009 H1N1 pandemic originated at a bird migration site.103 Some genes of the H9N2 virus isolated from migratory ducks in Hokkaido (Japan) are identical to the genes of the H3N2 virus.106 Recent cases of human infection with such highly pathogenic subtypes as H5N1, H7N7, H9N2 and H7N9 have also been reported.107-110
Due to the high contagiousness of avian influenza, the infection spreads rapidly between farms by mechanical transmission and by indirect contact factors, such as through contaminated equipment, transport, feed and cages. Infection among birds is transmitted through direct, close contact with the source of the pathogen. In this case, the fecal-oral route and the transmission of the pathogen by indirect contact predominate (for example, one gram of manure contaminated with the H5N1 virus can infect 1 million birds).94 In chickens, the avian influenza virus causes a severe course of the disease, whereas, in contrast, domestic ducks do not have clinical manifestations and a strong immune response.42 The rate of resorption of influenza viruses in natural reservoirs is quite significant.111 Given the fact that avian influenza viruses have 16 types of hemagglutinin and nine types of neuraminidase, there may be 144 subtypes of the pathogen with a free combination of different types of HA and NA segments. Scientists have identified at least 103 subtypes of such viruses112 which proves a significant frequency of reassortment. Therefore, in addition to the fact that domestic ducks can be a reservoir species for influenza viruses, these pathogens undergo constant reassortment in their bodies.113,44
Phylogenetic analysis of avian influenza virus genomes has shown that the causative agent in wild birds exists as a large pool of interconnected gene segments that form so-called “genomic constellations”.111 Any combination of viral segments can occur during the collection of virions from such genomic formations. In fact, in the body of wild waterfowl genomes of influenza viruses undergo constant mixing. It is likely that the constant presence of the virus in the body of wild migratory birds contributes to the high efficiency of reassortment.44 In 2015, the shift variant (reassortant) of the H5N2 virus, which contained the genetic material of the H5N8 virus of the Eurasian line and the H5N2 virus of the North American line, caused enormous damage to the US poultry industry. Approximately 50 million chickens and turkeys were killed in an effort to contain the infection. The epizootic affected large poultry farms with a high level of biological protection.13 The strain of influenza virus that caused the 2009 pandemic proved to be an unusual virus as it combined the genes of at least four strains of influenza A virus that infect humans, pigs and birds.114,51 During the monitoring studies of wild birds in the Azov-Black Sea region of Ukraine, the bird flu virus A/mallard/Novomychailivka/2-23-12/10 (H15N7) was isolated for the first time in Eastern Europe. The sequencing results showed that the isolate was low pathogenic and related to influenza viruses of various subtypes from Western Siberia, Western Europe, and Asia. Today, there is evidence of the transmission of influenza virus by birds through feathering during migration, which was hardly taken into consideration in earlier studies of the spread of the pathogen. Accordingly, adsorption by feathers can play an important role in understanding the ecology of the studied virus.115
Thus, avian influenza viruses are characterized by a constant change of hosts. Viruses easily cross the interspecific barrier, infecting poultry, pigs, horses, and humans. It is the constant reassortment of viruses in the body of birds that allows the pathogen to effectively overcome interspecific barriers, to adapt to changes in the environment and other host organisms. However, reassortment occurs not only in natural reservoirs. From wild birds, the flu virus is easily transmitted to poultry, which in turn infects pigs. Also, swine virus is often isolated from poultry, and avian virus is periodically isolated from pigs.116 Human influenza viruses have also been isolated from pigs. Cases of pigs becoming infected through contact with farmers – and vice versa – have been reported in the literature.117 Accordingly, pigs can be co-infected with different strains of avian and human influenza viruses, and then avian, swine and human influenza viruses can mix directly in their body.44,47 An example of such “mixer” for influenza viruses in pigs was the emergence of the reassortant virus in 2009.118 Reassortment of influenza viruses in the human body, which is not a natural reservoir of influenza viruses, is not common. However, reassortment in the human body contributed to the emergence and spread of amantadine-resistant subtype of influenza H3N2 virus.119 Reassortment of influenza viruses in the human body often led to the emergence of strains that caused epidemic outbreaks of influenza in 1947, 1951 and 2003–2004.44,120,121
Newcastle disease (ND) is the most common among small private households in Asia, Africa and South America. Today, Ukraine is officially free of ND. However, during laboratory studies, on occasion virus has been isolated from synanthropic birds, namely: pigeons – abstract and isolate paramyxovirus-1 (APMV-1), wild ducks – lentogenic (Hitchner’s form) and asymptomatic enterotropic forms of the disease.122 The differences between the pigeon variant (PPMV-1) and the classical paramyxovirus (APMV-1) are that these viruses with an index of intracerebral pathogenicity in one-day-old “sterile” chickens exhibit properties characteristic of lentogenic and mesogenic strains and, in most cases, cannot cause disease in adult chickens.123 The difficult epizootic situation with ND is a serious barrier to the exchange of genetic material obtained from poultry. New viruses that cause infectious diseases of birds necessitate a change in the approach to monitoring and diagnosis in industrial poultry farming. According to WHO, in the first decade of the 21st century, outbreaks of Newcastle disease have been reported in 87 countries around the world.124 Currently in Ukraine, cases of and deaths from the disease among birds have been registered in some small homesteads and individual farms keeping poultry of different species without complying with basic veterinary and sanitary requirements.125
When ingested, ND virus can cause depression and other symptoms similar to influenza.32,122 Severe conjunctivitis with retinitis, which causes swelling of the parotid lymph nodes, has been reported. In humans, ND can also manifest as inflammation of the eyes and purulent tonsillitis. In the case of aerosol vaccination of poultry in poultry farms, service personnel are advised to use respiratory masks and goggles to prevent the vaccine virus from entering the mucous membranes of the eyes and the respiratory system. Manifestations of Newcastle disease virus infection from poultry, wild ducks and pigeons in humans begins with acute fever, headache, lymphadenopathy, hyperemia and conjunctival chemosis, burning pain, serous or mucopurulent lesions and conjunctival follicles. The disease persists for 7–10 days.32,122,126 However, specialist literature includes reports of lesions in children, with the disease causing brain damage and even death.13 Humans are most frequently infected by airborne transmission, inhaling virus-contaminated dust, through dirty hands and the surface of the conjunctiva. However, it should be noted that the natural morbidity of humans is not high. While the disease is not frequent among humans, there is an occupational component (veterinary workers who deal with poultry and poultry farm workers become infected).32,126
Wild birds are a natural reservoir for the Newcastle disease virus since the virus has a wide range of hosts, affecting birds in both aquatic and terrestrial ecological complexes.127-130 Outbreaks of Newcastle disease occur worldwide. Significant outbreaks of the disease have been reported in Australia,131 Korea132 and Israel.133 In the course of monitoring studies conducted in the Russian Federation, the Newcastle pathogen was repeatedly isolated.127-130 The strains closest to NDV/Adigeya/Duck/8/2008 and NDV/Adigeya/Duck/15/2008 were previously isolated from domestic geese and ducks.134 The latter suggests that the same variants of the virus can infect wild birds and poultry alike. Therefore, it is possible that any genetic variant of the Newcastle disease virus be introduced across extensive geographical ranges.135,136 It is now proven that the range of potential hosts of the virus includes, primarily birds (Aves). Birds of the Galliformes and Columbiformes orders are natural reservoirs of the virus, but it has also been found in populations of more than 200 species of 27 orders. Wild migratory birds of Anseriformes and Charadriiformes orders are also carriers of the Newcastle disease virus, so there is a constant risk of the virus entering poultry farms or private yards where birds are kept.137 In autumn Newcastle disease virus mostly infects wild birds – 55.6%, whereas in autumn it is already 81.8%. The infection rate for the influenza virus in these periods stands at 35.6% and 15.3%, respectively.138
Bird paramyxoviruses are isolated from members of the families of sparrows, rails, parrots, as well as ducks, geese, chickens, pigeons and turkeys. The Newcastle disease virus (paramyxovirus type 1; APMV-1) is widespread in nature. While it has often been isolated from poultry, synanthropic and wild birds of many species, epizootics in wild birds are infrequent, despite the fact that the virus has been isolated from more than 50 species of wild birds. More than 230 species of birds are susceptible to natural and experimental infection.72,74 As for other serotypes, in many countries paramyxovirus-2 (Yucape viruses) has been isolated from poultry and wild birds, including rails, sparrows, ducks, turkeys, parrots, and the like. Paramyxovirus-3 strains were isolated from exotic birds, parrots, African and Australian finches, as well as waterfowl on the Atlantic, Pacific, and Baltic migration routes. Strains belonging to paramyxovirus-4 and paramyxovirus-5 were isolated from finches, pheasants and parrots in Japan, Israel and the USA. Paramyxoviruses-6 were isolated in Hong Kong from duck faeces, from water where the birds lived, as well as from wild ducks in Canada, Japan, Germany, Czechoslovakia and the United States. Isolates belonging to paramyxovirus-7 and paramyxovirus-8 were obtained from wild geese in Japan and the USA, as well as from the gray heron in Japan on the Pacific migration route. 72,75,139,140 According to the results of sequencing of the F 21 gene of the VND isolate obtained from wild birds and poultry in different regions of Ukraine between 1992 and 2011, they belonged to five genotypes, namely: I, II, VI, VII and XIV. Based on the structure of F0 protein cleavage sites it was determined that 5 isolates were velogenic and had a high degree of similarity to VND isolates which circulated in Russia for a long time. The results allowed scientists to make assumptions about their common origin. Isolates from pigeons as well as white-fronted geese were identified as velogenic and had a high degree of similarity to the epizootic strains that caused the 2008 outbreaks in West and Central Africa.141 It has been proven that some bird species, such as wild ducks, cormorants and pigeons, reserve the Newcastle disease virus and remain carriers for many years.142-147
Ukrainian scientists have conducted significant research on detecting paramyxoviruses (APMV) in wild birds. A study of 6,735 wild birds representing 86 species was conducted across different seasons of 2006–2011. Twenty viruses were isolated and subsequently identified as APMV-1 (9), APMV-4 (4), APMV-6 (3) and APMV-7 (4). The highest level of virus isolation was observed during autumn migration (61%). The level of excretion of pathogens was lower in winter (from December to March) (32%). No APMV strains were isolated from 1984 samples during spring migration, nesting and post-nesting (April to August). Sequencing and phylogenetic analysis of four APMV-1 and two APMV-4 viruses showed that one APMV-1 virus belonged to type 1 and was epidemiologically related to viruses from China, three APMV-1 type II viruses were epidemiologically related to viruses from Nigeria and Luxembourg, and one APMV-4 virus was associated with goose viruses from Egypt.148 During the autumn migrations of birds in the Azov and Black Sea basins, different serotypes of avian paramyxoviruses (APMV-1, APMV-4, APMV-6 and APMV-7) were isolated from migratory birds.149
As noted by D. Musyka (2013), performing seasonal transcontinental migrations, birds form large flocks, overcome distances of thousands of kilometers in a very short period of time, and fly through regions with different epizootic situations. From nesting, fattening, resting and wintering areas, transcontinental migrations can be encountered by sedentary and migratory birds.72 During long flights, birds fly along certain migration routes, stopping at places of mass permanent stops, where migratory directions of birds from different parts of the world converge.72,74 It is in such places that a large number of migratory birds of different species concentrate on a limited area, facilitating the circulation of pathogens. Even when resting for a short time, migratory birds are able to bring pathogens of infectious diseases into the migration zone. Local movements can facilitate the exchange of pathogens between migratory species and local communities. Native birds can be included in the circulation of pathogens that are not typical for the area. The exchange of pathogens is possible even between birds that do not form any clusters. This is often due to alimentary relationships, so-called food chains. Some representatives of wildlife live near humans as groups of synanthropic birds. They can be migratory, nomadic, and settled. At different times of the year and depending on climate conditions, synanthropic birds stay in certain areas, settlements, livestock facilities and other objects of the agro-industrial complex and can also pose a potential threat as a source of infectious diseases. Almost all livestock farms, including poultry farms, have a certain number of birds that permanently live and feed on their territory. During local travels over short distances, such birds can visit the territories of other poultry farms or other livestock facilities. During such mass flights from one facility to another, infectious agents can be transferred.72
For the most part, the causative agent of Newcastle disease is spread directly between infected and healthy birds.150 Isolates and strains of the virus are rather different from each other in virulence properties, but there are strains that cause almost a 100% mortality in sick birds. 75,151 In the European Union and the International Office of Epizootics, the main criterion for classifying Newcastle disease viruses by pathogenicity is the determination of the intracerebral pathogenicity index value in day-old chicks. This allows a quantitative analysis of viruses by specific values of compliance with the severity and calculation of pathogenicity. Further, with the development of molecular biological technologies, the method of polymerase chain reaction (PCR) in its various modifications, as well as sequencing, have become important tools in the classification of pathogenicity and phylogenetic analysis of viruses.152
Specialists of the Institute of Experimental and Clinical Veterinary Medicine (Ukraine) conducted serological tests of egg yolk extracts of slender-billed gulls and pied avocets, and established the seropositivity of these birds to paramyxovirus 1 at 49% of the samples.153 Serological tests of egg yolks of wild birds for the presence of antibodies to ortho- and paramyxoviruses showed that of the 70 samples of egg yolks taken by the authors from six species of wild birds of the Azov Sea, 51.4% tested positive for paramyxovirus infections, and 88.6% were found positive for orthomyxoviruses.154 Studies by Ukrainian authors have confirmed the thesis that wild migratory birds may be carriers of types of Newcastle disease virus that are new to Ukraine.155
An analysis of serological monitoring data collected in recent years has shown that the situation concerning Newcastle disease in the Russian Federation remains unstable. In fact, despite the high seropositive status of poultry on commercial poultry farms, there is an insufficient level of protection on small farms due to the lack of mass vaccinations, which poses a constant threat of disease.89,156 In this country, researchers have isolated genetic variants of type 1 and 2 Newcastle disease virus from birds. Moreover, type 2 virus had not previously been isolated in Russia. Type 1 viruses have been reported in Mongolia and the Taimyr Peninsula. While these locations are very far from each other, the areas are covered by the Central Asian Flyway. Since no type 1 virus has been isolated elsewhere, researchers believe that Newcastle disease virus (of this type) was transmitted by migratory birds, namely ducks that migrated along the Central Asian migration route. A significant number of viruses were isolated in Chukotka. The East Asian-Australian Flyway passes through this territory, crossing the territories of Australia, Indonesia, China, Japan, the USA and the Far East of Russia. The most likely source of Newcastle disease virus in Chukotka are birds migrating along this route, which demonstrates their active circulation in these areas. This hypothesis is also confirmed by the fact that strains of Newcastle disease virus isolated in the Far East in 2001–2002 are phylogenetically related to the strains previously isolated in the United States.157,158 The latter creates the preconditions for the introduction of pathogenic variants of the virus into the Far East of Russia. The presence of Newcastle disease virus in Western Siberia is most likely linked to large flocks of birds in the Chanov Lake system between the Novosibirsk and Omsk regions. Researchers point out that this is where the Black Sea-Mediterranean, East African-Eurasian and Central Asian flyways intersect. Phylogenetic analysis of NDV/Adigeya/duck/8 and NDV/Adigeya/duck/9 isolates showed that the highly pathogenic viruses belonged to type 2 genotype 7, while only type-2 viruses, genotypes 1 and 2 had previously been in isolated in Russia. In terms of phylogenetics, the closest strain to Adygea was Goose/China/2005. The latter indicates that the isolates originate from Southeast Asia or China and were introduced into the territory of the Russian Federation by migratory birds that migrate along the East African-Eurasian flyway. 159,157
The significant role wild birds play in the spread of low-pathogenic avian influenza viruses and Newcastle disease has been reliably confirmed.160 In a study of wild birds belonging to 21 species of sedentary, nomadic and migratory groups from 11 areas of the forest-steppe zone of the Altai Territory of the Russian Federation, specific antibodies to influenza virus were detected in 34.2% of the samples, and antibodies to Newcastle disease virus – in 60% of the samples.161 The same authors, studying 477 samples of blood sera (approximately 10 years), found specific antibodies to influenza viruses in 25.2% and to the Newcastle disease – in 69.1% of wild birds belonging to 26 different species from 15 areas of the steppe zone of the Altai Territory.162,163 In long-term studies of wild birds as possible carriers of influenza viruses and the Newcastle disease, Russian scientists have identified 13 strains of influenza A virus subtype H13N1 (four from seagulls, nine from cormorants) and three strains of Newcastle disease virus from cormorants.164
One study examined virulent Newcastle disease viruses (NDV) from Bulgaria and Ukraine over the period 2002–2013. All of these NDV isolates had the same cleavage site associated with virulence (‘113RQKR; F117’), and some had an intracerebral pathogenicity index value in the range of 1.61–1.96. The isolates were found to be the most closely associated with viruses circulating in Eastern Europe and Asia. Characteristically, most of these viruses are isolated from poultry (backyard farms), which points to the conclusion that there is a “home” or “urban” cycle of virus circulation. The molecular characteristics of the nucleotide sequence of genes for complete fusion of proteins suggests the circulation of virulent strains of NDV VIId subgenotype from Eastern Europe, with periodic introduction from Asia. In addition, these studies support the thesis that subgenotype VIId is much more widespread in Eastern Europe than previously thought. The presence of “backyard” livestock (individual farms) in these countries forces the relevant services to conduct continuous epidemiological monitoring of NDV on these farms, as such viruses have a high virulence potential and can be introduced into large poultry farms in various ways.165 The close genetic link between the isolates from Bulgaria, Ukraine, China, Israel, South Africa and Russia is explained by the migration of wild birds. In fact, the role of wild birds in the epidemiology of NDV has been confirmed by special studies.166
Phylogenetic analysis performed by Kazakh scientists showed that the virus isolated from birds which died at a poultry farm where livestock was vaccinated against Newcastle disease belonged to genotype VIId, whereas isolates obtained from unvaccinated birds on individual farms in Almaty, Zhambyl and North Kazakhstan regions belonged to genotype VIIb. The disease and death of poultry in Almaty, Zhambyl and North Kazakhstan regions of the Republic of Kazakhstan in 2010, 2012 and 2013 were caused by Newcastle disease virus. Studies have shown that Newcastle disease virus causes outbreaks in both vaccinated and unvaccinated birds. Outbreaks in vaccinated birds may have been due to decreased immunity.167,148 It should be noted that the strains of Newcastle disease virus used to make the vaccine belong to genotype II.168 Outbreaks may be due to decreased immunity, because in the case of a well-established vaccination schedule, classical vaccines belonging to genotype II provide 100% protection of birds despite the antigenic differences from the epizootic strain.169,170 It is believed that vaccination against the Newcastle disease should provide immunity against infection and suppress replication of the virus. However, according to some authors, while existing vaccines prevent clinical disease, they cannot stop the replication and spread of a virulent virus.171-173 An analysis of such studies shows that virus production was significantly lower only when a vaccine based on a certain genotype was used.174,175
Under the biological interaction programme, the genetic diversity and evolution of the virus responsible for the Newcastle disease was jointly investigated by The Southeast Poultry Research Laboratory (SEPRL) of the US Department of Agriculture, and veterinary laboratories in Russia, Pakistan, Ukraine, Kazakhstan and Indonesia. Information from Kazakhstan, the Russian Federation, and Ukraine has facilitated identifying possible migration routes for birds that can carry both virulent NDV (vNDV) and low virulence NDV to Europe. Further, genetically related NDV strains were isolated from wild birds in Ukraine and Nigeria, as well as from birds in the continental United States, Alaska, the Russian Federation, and Japan, which identifies wild birds as a possible mechanism for intercontinental transmission of low-virulence NDV. The recent discovery of new vNDV subgenotypes suggests that a new, fifth panzootic originated in Southeast Asia, spreading to the Middle East, and Eastern Europe.173
Measures to control and prevent the infectious diseases of birds with zoonotic potential in industrial poultry farming
In case of an outbreak of Newcastle disease or highly pathogenic influenza, immediate action must be undertaken. Certified diagnostic laboratories and a rapid alert system are needed to mobilize and implement effective anti-epizootic measures.94 As international experience shows, the modern methods of combating Newcastle disease do not differ from those applied to tackle influenza (Table 1, Table 2). These control methods are based on the provisions of Directive 92/66/EEC. In case of an outbreak of the disease, each country, depending on the epizootic situation, carries out a full or partial sanitary slaughter of birds that are infected or suspected of being infected within a three-kilometer radius (protection zone). Vaccination and systematic control of poultry by serological methods176 are performed within a radius of 10 kilometers (surveillance zone). Nowadays, specialists in many countries are adopting the American approach to eliminating flu outbreaks, i.e. destroy the infected herds (“stamping-out”) within 24 hours after the disease has been detected.36
Table (2):
Prevention and eradication measures of Highly Pathogenic Avian Influenza.
Measures |
Ukraine |
Russia |
Kazakhstan |
Belarus |
Source |
|---|---|---|---|---|---|
General preventive measures |
Biosecurity measures and closed operating model of large poultry farms to prevent the entry of the virus with poultry, staff, feed etc. Creating buffer zones without poultry around farms Continuous monitoring of wild birds, identification of new subtypes of viruses Risk analysis and reduction |
Biosecurity measures and closed operating model of large poultry farms to prevent the entry of the virus with poultry, staff, feed etc. Creating buffer zones without poultry around farms Epizootic monitoring of virus circulation in industrial farms |
Biosecurity measures and closed operating model of large poultry farms to prevent the entry of the virus with poultry, staff, feed etc. Creating minimum 5 km buffer zones without poultry around farms Depopulation of wild birds around large poultry farms by hunting |
Biosecurity measures and closed operating model of large poultry farms to prevent the entry of the virus with poultry, staff, feed etc. Creating buffer zones without poultry around farms |
[80, 138, 180-182] |
Laboratory diagnostic |
Serological tests: reaction of diffuse precipitation, reaction of hemagglutination delay, direct and blocking ELISA; identification of the pathogen into 9-11-day chicken embryos with subsequent identification by RDP, ELISA, reaction of hemagglutination delay, neuraminidase inhibition reaction, RT-PCR or RT-PCR in real time and determination of the virulence of the isolated strain according to the index of intravenous pathogenicity on chickens The isolation of influenza virus in cell culture is allowed |
Testing pathological material and serum: isolation of the virus, detection of RNA of the virus by RT-PCR or RT-PCR in real time, detection of antibodies to the virus in the hemagglutination reaction, if it is known for sure that they are not a consequence of vaccination |
Testing pathological material and serum, test systems are used: reaction of hemagglutination delay, ELISA and RT-PCR for identification and typing of virus subtypes |
Testing samples of cloacal or tracheal washings, internal organs and blood serum, test systems are used: reaction of hemagglutination delay, ELISA and RT-PCR also virus isolation |
[36, 180-182] |
Response in case of outbreak |
Informing the state veterinary service, laboratory confirmation of the diagnosis. Infected zone – stamping-out measures, slaughtering and burning of poultry population “Buffer zone” (“protection zone”) with a radius of 3 km from the borders of the infected zone – isolated of poultry, ban on transportation of poultry and poultry products, optional slaughter of poultry, monitoring observations “Observation zone” (“surveillance zone”) a radius of 10 km including the protection zone –monitoring observations, closed operational type of farms, based on the results of the risk analysis additional restriction zones may be established The duration of the quarantine is 21 days |
Informing the state veterinary service, laboratory confirmation of the diagnosis. Infected zone – stamping-out measures, slaughtering and burning of poultry population “Threatening zone” with a radius of 5 km – isolated keeping of poultry, control over transportation of birds and poultry products, monitoring observations, vaccination (optional) “Observation zone” a radius of 10 km –monitoring observations, closed operational type of farms, vaccination (optional) The duration of the quarantine is 21 days |
Informing the state veterinary service, laboratory confirmation of the diagnosis. Infected zone and territory with a radius minimum 8 km – stamping-out measures, slaughtering and burning of poultry population, quarantine with a ban on the movement of poultry products. “Buffer zone” with a radius minimum 25 km – restrictions on the movement of poultry and poultry products, closed operation type of farms, increased surveillance. “Surveillance zone” with a radius of 50 km – closed mode of operation of farms, increased surveillance. The duration of the quarantine is 21 days |
Informing the state veterinary service, laboratory confirmation of the diagnosis. Infected zone – stamping-out measures, slaughtering and burning of poultry population
“Threatening zone” with a radius of 5 km – isolated keeping of poultry, control over transportation of birds and poultry products, monitoring observations, vaccination (optional) “Observation zone” a radius of 10 km –monitoring observations, closed operational type of farms, vaccination is forbidden The duration of the quarantine is 21 days
|
[36, 94, 176] |
Vaccination |
Vaccination is forbidden |
Inactivated vaccine used in the “threat zone” аs an additional effort. |
Inactivated vaccine used in the threat zone”, also for poultry in private backyards within a radius of 20 kilometers from existing poultry farms and along the route of flight of wild birds |
Inactivated vaccine used in the threat zone” taking into account the epizootic situation (optional) |
[13, 36] |
Protection of staff |
Personal protective equipment, risk notification, daily medical examination |
Personal protective equipment, workers under 18 and over 65 are not allowed |
Personal protective equipment, workers under 18 and over 65 are not allowed |
Personal protective equipment, compliance with hygiene rules |
[94, 193, 215, 236] |
In industrial enterprises (large poultry farms), if an outbreak of influenza or Newcastle disease occurs, birds are slaughtered in poultry houses using generators and gas, disinfectants are used during the cleaning and packing of carcasses, equipment is disinfected, staff receives disposable overalls, special transport is used. All these activities are carried out in a few hours, as the carcasses simply begin to decompose in the warm season. After these measures have been undertaken, dry cleaning, washing, disinfection and drying takes place, and the limiting period begins before a new batch of birds is brought in.36,177,138
In Russia, the strategy to combat bird flu is also based on radical measures – destroying all susceptible livestock. As an additional effort, poultry in at-risk areas is vaccinated. In this country, vaccines that are made from the epizootic strain H5N1 obtained during the outbreak of highly pathogenic influenza in 2005 are used in such areas. Researchers advise that the risks associated with the flows of wild birds are largely unmanageable. The genetic evolution of influenza viruses also cannot be controlled, and it often ends in the emergence of new reassortants. In such case, it is essential that the identified risks be managed and appropriate measures be implemented at each poultry farm, as migratory birds can leave their droppings anywhere. These days researchers are linking the disease to seasonality even less than before, as the virus survives in manure at +4°С for more than 50 days in the environment and more than 100 days in frosty weather. Although synanthropic birds (crows, sparrows, tits) do not migrate, they can participate in the transmission of the pathogen to poultry. Oftentimes the delayed response of veterinarians and poultry owners reduces the effectiveness of disease-control actions. The problem is especially serious in the case of subtypes H5N8 and H5N2, which are characterized by a fulminant disease course. Untimely diagnosis leads to a delayed introduction of specific measures. In addition to this, secondary outbreaks of the virus can occur from such focuses of infection. Insufficient outreach to the population that keeps poultry on individual farms leads to such owners withholding information about the deaths of chickens, ducks, geese, etc. A dead bird disposed of in violation of sanitary rules becomes a target for wild birds and birds of prey, which severely aggravates the epizootic situation. 80,91,178
General preventive measures (Table 1) should guarantee strict compliance with the rules aimed at ensuring a closed operating model of large poultry farms, as well as their biological and sanitary protection.80 In general, the prevention of infectious diseases in large industrial poultry enterprises includes high-quality veterinary and sanitary measures; compliance with poultry farming standards; creating favourable housing conditions; the use of complete and safe (in the matter of pathogenic microorganisms, fungi and products of their vital activity) feed that correspond to the age and direction of cultivation; stress prevention; the use of vitamin and mineral supplements, probiotics, phyto-preparations, drugs that support the immune system such as tonics, drugs that increase resistance, anti-stress drugs and, accordingly, specific prevention. 13,179,180 Only strict technological discipline and high veterinary and sanitary production culture as well as full-fledged feeding promote the creation of reliable immune protection in a herd.181,182
An analysis of the production technology of poultry products indicates that poultry farms are stocked with day-old chicks purchased from sources that are safe in veterinary and sanitary terms. Before bringing in the next batch of birds, complete disinfection of the buildings is carried out by cleaning the buildings (including the removal of coop bedding). The territory of almost all large poultry farms is surrounded by 2.5-3-meter fences that run along the perimeter of the borders of poultry farms, thus protecting the enterprise from the intrusion of outsiders, animals and birds. Entry roads to poultry farms are always paved. There are no open reservoirs on the territory of poultry farms so as not to create living conditions for waterfowl. In each poultry house and feed storage, there are ventilating and technological apertures equipped with frames with a small metal grid which prevents synanthropic or wild birds from entering the room. If a dead wild bird is found, its carcass is disposed of.183 The whole set of veterinary and sanitary measures described above is implemented.138,183
In Ukraine, the prevention of influenza virus on poultry farms also relies on a set of measures implemented by representatives of the State Food and Consumer Service and the veterinary services of poultry enterprises. As an additional step, these measures also include continuous monitoring of wild birds, identification of new subtypes of viruses to assess the risk, creating buffer zones throughout the farm and production areas, preventing rodents and wild birds from entering the poultry farm, using barriers on all transport routes and staff entry routes, preventing outsiders from entering the poultry farm and reducing production traffic at poultry farms. Potential carriers of the virus include staff, factors of virus transmission can be equipment, feed, visitors, transport, air, etc. The “biosecurity system” suggests that staff cannot enter the farm area freely. People leave all their clothes and shoes in the “quarantine zone”, after which they shower and change into overalls and special shoes.36 Thus, large poultry complexes must have in place a comprehensive control system comprising epizootiological monitoring of the technological cycle of production, microbiological and virological monitoring of chicken breeding (in broiler farms), diagnostic monitoring (serological testing), microbiological testing, vaccination, disinfection, and derivatization.138
In addition to the above measures, as part of the system of ensuring veterinary welfare on poultry farms, an analysis of the risks of contamination of products is performed at each stage of the technological process. Thus, throughout the technological chain of feed production and until ready feeds are placed in feeding troughs, a continuous process of its inoculation with microflora takes place. All the facilities involved in the production cycle can be identified as critical risk points – hatcheries, poultry houses, feed mills, etc. Veterinary specialists pay special attention to slaughterhouses, recycling houses, and the buildings where autopsies are performed. Protective measures are implemented in all such buildings and the spread of pathogens is prevented owing to constant disinfection.138,183
In the context of large-scale poultry farming, typical, for example, for Russia, vaccination is one of the main means of preventing infectious diseases (Table 1). Currently, up to 4-16 (taking into account serovars) infections (viral, bacterial, parasitic) are prevented through vaccines. Intense immunity against most of them is achieved by repeated administration of vaccines.13,179,180 Researchers note that the emerging situation with bird flu is a unique phenomenon, as the frequency and severity of bird flu outbreaks do not decrease from year to year. Over time, it may even be necessary to vaccinate people working in the poultry business with vaccines of subtypes that can potentially infect humans.94
The experience of poultry farming establishments from different countries shows that only a healthy bird can have a high level of productivity. Specific prophylaxis with the use of vaccines also facilitates preventing such cross-border diseases as Newcastle disease in poultry (according to the relevant schemes in poultry farms, parent flock, poultry, and broiler eggs are vaccinated). However, specific prevention of highly pathogenic and low pathogenic avian influenza in Ukraine is not implemented. In this case, compliance with biological safety and undertaking appropriate sanitary measures at poultry farms play a key role.13
Large industrial farms often stock their livestock with breeding and hybrid birds, using eggs from different European countries. In different countries, vaccines and diagnostic tests may differ even across one cross-vaccination. This means that different vaccines and vaccination schemes may be used within a single company. Vaccination is always stressful as a significant amount of vaccine virus is released into the environment (in the case of live vaccines). It is also necessary to take into account the antigenic incompatibility of vaccine strains with the ones circulating on farms. The negative factors that can be at play on farms are the immunosuppressive state of poultry, vaccine regimens violations, and the presence of latent infections with persistent viruses. In addition to this, veterinary and sanitary problems may arise on such farms: violation of sanitary requirements when preparing buildings for bringing in poultry; violation of feeding rules, stress; mixing birds of different ages or from different countries or different suppliers within a single flock; unreasonable introduction of new vaccines, change of vaccines or their manufacturers; violation of guidelines for the use of vaccines; lack of diagnostic monitoring tests; lack of specialist attention to immunosuppressive conditions that affect birds’ productivity and health.184 Fundamentally, protection of poultry flocks on industrial farms relies on compliance with a high level of biosecurity and vaccination of livestock, high-quality and comprehensive monitoring, as well as compliance with veterinary and sanitary requirements and standards. Cluster approach to the development of the poultry industry in a certain area is effective in the case of an infectious disease outbreak only when the feed mill, incubator, processing enterprise and other members of the cluster interact on an exclusive basis. Such a requirement is necessary, as in such case the company operates in isolation from other entities, and even if the products are exported from this area, they are completely safe for humans and birds.13,36
In general, the optimal level of outlays for veterinary measures in relation to the cost structure of poultry products is 2–4%. However, there are certain veterinary and sanitary problems that, as a rule, arise in the case of significant savings and outlays for veterinary preventive measures of less than 2% of the cost structure. Any violations related to poor quality of vaccination, veterinary and sanitary measures, poor feed quality, savings on ventilation systems, lighting, heating, equipment failure, insufficient training of staff and specialists – always lead directly or indirectly to significant veterinary problems and economic losses.184
However, vaccination remains the main component in the complex of special veterinary and sanitary measures. Therefore, bird treatment schemes in poultry farms mostly constitute programs or technological maps of vaccination.185 Each poultry farm has its own technology for the specific prevention of infectious diseases. Following the technology allows to achieve a full immune response, suppress subclinical disease course, reduce the incidence of secondary infections and minimize the use of veterinary drugs for therapeutic and prophylactic purposes. Immunization of birds against Newcastle disease is carried out by practically all large poultry enterprises in Russia without exception.186 Most poultry farms vaccinate poultry three times, after a complex vaccination against Marek’s and Newcastle diseases at the age of one day; according to another scheme – twice; the rest of poultry farms carry out a single vaccination, in this case, one of them is a comprehensive vaccination against Newcastle disease and infectious bronchitis. In Russia, vaccines of both Russian and foreign origin are used: “AVIVAK-NH” strain “La Sota” (NVP “Avivak”), “AVIVAK-NH” strain “Bor-74 VDNKI” (NVP “Avivak”), “ND La-Sota” (ZOETIS), “BIO-VAC NDV 6/10” (FATRO, Italy), “ND C2” (OOO “Intervet” MSD Animal Health), “HIPRAVIAR® CLON / H120”, LABORATORIOSHIPRA, SA., Spain).187,188
The use of Newcastle disease vaccines which contain any genotype of the virus protects poultry from death and clinical symptoms, however, in this case the release of the virus into the environment is not prevented, which can contribute to the spread of infection, especially where there are genetic structure differences between the field virus and vaccine strains. Therefore, vaccines made from phylogenetically similar strains of the Newcastle disease virus circulating in the region should be used for immunising poultry.189-191 In industrial poultry farming, observing the intervals between vaccinations is essential. Veterinarians in most poultry farms face the problem of developing vaccine prophylaxis schemes taking into account the origin of the parent flock, constant serological tests for antibody titers and the combination of vaccines.192
A certain role may also be played by specific prevention with the use of exclusively inactivated vaccines in the system of measures to prevent and control highly pathogenic avian influenza.193 In recent years, Ukrainian scientists have developed and successfully tested biological products based on domestic strains for the prevention of highly pathogenic avian influenza in the form of mono- and bivalent194,195 as well as trivalent196 vaccines against highly pathogenic avian influenza and Newcastle disease. However, such biological drugs against avian influenza are not yet legally permitted for use in the country.
The ideal vaccine against avian influenza should protect the bird from the disease, stimulating a protective immune response, as well as prevent the bird from contamination.197 However, the currently available commercial influenza vaccines are not able to prevent infection and induce sterilizing immunity.198
In Russia, preventive vaccination against avian influenza is allowed. For example in 2015,57,84 million of the country’s birds were vaccinated against influenza, and nearly 19 million birds were revaccinated.199 In Russia, preventive vaccination is applied in order to prevent highly pathogenic influenza in individual households located in areas at a higher risk of the disease. Given that the main source of influenza virus is wild migratory birds, the risk of highly pathogenic avian influenza pathogens entering Russian territory is permanent. In Russia, the Newcastle disease virus is detected in pigeons and chickens almost every year.
The results of serum testing carried out on four industrial enterprises of the Republic of Crimea revealed a high level of intensity of immunity to Newcastle disease in poultry. At the same time, the authors of the study note that diagnostic titers of antibodies to the disease were found in poultry blood serum collected from individual farms in three settlements where vaccination against Newcastle disease was not carried out. The latter indicates that the causative agent of the disease circulates in poultry from individual farms.200 Russian researchers have described outbreaks of Newcastle disease on poultry farms that did not vaccinate birds against the disease. The tests of antibody titers in poultry showed high concentrations of post-infection antibodies. The virus was isolated in a polymerase chain reaction.201
In Ukraine, the poultry kept on poultry farms is immunised against Newcastle disease with vaccines from strains “ND Clone 30”, “La-Sota”, “HIPRAVIAR-B1/H120”, “VH”, “VG/GA” produced in different foreign countries. It was found that the intensity of poultry’s immunity in laying hens was 100%, for broiler chickens it ranged from 89 to 100%, and for quail it stood at 85–87%.202 Ukrainian scientists B.T. Stehnii et al.203,154 studied the epizootic situation and post-vaccination immunity against Newcastle disease in several industrial poultry farms in different regions of Ukraine. In the Ivano-Frankivsk region, 255-day-old birds were examined for the presence of antibodies to the ND virus; the antibodies were detected in dilutions of 1: 256–1: 32768, in which case the CV ratio was 12.1 and the immunity intensity was 100%. After 30 days, the authors tested the blood serum of chickens from the same poultry house again. At the time of the tests, titers to ND virus ranged from 10 to 13 log2, and the intensity of immunity was also 100%. The antibody titers were in the range of 1: 64–1: 8192, CV was 31.86, and immunity was 95% in chickens aged 349 days kept on the same poultry farm, but in a different poultry house. The authors examined the blood serum of chickens aged 232 and 236 days kept in two poultry houses on farms in the Chernivtsi region. 100% intensity of immunity was found in both cases. In young chickens, antibody titers ranged from 8 to 13 log2, in older birds – from 9 to 13 log2. During the testing of blood serum taken from 107-day-old chickens from poultry farms in the Ivano-Frankivsk region, the intensity of immunity was 100%, whereas antibody titers, in this case, were in the range of 6–10 log2. A test of blood serum collected from poultry farms in the Kharkiv region revealed the presence of antibody titers in the range of 1:32–1:4096, although the intensity of immunity was 100%.203
Belarusian scientists note that the territory of the Republic of Belarus is free from highly pathogenic influenza and Newcastle disease, while these diseases are registered in the Russian territory almost every year. Scientists in the Republic of Belarus believe that the success in combating these diseases was achieved through vaccinations and monitoring.204 There is currently no systematic monitoring of Newcastle disease in Russia.205 For the prevention of avian influenza at large poultry enterprises of the Republic of Belarus, among other measures, general veterinary and sanitary measures aimed at improving the biological safety of these production facilities are strictly adhered to, and the following tests are performed on a regular basis: testing of pathological material and hatching eggs to detect highly pathogenic influenza virus; serological tests for the presence of specific antibodies in the serum of birds from poultry farms, poultry, and wild birds, as well as day-old chicks imported into Belarussian territory; poultry workers are prohibited from breeding poultry in their individual yards; it is prohibited to import live poultry, products of poultry processing as well as feed and feed additives from countries affected by highly pathogenic avian influenza into Belarussian territory.206
As already mentioned, the policy of some poultry farms in working with poultry is to only use inactivated vaccines against Newcastle disease. It is believed that such drugs do not cause immunosuppression, there is no replication of live vaccine virus, which eliminates the negative impact factors of vaccination on the body.207 Although in Russia and Kazakhstan, the leading role in poultry breeding and rearing belongs to specialized poultry enterprises, a significant part of poultry is kept on individual, small-scale farms. Such farms often take on the functions of “incubators” where no sanitary requirements are observed, and eggs of unreliable origin are often used.208 Such private entrepreneurs often ignore any anti-epidemic and special prevention measures. Researchers point out that preventive immunisation of pigeons, pheasants, guinea fowl, turkeys, and chickens in individual poultry farms must be carried out due to the significant economic damage caused by Newcastle disease, the spread of the virus and the danger of introducing the virus from small individual farms to large poultry farms. The purpose of such vaccination is to achieve the longest possible group immunity, which prevents virulent strains of the virus from being introduced in such groups of birds with subsequent reproduction and spread of infection.209
Nowadays industrial poultry farming uses Newcastle disease vaccines that do not require revaccination. A single Vectormune® HVT-NDV vector vaccine dose administered in an incubator is absolutely sufficient for stable immunity against the disease to form and develop, which reduces the impact of stress as compared to mass vaccinations of poultry by watering. According to Ukrainian scientists, when a vector vaccine is used, horizontal spread of the vaccine virus does not occur.210
In large poultry farms, the concentration of several pathogens (infectious chicken anemia, infectious bursal disease, Marek’s disease) often leads to severe immunosuppressive conditions, accompanied by less effective protection of birds following vaccinations against infectious diseases, a significant increase in mortality, susceptibility to secondary infections, and the threat of infectious diseases, especially Newcastle disease.211 Therefore, veterinarians should take this aspect into account when working with poultry.
In modern realities, the use of hatching eggs is always associated with risks of transboundary spread of infectious diseases, especially avian influenza and Newcastle disease.212 Transmission of low-pathogenic avian influenza viruses through hatching eggs of agricultural birds is highly probable, as the surface of eggs and containers may become contaminated with these agents. A high probability of influenza virus spreading through a marketable egg was observed in quails, turkeys, geese, and chickens.213 The analysis of the steps taken by European Union Member States in the fight against avian influenza214,213 shows that the set of measures also includes a ban on egg displacement, regardless of the pathogenicity of influenza (highly- or low pathogenic). In this case, the egg, as well as poultry, feeds, meat, people, and pathogen-containing objects are identified as potential sources for the recovery of influenza viruses within or spread between farms. However, breeding enterprises that produce hatching eggs and day-old chicks for export perform tests in accordance with Article 10.4.32 of the Code. 213,215 In this case, it is noted that, according to said provision, additional monitoring requirements to confirm the farm’s status as satisfactory in terms of avian influenza include evidence of no infection with highly pathogenic and low pathogenic influenza viruses. Poultry kept on such enterprises must be tested for the virus based on random samples, using serological test methods in accordance with the general requirements of the Code. The tests and analyses, depending on the risk of infection, should be conducted on a regular basis, but at least every 21 days.215 It is necessary to use supervision based on clinical examinations of livestock in such work. Chapter 10.4 of the Code states that the purpose of clinical surveillance is to identify clinical signs of avian influenza, primarily highly pathogenic influenza, at herd level. The following production indicators are monitored to help detect flu in the early stages of infection: increased death rate; reduction of feed or water consumption; detection of respiratory symptoms of the disease; reduced egg production. Reduced feed consumption and egg production is basically the only indicator suggesting that the herd may be infected by low-pathogenic influenza virus. Clinical supervision and laboratory tests should complement each other and be conducted consistently to obtain unequivocal confirmation. The result of a serological examination (random or probability) allows proving with confidence the absence of infection caused by influenza viruses in the country, zone, or compartment. In this regard, careful documentation of tests conducted213,215 will be of particular importance. The summary of HPIV and Newcastle diseases general preventive measures, laboratory diagnostic rules, response in case of outbreak, vaccination strategies in the countries are shown in the Table 2 and Table 3 accordingly.
Table (3):
Prevention and eradication measures of Newcastle diseases.
Measures |
Ukraine |
Russia |
Kazakhstan |
Belarus |
Source |
|---|---|---|---|---|---|
General preventive measures |
Biosecurity measures and closed operating model of large poultry farms to prevent the entry of the virus with poultry, staff, feed etc Routine vaccination large farms and backyard poultry |
Biosecurity measures and closed operating model of large poultry farms to prevent the entry of the virus with poultry, staff, feed etc. |
Biosecurity measures and closed operating model of large poultry farms to prevent the entry of the virus with poultry, staff, feed etc. |
Biosecurity measures and closed operating model of large poultry farms to prevent the entry of the virus with poultry, staff, feed etc. |
[206-209, 213] |
Laboratory diagnostic |
The method of fluorescent antibodies on longitudinal sections of the trachea, peroxidase method on a brain slice using antibodies, detection of antibodies in unvaccinated birds in the hemagglutination delay reaction or ELISA, PCR, isolation of the virus on chicken embryos or laboratory animals with next identification, and determination of strain virulence |
Isolation of the virus on chicken embryos with next identification, PCR, method of fluorescent antibodies, serological studies in unvaccinated birds: ELISA, hemagglutination delay reaction |
Method of fluorescent antibodies, serological studies in unvaccinated birds: ELISA, hemagglutination delay reaction, isolation of the virus on chicken embryos with next identification, PCR, |
Isolation of the virus on chicken embryos with next identification, PCR, method of fluorescent antibodies, serological studies in unvaccinated birds: ELISA, hemagglutination delay reaction |
[189-192, 201, 202, 206] |
Response in case of outbreak |
A dangerous zone with a radius of at least 3 km is established around the affected poultry farm, which is part of the 10 km observation zone In affected poultry farm, stamping-out is used for sick and suspicious birds, eggs are also destroying Healthy birds from unaffected poultry houses are slaughtered for meat immediately or after the end of the technological cycle (optional) In the protection zone, observation and vaccination of birds is carried out. |
In affected poultry farm, stamping-out is used for sick and suspicious birds
In the 3 km protection zone – observation and vaccination of birds is carried out. In 10 km observation zone – use closed operational model of farms, vaccination of birds population |
In affected poultry farm, stamping-out is used for sick and suspicious birds
In the 3 km protection zone – observation and vaccination of birds is carried out. In 10 km observation zone – use closed operational model of farms, vaccination of birds population |
In affected poultry farm, stamping-out is used for sick and suspicious birds
In the 3 km protection zone – observation and vaccination of birds is carried out. In 10 km observation zone – use closed operational model of farms, vaccination of birds population |
[187, 204] |
Vaccination |
All poultry on poultry farms and personal backyard farms are routinely vaccinated under the control of the state veterinary service
Live and inactivated vaccines are used, the vaccine must be authorized at the state level Periodic serological control of post-vaccination immunity tension of birds is carried out in poultry farms, personal backyard farms using the hemagglutination delay reaction |
Мono- and polyvalent live and inactivated vaccines are used for mandatory immunization of large and backyard poultry farms
Periodic serological control of post-vaccination immunity tension of birds is carried out in poultry farms, personal backyard farms |
Мono- and polyvalent live and inactivated vaccines are used for mandatory immunization of large and backyard poultry farms
Periodic serological control of post-vaccination immunity tension of birds is carried out in poultry farms, personal backyard farms |
Мono- and polyvalent live and inactivated vaccines are used for mandatory immunization of large and backyard poultry farms
Periodic serological control of post-vaccination immunity tension of birds is carried out in poultry farms, personal backyard farms |
[187, 203, 206, 209] |
Protection of staff |
Personal protective equipment, risk notification, daily medical examination, workers under 18 and over 65 and pregnant women are not allowed |
Personal protective equipment, risk notification, daily medical examination |
Personal protective equipment, risk notification, daily medical examination |
Personal protective equipment, risk notification, daily medical examination |
[210, 215, 237] |
Potential role of intensive bird growing during outbreaks of viral zoonosis
In the conditions of intensive poultry farming, an optimal indoor microclimate is necessary, as birds’ bodies are characterized by a fairly high level of metabolism and are rather sensitive to changes in the external environment. Therefore, one of the most important tasks in poultry farming is to create favourable conditions in poultry houses in order to increase poultry productivity, reduce morbidity, mortality, and culling.216,217 For each poultry species and depending on age, there are certain ranges of values of microclimate parameters which require the minimum amount of energy to maintain biological processes at an optimal level, i.e. the so-called biological comfort zone. The lower limits of such a critical zone determine the lower critical values of microclimate parameters, at which the organism begins to increase its biological activity in various ways (increased feed and water consumption, muscle activity, etc.), which ultimately leads to an increase in heat production, and, accordingly, the body’s heat consumption due to a decrease in productivity.218 The use of modern tunnel ventilation systems of leading manufacturers makes it easy to regulate air speed, allowing to create an optimal microclimate and comfortable conditions for poultry, adapted to their age.219 Microclimate indicators and the corresponding maximum productivity indicators can be achieved only if modern technologies are used in aviculture and at large poultry farms. However, it is necessary to consider the fact that large poultry enterprises produce a significant amount of harmful gases, manure and other biological waste. In this case, the production technology should ensure that the workers of such enterprises and the population living within a certain distance from poultry farms are protected. Furthermore, while it is much easier to establish biological safety measures and prevent infectious diseases in industrial enterprises than in household plots, the losses caused by highly-contagious pathogens are many times higher.24 Intensive farming creates opportunities for rapid passage of the pathogens to hundreds of thousands of aves and increase their virulence. Pathogens of infectious diseases with cross-border characteristics and zoonotic potential (avian influenza and Virulent Newcastle disease) are particularly dangerous for modern poultry farming.36 Consequently, the veterinary component acquires major significance in ensuring the smooth functioning of industrial poultry enterprises. The zoonotic nature of these infections makes their relevance all the more considerable. One Health now actively intervenes at all levels (local, national, global) in order to prevent the occurrence of such dangerous zoonoses. The significance of zoonoses in the global modern world will only increase in the future.29-31
Thus, an increase in the productivity of broiler poultry farming is associated with the optimization of feeding, maintenance and metabolism.220 Large-scale poultry farming provides for the harmonious development of the industry, uses the most modern production technologies and keeps the cost of obtaining products (meat and eggs) low. Poultry products are affordable and fully meet the needs of the population. The technology of raising poultry is optimized and facilitates obtaining the maximum amount of products at the lowest cost and in a short time. Although the costs of meat and egg production in private household plots are several times higher than in large poultry enterprises,11 it is not possible to significantly reduce the number of such low-commodity farms in the former USSR.
The potential role of wild fowl in reserving pathogens of the two significant zoonotic diseases in question has been proven by numerous studies.25-29 For example, the real-time situation in places of aves concentration can be seen based on data on the allocation of positive samples in the structure of the migration range of wild fowl, the placement of poultry farms, recreational objects and other establishments, and satellite images using GIS technology, as well as documentation. Based on such studies, Russian scientists have identified 27 zones of high risk of infection of birds, humans and farm animals. The authors indicate that the zones were not determined by geographical area, but rather by the extent to which they pose an increased epidemiological threat, namely the zoonotic component.221 Now, the zoonotic component to be taken into consideration is that in addition to monitoring the variability of the virus (for example, avian influenza) circulating in the human population (to track drift variants of the virus that can evade the immune response), constant monitoring of these pathogens that circulate among different hosts is crucial. Control of the influenza virus circulating among wild fowl is necessary to monitor the emergence of new variants of the virus in the natural reservoir, and among poultry – to track the influenza virus that can infect people who come into contact with birds. In addition, it is important to monitor the circulation of the influenza virus in populations of potential intermediate hosts (primarily pigs) to identify the variants of the influenza virus that can infect humans and be successfully transmitted from person to person, causing epidemics and pandemics.222 In household plots of citizens who often keep pigs and different types of poultry at the same time, this might be a viable solution (Figure 3).
Geographically speaking, Ukraine occupies a central place in Europe and is located at the crossroads of migration routes of wild fowl of many species, and has favorable climatic conditions for the existence of many living beings, including birds.72,223 According to the latest data, the ornithological fauna of Ukraine is represented by aves of 414 species, 207 of which nest on its territory. The wild fowl is often a reservoir of avian influenza and Virulent Newcastle disease pathogens. Three out of 14 transcontinental global migration flows pass through the territory of Ukraine, and almost the entire territory of the country is located within the main migration routes of aves from North Asia and Europe to the Mediterranean, Africa and South-West Asia. Ukraine lies at an intersection of the flight directions of birds from the Baltic and Caspian seas to the Black and Mediterranean seas, from Western Siberia and Kazakhstan to Western Europe and North Africa. The most intense ornithological situation concerns the south of Ukraine, namely the Azov-Black Sea coast, which is where aves flying from the north of Europe to the south concentrate, settling in numerous waterbodies along the sea. On the other side, the main migration route of aves from Kazakhstan, the Caspian Sea and South-Eastern Europe, and through the mouth of the Don further to the south-west of Europe passes along the Azov-Black Sea coast. The ornithological situation on the coast is one of the most intense in Eastern Europe. A significant number of waterfowl nest and winter in the south of Ukraine. 72,74,224 In fact, the entire south of the country is at an increased risk of spreading infections carried by wild fowl throughout the year. The second most critical region where seasonal migrations cause a tense ornithological situation is located within Polissia, in the floodplain of the Desna River and along the Dniprovskyi cascade of reservoirs. Several migration routes leading to Western Europe from Northwestern Siberia and direct continental routes from the Baltic states and Scandinavia and Northern Eastern Europe also intersect here.72,223
As already noted in the previous sections, in the industrial poultry industry of the former USSR countries (Ukraine, Russian Federation, Kazakhstan, Belarus), Virulent Newcastle disease is controlled by vaccines and constant serological monitoring. However, the situation in the private farms owned by citizens remains mostly uncontrolled. Researchers from the Russian Federation point to the fact that the infection has been confirmed in the Krasnodar and Stavropol territories and in the Chechen Republic. The virus circulates on household farms owned by families living in the south of the Russian Federation. Preventing avian influenza on poultry farms requires a strict permit regime, staff training and hygiene. Even if poultry farm employees do not keep poultry at home, their neighbours often do. The virus can infect the poultry population and spread into the premises of poultry enterprises. Household plots are at the highest risk of infection. Birds kept on such farms can come into contact with wild fowl, which is the primary source of the avian influenza virus. Owners of small household plots cannot preclude contact between poultry and wild birds and ensure that poultry is kept without open-air run. Scientists note that as a primary measure to prevent the virus from entering the territory of poultry farms, biological safety rules must be strictly observed.225 Synanthropic aves species are a kind of vector of transmission of the virus between the reservoir of infection in wild avifauna and susceptible poultry. However, if household plots do not engage in poultry farming, influenza outbreaks among poultry are not observed, as there are no susceptible organisms whose presence would lead to an inevitable disease in case of a natural outbreak.226
Successful vaccination of susceptible livestock and its quality control are important parts of the program of specific prevention in poultry farms of the former USSR countries. The effectiveness of vaccine prevention programmes is assessed on the basis of a number of factors, the main ones being: the general state of health of poultry, high indicators of safety and productivity of vaccinated poultry. The quality of vaccinations performed in broilers and laying hens is assessed by conducting routine serological monitoring (primarily Virulent Newcastle disease) to control the intensity of post-vaccination immunity in vaccinated poultry.227 Farms and household plots are always at risk of poultry disease. This is one of the reasons why large industrial enterprises vaccinate the entire poultry population. Household plots are often located at a small distance from industrial poultry enterprises, and synanthropic poultry can carry the causative agent of the disease.228 Monitoring studies of poultry from household plots are conducted in Ukraine and Belarus, but not in the Russian Federation.205 Another problem in the specific prevention of Virulent Newcastle disease is the discrepancy between the vaccine strains used on poultry and those that are constantly circulating in these areas.189-191
Virulent Newcastle disease has not been present on the territory of Ukraine for a long time (more than 13 years) due to preventive vaccinations.141,203 At present, the disease occurs mainly in a subclinical form, which is manifested by a slight respiratory pathology, a significant difference in the titers of antibodies to the pathogen in one herd, and the occurrence of secondary infections (colibacteriosis) against the background of the subclinical course of the disease. The subclinical form of Virulent Newcastle disease contributes to the activation of other viral pathogens – avian infectious bronchitis virus, metapneumovirus infection, and mollicutes (respiratory mycoplasmosis). The problem is particularly relevant for large poultry holdings.184 An urgent scientific and practical task consists in zoning the territory according to the levels of epizootic risk of Virulent Newcastle disease. Assessing the risk of entry of the pathogen from the outside and the nature of its spread within the state is an important task in the development of anti-epizootic measures aimed at preventing, localizing and eliminating epizootics.229,230
Currently, Russian researchers are particularly concerned about the fact that in 2018 poultry enterprises with a high level of veterinary and sanitary protection and zoned production locations (Rostov and Penza regions) were affected by avian influenza. Upon detecting the influenza virus, quarantine measures were introduced at eight large poultry farms. Epizootics of highly pathogenic avian influenza have become widespread, as 82 outbreaks have been documented in 15 regions of the Central, Southern, and Volga Federal districts. The Veterinary Medicine Service was particularly concerned about the infection pervading large poultry enterprises with existing biosecurity systems and sanitary zones (eight such outbreaks in 2018).231
According to the recommendations of the OIE, in the event of an outbreak of influenza, veterinary medicine workers should conduct a “stamping-out” of poultry from the focus of the infection and potential carriers of the virus. In this case, birds kept in household plots are allowed to be vaccinated. However, the OIE does not recommend exporting poultry after vaccination. That is why almost all countries prohibit the import of poultry products (except for heat-treated ones) from regions affected by avian influenza. In this case, veterinary services impose self-restrictions on exports in accordance with multilateral and bilateral trade agreements, but the host country has the right to introduce stricter measures. For example, an importing country may prohibit the import of poultry products not only from a separate administrative unit, but also from another importing country. If avian influenza is detected in wildlife but not in poultry, the importing country may impose an embargo and prohibit import for a period exceeding the agreed time limits of quarantine measures.36
Currently, it is proved that the zoonotic potential of influenza viruses in humans is realized through subtypes A(H5N1), A(H7N9), A(H9N2), A(H1N1), A(H1N2), and A(H3N2). Often, these human-pathogenic subtypes are reassortant forms of interaction between avian and swine influenza viruses. The disease is very difficult to control and prevent, because wild migratory fowl are simply layered with different subtypes of this pathogen. The major flow of aves twice a year (spring and autumn migration) between continents only contributes to the spread of the pathogen.32 Preventive vaccination against influenza, while improving the epizootic situation for this disease in household plots (for example, in the Russian Federation), does not reduce the risk of the disease.199 Thus, it should be concluded that outbreaks of avian influenza will continue to occur in the future, which will require joint work of veterinary and medical services, epidemic and epizootic monitoring, constant risk assessment and response measures.
It can also be stated that the development of the poultry industry, the emergence of new technologies for keeping and feeding poultry, as well as the achievements of biotechnology, not only will fail to reduce the range of infectious diseases, but on the contrary, extend it due to the emergence of new diseases (infectious anemia of chicken, hepatitis E, astrovirus infection, etc.). New strains or serovariants of already known infectious diseases appear: infectious bronchitis of chickens – serovariants D 388, 4/91, QX, CR88, it-02; Virulent Newcastle disease – 7 serological variants; infectious bursal disease; metapneumovirus infection – serovariants A and B; reovirus infection – Polish variant; influenza – H5N1, H7N9); and the number of infections caused by reassortant viruses is also growing (infectious bronchitis of chickens-isolate 062545/09 Swedish and various reassortants of influenza virus).184 All these pathogens present in industrial poultry farming increase the biological load on the immune system of poultry and manifest themselves as additional stressors.
Currently, in many developing countries (in Africa, Asia and South America, including Ukraine, the Russian Federation, Kazakhstan, Belarus), a significant part of poultry is kept in household plots and farms. In addition, part of this poultry is often a hidden reservoir of the causative agent of Virulent Newcastle disease. This situation seriously impedes the development of large-scale industrial poultry farming. After several passages of the causative agent of this disease vertically through the eggs, chickens become its natural reservoir. This is facilitated by the constant vaccination of aves. The phenomenon is confirmed by reports that vaccinated hens remain latent carriers of the causative agent of Virulent Newcastle disease.232 Poultry from household plots and synanthropic poultry can be a kind of “bridge” between wild fowl and aves from industrial poultry enterprises in the transmission of influenza viruses. In Ukraine, it is in rural areas that a significant number of private yards and farms where poultry is kept are concentrated, with practically no control by the veterinary service. In such plots, the population (“backyard poultry”) lacks basic biological safety measures, there are no disinfection gates, sometimes even fences, often even employees of large industrial poultry enterprises keep poultry in their yards.36 In these households, the spread of avian influenza and Virulent Newcastle poultry disease is influenced even by meteorological factors, because there are no biosecurity systems and a controlled microclimate like in industrial enterprises.233
Thus, analyzing outbreaks of avian influenza and Virulent Newcastle disease in the former USSR, a distinctive chain can be observed: wild migratory fowl – poultry of household plots and synanthropic aves – poultry of industrial enterprises.
The occurrence of avian influenza or Virulent Newcastle disease in any poultry farm causes significant economic damage not only to the farm, but also to the entire region, and ultimately to the country as a whole. The destruction of the entire poultry population affected and closing the poultry enterprises, restrictions on the activities of other farms in the risk zone, the costs of liquidation and anti-epizootic measures are the leading components of economic losses in case of occurrence of these two cross-border diseases of poultry with zoonotic potential. Subsequently, the resumption of activities of such enterprises affected by outbreaks of avian influenza or Virulent Newcastle disease requires significant investment.234 The very emergence of new and repeated (already eliminated outbreaks) of avian influenza now indicates not only a low level of biosafety at enterprises, but also often the lack of competence or inability of the veterinary service of poultry enterprises themselves to provide reliable protection against epizootics.36 For veterinary medicine workers, the problems of biosafety and biosecurity is an urgent problem, they primarily concern industrial poultry farms and enterprises where products are obtained for human consumption.235
Zoonotic viral diseases of poultry
highly pathogenic avian influenza and Newcastle disease, although to varying degrees, pose a threat to human health. These diseases are a public health problem, especially bird flu, which poses a constant pandemic threat. Modern approaches to managing such threats are based on the principles of the One Health concept.236 In both cases, large poultry farms may have a potential role in the spread of viral zoonoses to the public through poultry products or through contamination of personnel. The large number of birds in industrial farms, the close contact with birds of large numbers of personnel, and the large amount of potentially contaminated waste create an environment conducive to the rapid and significant spread of viral zoonoses. Also, endemic zones can be created around large poultry farms, and the bird population, even with vaccination, can also become endemic for Newcastle disease.237
In Ukraine, Russia, Kazakhstan and Belarus, the threat of viral zoonoses originating from birds is real and requires control by the state veterinary medicine and Public Health system. The topic of reducing the risks of the spread of Highly pathogenic avian influenza and Newcastle disease to large poultry farms and protecting the population from these diseases need to be studied.
ACKNOWLEDGMENTS
The authors would like to thank Management and Staff of State Scientific and Research Institute of Laboratory Diagnostics and Veterinary and Sanitary Expertise for theie help in collecting and processing data.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by a grant from Tiny Beam Fund (Award 2020, No. 11). The authors thank Stowarzyszenie “Otwarte Klatki” (“Open Cages” Association).
DATA AVAILABILITY
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
Not applicable.
- Halanets V. Poultry farming as the most capable industry of stock-raising. Agrarian economy. 2014;7(3–4):37–42. Accessed February 23, 2022. http://agrarianeconomy.lnau.edu.ua/images/docs/ae_2014_7_3-4/ae_2014_7_3-4_8.pdf.
- Detsiura SO. Ukraine in the world production of poultry products. The Economy of Agro-Industrial Complex. 2012;2:133–139.
- Burdeniuk T., Svirsky V. Competitiveness analysis of poultry market in Ukraine. Bulletin Zaporizhzhia National University. Economic sciences. 2013;2(18):145–152. Accessed February 23, 2022. https://web.znu.edu.ua/cms/index.php?action=category/browse&site_id=5&lang=ukr&category_id=1154&path=ves-arkhiv/2013/ekonomichni-nauki/ekonomichni-nauki—–2–2013-r-&category_code=ekonomichni-nauki—–2–2013-r-.
- Mead G., ed. Poultry meat processing and quality. Woodhead Publishing, 2004:2–18.
- Piroh SV. Efficiency of meat poultry enterprises. Economy and state. 2017;10:90–91. Accessed February 23, 2022. http://www.economy.in.ua/?op=1&z=3889&i=18.
- Gavrik O. Foreign experience of business and directions poultry adapt it in domestic practice. Economics and management of agro-industrial complex. 2015;2:136–142. Accessed February 23, 2022. https://econommeneg.btsau.edu.ua/en/content/foreign-experience-business-and-directions-poultry-adapt-it-domestic-practice.
- Polehenka MA. Formation of the logistic system of poultry enterprises. Economy and state 2017;10:95–98. Accessed February 23, 2022. http://www.economy.in.ua/?op=1&z=3891&i=20.
- Sharma RK. Role and relevance of rural family poultry in developing countries with special reference to India. Family poultry. 2007;17.1-2: 35–40. Accessed February 23, 2022. https://www.fao.org/3/aq611e/aq611e.pdf#page=37.
- Zabolotny VS. Informational analytical providing the management of competability of poultry products under globalization. Economics and management of agro-industrial complex. 2013; 11(106):65–68. Accessed February 23, 2022. https://econommeneg.btsau.edu.ua/en/content/informaciyno-analitichne-zabezpechennya-upravlinnya-konkurentospromozhnistyu-produkciyi.
- Piroh S. Factors of economic efficiency of poultry. Investments: practice and experience. 2017;10:61–63. Accessed February 23, 2022. http://www.investplan.com.ua/pdf/10_2017/13.pdf.
- Petrov YuI. Poultry farming of Ukraine: state and prospects of development. Modern poultry farming. 2011;11:3–5.
- Sendetska SV. Poultry farming in private farms: problems and prospects. Scientific Messenger of Lviv National University of Veterinary Medicine and Biotechnologies named after S.Z. Gzhytskyj 2014;1(58,2):130–134.
- Fedotov A. What is the danger of pseudoplague? Weekly «Arguments and facts». AiF-Kursk. 2020;5: 2-3. Accessed February 23, 2022. https://chr.aif.ru/kursk/incidents/chem_opasna_psevdochuma_v_kurske_
zafiksirovana_massovaya_gibel_kur. - Fisinin VI. Innovative designs and their development in industrial poultry farming. Poultry farming in Russia in 2011: state and prospects of innovative development until 2020: materials of the XVII international scientific and practical conference of VNAP, Sergiev Posad: VNITIP. 2012:7–17.
- Gogoladze D., Kotlyar P., Serova N. Industrial poultry farming in Russia – realities and possible threats. Poultry and poultry products. 2015;4:8–10.
- Sharipov R, Siganbaeva M, Rakhimzhanova D. Analysis of the state and problems of poultry farming in the Republic of Kazakhstan. Kombikorma. 2019;5:19–20.
Crossref - Pokush LV, Koval TYu. Poultry-farming branch development in the Republic of Belarus taking into account world tendencies. Problems of Economics : collection of scientific papers. 2012; 1(14):136-141. Accessed February 23. https://elc.baa.by/problemy_ekonomiki/14_2012.pdf
- Kosyanenko SV. Status and prospects of poultry farming in the Republic of Belarus. Agrarian economy. 2015;3:49–55.
- Tiginyanu ME. Development of poultry farming in the Republic of Belarus. New Horizons. Proceedings of the 3rd Belarusian-Chinese Youth Innovation Forum. 2016: 240–241. Accessed February 23, 2022. https://rep.bntu.by/bitstream/handle/data/40479/Razvitie_pticevodstva_v_
Respublike_Belarus.pdf?sequence=1&isAllowed=y - Velichko OV. Problems and prospects of poultry development in Ukraine. Formation of market visibility in Ukraine. 2012;2(129):119–124.
- Fasinin VI, Stollyar TA, Buyarov VS. Innovative projects and technologies in meat poultry farming. OlelGAU Bulletin. 2007;1:6–12.
- Boltianskyi OV, Boltianska NI. Influence of microclimate parameters on productivity of bird. Studies of Tavria State Agrotechnological University. 2012;12(3):159–163.
- Egorov IA, Buyarov VS. Development of new directions in the field of selection, feeding and technology of broiler poultry farming. OlelGAU Bulletin. 2011; 6(11): 17–23.
- Fisinin VI, Trukhachev VI, Saleeva IP, Morozov VU, et al. Microbiological risks related to the industrial poultry and animal production (review). Agricultural biology. 2018;53(6):1120–1130.
Crossref - Muzyka DV. Epizootological monitoring of viral diseases in wild birds in Ukraine: PhD thesis, Kharkiv, 2006; 1-20.
- Rusev IT. The role of migratory birds in the introduction and spread of highly pathogenic avian influenza in Ukraine. Bulletin of Sumy State University. Medicine. 2006;8(92):29–41. Accessed February 23, 2022. https://essuir.sumdu.edu.ua/bitstream/123456789/12229/1/05%20Rusev%20p29-41.pdf
- Stegniy BT, Muzyka DV, Stegniy AB, et al. The epizootic monitoring of avian virus diseases in the AR Crimea. Veterinary medicine. 2013;97:233–236. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/97/3_93.pdf
- Pribytkova KV. Improving the immunoprophylaxis of influenza and Newcastle disease in birds using inactivated vaccines in combination with Miramistin: PhD thesis.Voronezh, 2011; 1-20.
- Wang LF, Crameri G. Emerging zoonotic viral diseases. Rev. sci. tech. Off. int. Epiz. 2014;33(2):569–581.
Crossref - Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993.
Crossref - Woolhouse ME, Haydon DT, Antia R. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol., 2005;20:238–244.
Crossref - OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals / ed. OIE Biological Standards Commission and adopted by the International Committee of the OIE. – Paris: rue de Prony, 2015:8. Accessed February 23, 2022. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access
- Wernike K, Hoffmann В, Beer М. Single-tube multiplexed molecular detection of endemic porcine viruses in combination with background screening for transboundary diseases. J. Clin. Microbiol. 2013;51(3):938–944.
Crossref - Beato MS, Capua I. Transboundary spread of highly pathogenic avian influenza through poultry commodities and wild birds: a review. Rev. Sci. Tech. 2011;30(1):51–61.
Crossref - Stegniy BT, Gerilovych AP, Kucheryavenko RO. Transmissible diseases of animals: international experience of monitoring, forecasting, response and scientific support of the problem in Ukraine. Veterinary medicine. 2013;97:12–15. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/97/1_1.pdf
- Davleev A. Poultry farming under threat of H5N8. Agroinvestor. 2017;6:20–26. Accessed February 23, 2022. https://www.agroinvestor.ru/markets/article/27731-ptitsevodstvo-pod-ugrozoy-h5n8
- Cumulative number of confirmed human cases of avian influenza A (H5N1) reported to WHO (2013). World Healt Organization. 10–12. Accessed February 23, 2022. https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2021-15-april-2021
- Stegniy BT, Muzyka DV, Stegniy AB et al. Study of wild birds central black sea for availability antibodies to ortomixovirus infections and paramyxovirus serotype 1. Veterinary medicine. 2014;99:24–27. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/99/1_6.pdf
- Shmarov MM, Sedova ES, Verkhovskaya LV, et al. Induction of a Protective Heterosubtypic Immune Response Against the Influenza Virus by using Recombinant Adenoviral Vectors Expressing Hemagglutinin of the Influenza H5 Virus. Acta Naturae. 2010;2(1):111–118.
Crossref - Shein SA. The issue of the threat of the spread of diseases of animals and birds in the Russian. Farm Animals. 2013;3–4:28–36.
- Marchenko VYu, Goncharova NI, Evseenko VA et al. Overview of the Epidemiological Situation on Highly Pathogenic Avian Influenza Virus in Russia in 2018. Problems of Particularly Dangerous Infections. 2019;1:42–49.
Crossref - Kida H, Yanagawa R, Matsuoka Y. Duck influenza lacking evidence of disease signs and immune-response. Infect. Immun. 1980;30:547–553.
- Latorre-Margalef N, Gunnarsson G, et al. Effects of influenza A virus infection on migrating mallard ducks. Proc. R. Soc. 2009; 276:1029–1036.
Crossref - Popov NN, Kolotova TYu, Davydenko MB. Reabsorption of influenza virus: mechanisms and significance for overcoming the interspecific barrier. Veterinary medicine. 2017;103:202–208. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/103/3_46.pdf
- Jourdain E, Gunnarson G, Wahlgren J et al. Influenza virus in a natural host, the mallard: experimental infection data. PLoS ONE. 2010;5: e8935.
Crossref - van Dijk JG, Fouchier RA, Klaassen M, Matson KD. Minor differences in body condition and immune status between avian influenza virus-infected and noninfected mallards: a sign of coevolution? Ecol Evol. 2015;5(2):436–485.
Crossref - Bengtsson D, Safi K, Avril A, et al. Does influenza A virus infection affect movement behaviour during stopover in its wild reservoir host? R Soc Open Sci. 2016;10:3(2):150633.
Crossref - Sivaj M., Sharshov KA, Prokudin AV et al. Influenza A virus in wild bird populations in southern Western Siberia (2009–2010). Current issues of veterinary biology. 2016;1: 38-44. February 23, 2022. http://invetbio.spb.ru/avvb/AVVB_2016_01.pdf
- Perez-Ramirez E, Gerrikagoitia X, Barral M, Hofle U. Detection of low pathogenic avian influenza viruses in wild birds in Castilla-La Mancha (south central Spain). Veterinary microbiology. 2010;146(3–4):200–208.
Crossref - Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 2010;7(6):440–451.
Crossref - Rybalko SL, Krasnobaev EA, Zherebczov E`N, et al. The current state of the problem of influenza A H1N1. Ukraine. Health of the nation. 2010:169–178.
- Gibbs SE. Avian biology, the human influence on global avian influenza transmission, and performing surveillance in wild birds. Anim. Health Res. Rev. 2010;11(1):35–41.
Crossref - Dzhavadov ED, Dmitrieva ME. Avian influenza. St. Petersburg: First publishing and printing holding, 2011;188p.
- Ilyushina NA, Rudneva IA, Shilov AA, et al. Postreassortment changes in a model system: HA–NA adjustment in an H3N2 avian-human reassortant influenza virus. Arch Virol. 2005;150:1327–1338.
Crossref - Muzyka DV, Nevolko OM, Herilovych AP, et al. Highly pathogenic avian influenza in the world and in Ukraine. Veterinary medicine. 2017;103:198–201. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/103/3_45.pdf
- Capua I, Alexander DJ. Avian Influenza and Newcastle Disease: Field and laboratory Manual. Italy: Springer, 2009:198.
Crossref - Chvala IA, Scherbakova LO, Abramova LY, et al. Characteristics of influenza A infection caused by A/chicken/Primorsky/85/08 H5N1 virus in mallards. BirdFlu 2009: Avian Influenza and Human Health. Oxford, UK. 2009:23–25.
- Chvala IA, Abramova LYu, Babin YuYu, et al. Features of influenza A infection in young waterfowl caused by A/chikken/Primorsky/85/08/H5N1. Veterinariya. 2010;12:28–30.
- Shi WF, Gibbs,MJ, Zhang YZ, et al. Genetic analysis of four porcine avian influenza viruses isolated from Shandong, China. Archives of virology. 2008;153:211–217.
Crossref - Chvala IA, Shherbakova LO, Shul`pin MI, et al. Features of the course of influenza A in mallard ducks, caused by A/chikken/Primorsky/85/08 H5N1. Veterinariya. 2009;10:25–26.
- Frolov AV, Gusev AA, Shhelkanov MYu. Methodological approaches to the monitoring and diagnosis of influenza type A birds. International scientific and practical journal: epizootology, immunobiology, pharmacology, sanitation. 2017;2:22–27.
- Alexander DJ. Summary of avian influenza activity in Europe, Asia, Africa, and Australia, 2002–2006. Avian Dis. 2007;51(1):161–166.
Crossref - Zhao K, Gu M, Zhong L, et al. Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet. Microbiol. 2013;163(3–4):351–357.
Crossref - Abramova LYu. Features of influenza in different species of birds in experimental conditions and effectiveness of methods of pathogen detection. PhD thesis. Vladimir, 2011; 1–185.
- Ramey AM, Reeves AB, TeSlaa JL, et al. Evidence for common ancestry among viruses isolated from wild birds in Beringia and highly pathogenic intercontinental reassortant H5N1 and H5N2 influenza A viruses. Infect. Genet Evol. 2016;40:176–185.
Crossref - Verhagen JH, Herfst S, Fouchier RA. Infectious disease. How a virus travels the world. Science. 2015;347(6222):616–617.
Crossref - Marchenko VY, Susloparov IM, Kolosova NP, et al. Influenza A(H5N8) virus isolation in Russia, 2014. Arch. Virol. 2015;160(11):2857–2860.
Crossref - World Organisation for Animal Health (OIE). Update on highly pathogenic avian influenza in animals (typeH5 and H7). Paris: OIE; 2017.
- Marchenko VYu, Susloparov IM, Sapronova NYu, et al. Characterization of avian influenza H5N8 virus strains that caused the outbreaks in the Russian Federation in 2016–2017. Problems of Particularly Dangerous Infections. 2017;3:68–74.
Crossref - Shestopalov AM, Sharshov KA, Varkentin AV, et al. Results of long-term (2006-2016) avian influenza surveillance in wild birds of Uvs Nuur lake. South of Russia: ecology, development. 2016;11(3):106–119.
Crossref - Koopmans M, Wilbrink B, Conyn M, et al. Transmission of H7N7 avianinfluenza A virus to human during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;393(9409):587−593.
Crossref - Muzyka DV. Wild birds as one of the main distribution factors of birds, animals and people pathogens. Veterinary medicine. 2013;97:34–36. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/97/1_10.pdf
- Petrashkevich VG. Biological characteristics of avian influenza and the problems of laboratory diagnostics (review). Ecology and Animal World. 2018;1:27–31. Accessed February 23, 2022. https://animal.belal.by/jour/article/view/7?locale=ru_RU
- Korniienko LE, Nalyvaiko LI, Nedosiekov VV, et al. Infectious diseases of birds. 2013; Kherson: FOP “Grin DS Publisher”: 528 p.
- Zeinalova S, Bvdullaiev Y, Huliiev F, et al. Biomonitoring of avian influenza and Newcastl disease in Bardinsky district, Azerbaijan. Veterinary medicine. 2013;97:34–36. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/97/1_21.pdf
- Muzyka D, Pantin-Jackwood M, Spackman E, et al. Isolation and Genetic Characterization of Avian Influenza Viruses Isolated from Wild Birds in the Azov-Black Sea Region of Ukraine (2001–2012). Avian Diseases. 2016;60(1s):365–377.
Crossref - Stegniy BT, Muzyka DV, Shutchenko PO, et al. Cellular humoral mediated immunity and distribution of viral antigens in chickens after infection with a low pathogenic avian influenza virus (LPAIV H4N6) ssolated from wild ducks. Journal for Veterinary Medicine, Biotechnology and Biosafety. 2015;1(4): 28–34. Accessed February 23, 2022. http://jvmbbs.kharkov.ua/archive/2015/volume1/issue4/article5.php
- Muzyka D, Pantin-Jackwood M, Starick E, Fereidouni S. Evidence for genetic variation of Eurasian avian influenza viruses of subtype H15: the first report of an H15N7 virus. Arch Virol. 2016;161(3):605–612.
Crossref - Shhelkanov MYu, Kirillov IM, Shestopalov AM, et al. Evolution of influenza A / H5N1 virus (1996–2016). Problems of virology. 2016;61(6):245–256.
Crossref - Irza VN. Avian Influenza: Potential Threats in 2018. Veterinary and life. 2019;4(11):1–3.
- Kulak MV, Ilinykh FA, Zaykovskaya AV, et al. Surveillance and Identification of Influenza A Viruses in Wild Aquatic Birds in the Crimea, Ukraine (2006–2008). Avian Diseases. 2010;54(3):1086–1090.
Crossref - Czivanyuk MA, Sosipatorova VYu, Altunin DA, et al. The results of monitoring studies on influenza among wild and synanthropic birds in the Russian Federation in 2015. Proceedings of the Federal Center for Animal Health, (FGBU VNIIZZH): Vladimir. 2016;14:107–117.
- Li X, Zhang Z, Yu A, et al. Global and local persistence of influenza A (H5N1) virus. Emerg. Infect. Dis. 2014;20(8):1287–1295.
Crossref - Jeong J, Kang HM, Lee EK, et al. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during. Vet. Microbiol. 2014;173(3–4):249–257.
Crossref - Mulatti P, Zecchin B, Monne I, et al. H7N7 Highly Pathogenic Avian Influenza in Poultry Farms in Italy in 2016. Avian Dis. 2017;61(2):261–266.
Crossref - Harder T, Maurer-Stroh S, Pohlmann A, et al. Influenza A (H5N8) virus similar to strain in Korea causing highly pathogenic avian influenza in Germany. Emerg. Infect. Dis. 2015;21(5):860–863.
Crossref - Marchenko V, Goncharova N, Susloparov I, et al. Isolation and characterization of H5Nx highly pathogenic avian influenza viruses of clade 2.3.4.4 in Russia. Virology. 2018;525:216–223.
Crossref - Volkova MA, Chvala IA, Yaroslavczeva PS, et al. Serological monitoring of avian influenza in the Russian Federation in 2017–2018. Veterinary Science Today. 2019;2:3–11.
Crossref - Volkova MA, Chvala IA, Osipova OS, et al. Serological monitoring of avian influenza and Newcastle disease in the Russian Federation in 2019. Veterinary Science Today. 2020;2:76–82.
Crossref - Volkov MS, Varkentin AV, Irza VN, Starova AS. Outbreak of highly pathogenic avian influenza H5N1 in the Altai Territory in 2014: causes and elimination experience. Veterinary Science Today. 2015;3 (14): 62–65. Accessed February 23, 2022. https://veterinary.arriah.ru/jour/article/view/209
- Sosipatorova VYu, Altunin DA, Czivanyuk MA, Chvala IA. Study of the features of the pathological process in chickens caused by an isolate of the avian influenza virus A/duck/Altai/469/14 H5N1. Veterinary Science Today. 2016;1:51–54. Accessed February 23, 2022. https://veterinary.arriah.ru/jour/article/view/233/234
- Andriyasov AV, Pchelkina IP, Chvala IA, Drygin VV. Study of the primary structure of the genome of isolates of highly pathogenic avian influenza A/H5N1 virus isolated in Russia in 2007. Proceedings of the Federal Animal Health Center. 2014;12:60–76.
- Chvala IA, Andriyasov AV, Zinyakov NG, et al. The breeding and study of the A/H5N1 avian influenza virus that caused outbreaks in the Altai territory in 2014. Veterinary Science Today. 2017;1:23–29.
- Zelenkova GA, Karanty`sh GV, Tambiev TS, et al. Avian influenza: ecology, morphology, molecular markers of virus pathogenicity, modern epizootic situation. Veterinary patology. 2018;1(63):5–17.
- Olsen B, Munster VJ, Wallensten A, et al. Global patterns of influenza A virus in wild birds. Science. 2006;312:384–388.
Crossref - Webster RG, Bean WJ, Gorman OT., et al. Evolution and ecology of influenza A viruses. Microbiol. 1992;56:152–179.
Crossref - Tong S, Li Y, Rivailler P, Conrardy, C., et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U.S.A. 2012;109(11):4269–4274.
Crossref - Diagnostic Techniques and Vaccines for Foot-and-Mouth Disease, Classical Swine Fever, Avian Influenza and some other important OIE List A Diseases. Report of the Scientific Committee on Animal Health and Animal Welfare. Accessed February 23, 2022. https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scah_ out93_en.pdf
- Claas ECJ, deJong JC. vanBeek, et al. Human influenza virus A/HongKong/156/97 (H5N1) infection. Vaccine. 1998;16(9–10):977–978.
Crossref - Mandler J, Gorman OT, Ludwig S, et al. Derivation of the nucleoproteins (NP) of influenza A viruses isolated from marine mammals. Virology. 1990;176(1):255–261.
Crossref - Reperant LA, Rimmelzwaan GF, Kuiken T. Avian influenza viruses in mammals. Rev. Sci. Tech. OIE. 2009;28:137–159.
Crossref - Zhou J, Sun W, Wang J, et al. Characterization of the H5N1 highly pathogenic avian influenza virus derived from wild pikas in China. J. Virol. 2009;83:8957–8964.
Crossref - Gibbs AJ, Armstrong JS, Downie JC. From where did the 2009 ‘swine-origin’ influenza A virus (H1N1) emerge? Virol. J. 2009;6:207.
Crossref - Fatkhuddinova MF, Sattorova MKh, Alimova BO. On the relationship of human, animal and avian influenza according to complex seroepidemiological and virological studies. Medical Bulletin of the National Academy of Sciences of Tajikistan. 2018;1:86–90.
- Chumachenko DI, Bondareva DH, Sokolov OO. Methods of assessing epidemic situations and predicting the development of influenza and SARS. Radioelectronic and computer systems. 2007;2(21):111–115.
- Liu JH, Okazaki K, Shi WM, Kida H. Phylogenetic analysis of hemagglutinin and neuraminidase genes of H9N2 viruses isolated from migratory ducks. Virus Genes. 2003;27:291–296.
Crossref - Ma MJ, Yang XX, Xia X, et al. Comparison of commercial influenza A virus assays in detecting avian influenza H7N9 among poultry cloacal swabs. Journal of Clinical Virology. 2014;59(4):242–245.
Crossref - Du Ry van Beest Holle M, Meijer A, Koopmans M, de Jager CM. Outbreak report: human-to-human transmission of avian influenza A/H7N7, The Netherlands, 2003. Eurosurveillance. 2005;10(12):584.
Crossref - Cheng VC, Chan JF, Wen X, et al. Infection of immunocompromised patients by avian H9N2 influenza A virus. Journal of Infection. 2011;62(5):394–399.
Crossref - Li FC, Choi BC, Sly T, Pak AW. Finding the real case-fatality rate of H5N1 avian influenza. Journal of Epidemiology and Community Health. 2008;62(6):555–559.
Crossref - Dugan VG, Chen R, Spiro DJ, et al. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog. 2008;4:e1000076.
Crossref - Munster VJ, Baas C, Lexmond P, et al. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007;3:e61.
Crossref - Huang K, Zhu H, Fan X, et al. Establishment and lineage replacement of H6 influenza viruses in domestic ducks in southern China. J. Virol. 201286(11):6075–6083.
Crossref - AlHajjar S, McIntosh K. The first influenza pandemic of the 21th century. Ann. Saudi Med. 2010;30(1):1–10.
Crossref - Gulyaeva MA, Sharshov KA, Sobolev IA, Yurlov AK, et al. The isolation of influenza a virus from plumage of waterfowl during autumn migration. South of Russia: ecology, development. 2018;13(3):134–141.
Crossref - van Reeth K, Verh K. Avian influenza in swine: a threat for the human population? Verh K Acad Geneeskd Belg, 2006;68(2):81–101. Accessed February 23, 2022. https://pubmed.ncbi.nlm.nih.gov/16800240
- Karasin AI, Carman S, Olsen CW. Identification of human H1N2 and human-swine reassortant H1N2 and H1N1 influenza A viruses among pigs in Ontario, Canada 2003 to 2005. J Clin Microbiol. 2006;44(3):1123–1129.
Crossref - Garten RJ, Davis C., Russel CA, et al. Antigenic and genetic characteristics of swine-origin A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201.
Crossref - Simonsen L, Viboud C, Grenfell BT, et al. Jennings The genesis and spread of reassortment human influenza A/H3N2 viruses conferring adamantane resistance. Mol Biol Evol. 2007;24:1811–1831.
Crossref - Nelson MI, Viboud C, Simonsen L, et al. Multiple reassortment events in the evolutionary history of H1N1 influenza A virus since 1918. PLoS Pathog. 2008;4:e1000012.
Crossref - Kreibich A, Stech J, Hundt J, et al. Avian Influenza Virus H3 Hemagglutinin May Enable High Fitness of Novel Human Virus Reassortants. PLOS ONE. 2013;8(11):1–9.
Crossref - Stegniy MIu, Voroshylov IS. Transmissible infection of humans with Newcastle disease from birds. Veterinary medicine. 2012;96:126–128. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/96/49.pdf
- Abdulla AKh, Stegni`j BT. Comparative study of Newcastle disease field viruses isolated from chickens and pigeons. Veterinary medicine. 2014;98:34–39.
- Abdyldaeva RT, Akmatova EK, Atambekova ZhA, Kamarli AA. Diagnosis of Newcastle disease with the use of polymerase chain reaction (PCR). Bulletin of the Altai State Agricultural University. 2016;6(140):137–141.
- Stegniy AB. Comparative studies of biological properties of epizootic velogenic and mesogenic strains of Newcastle disease virus isolated in Ukraine. Veterinary medicine. 2014;98:71–75. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/98/rubrika2.php
- Nelson CB, Pomeroy BS, Schrall K, et al. An Outbreak of Conjunctivitis Due to Newcastle Disease Virus (NDV) Occurring in Poultry Workers. Am J Public Health Nations Health. 1952;42(6):672–678.
Crossref - Usachyov EV, Fedyakina IT, Shhelkanov MYu, et al. Molecular genetic characteristics of Newcastle disease virus Stern/Astrakhan/2755/2001, isolated in Russia. Molecular Genetics, Microbiology and Virology. 2006;1:14–20.
- Shhelkanov MYu, Ananev VYu, Lvov DN, et al. Comprehensive ecological and virological monitoring in the Primorsky Krai (2003–2006). Virological issues. 2007;52(5):37–48.
- Shhelkanov MYu, Usachyov EV, Fedyakina IT, et al. Newcastle disease virus in wild bird populations in the southern Primorsky Krai during the autumn migrations of 2001-2004. Virological issues. 2006;51(4):37–41.
- Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430(6996):242–249.
Crossref - Gould AR, Kattenbelt JA, Selleck P, et al. Virulent Newcastle disease in Australia: molecular epidemiological analysis of viruses isolated prior to and during the outbreaks of 1998—2000. Virus Res. 2001;77(1):51–60.
Crossref - Lee YJ, Sung HW, Choi JG. et al. Molecular epidemiology of Newcastle disease viruses isolated in South Korea using sequencing of the fusion protein cleavage site region and phylogenetic relationships. Avian Pathol. 2004;33(5):482–491.
Crossref - Wiseman A, Berman EM, Klement E. Risk factors for Newcastle disease in broiler farms in Israel. Prev Vet Med. 2018;149:92-97.
Crossref - Miller PJ, Decanini EL, Afonso CL. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect. Genet. Evol. 2010;10(1).26–35.
Crossref - Hu S, Ma H, Wu Y, Liu, et al. A vaccine candidate of attenuated genotype VII Newcastle disease virus generated by reverse genetics. Vaccine. 2009;27(6):904–910.
Crossref - Silko NYu, Glushhenko A V, Shestopalova LV, et al. Biological properties of velogenic strains of Newcastle disease virus isolated from birds in the North Caucasus. Virological issues. 2013;1:45–48.
- Glushhenko AV, Yurchenko KS, Yurlov AK, et al. The role of wild birds in preservation and prevalence of avian paramyxovirus Serotype 1 (Newcastle disease viruses) in Siberia and the Far East, Russia. South of Russia: ecology, development. 2016;11(2):50–58.
Crossref - Borisenkova AN, Rozhdestvenskaya TN. Program for ensuring the epizootic welfare of poultry farms for bacterial diseases of birds. 20 years in poultry farming, St. Petersburg: Research and Production Enterprise AVIVAK. 2010:75–85.
- Miller PJ, Dimitrov KM, Williams-Coplin D, et al. International Biological Engagement Programs Facilitate Newcastle Disease Epidemiological Studies. Front Public Health. 2015;3:235.
Crossref - Stegniy BT, Voroshylov IS. Virological indication of Newcastle disease in primary culture fibroblasts (FEC),the study of infectious properties of field isolates and storage. Veterinary medicine. 2013;97:141–142. Accessed February 23, 2022. http://jvm.kharkov.ua/sbornik/97/2_57.pdf
- Stegniy BT, Bolotin VI, Herilovych AP, et al. Determination of patho- and genotype of Newcastle disease viruses isolated in Ukraine in 1992-20. Veterinary medicine. 2013;97:34–36. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/97/1_13.pdf
- Lancaster JE. A history of Newcastle disease with comments on its economic effects. World Poultry Sci J. 197632:167–175.
Crossref - Spradbrow PB. Geographical distribution. In Newcastle Disease. Edited by Alexander DJ. Boston: Kluwer Academic Publishers. 1988:247–255.
Crossref - Fadly AM, Glisson JR, McDougald LR, et al. Diseases of poultry. Blackwell Pub., Ames, Iowa, 2008;12th ed.:1283.
- Miller PJ, Afonso CL, Spackman E, et al. Evidence for a new avian paramyxovirus serotype 10 detected in rockhopper penguins from the Falkland Islands. J. Virol. 2010;84(21):11496–11504.
Crossref - Briand FX, Henry A, Massin P, Jestin V. Complete genome sequence of a novel avian paramyxovirus. J. Virol. 2012;86(14):7710.
Crossref - Pchyolkina IP, Kolosov SN, Manin TB., et al. Newcastle disease virus isolated in wild and synanthropic bird populations in the territory of Russia in 2008. Veterinary medicine. 2009;92:417–422.
- Muzyka D, Pantin-Jackwood M, Stegniy B, et al. Wild bird surveillance for avian paramyxoviruses in the Azov-black sea region of Ukraine (2006 to 2011) reveals epidemiological connections with Europe and Africa. Appl Environ Microbiol. 2014;80(17):5427–5438.
Crossref - Muzyka D, Pantin-Jackwood M, Spackman E, et al. Avian Influenza Virus Wild Bird Surveillance in the Azov and Black Sea Regions of Ukraine (2010–2011). Avian Diseases. 2012;56(4s1):1010–1016.
Crossref - Kaleta EF, Baldauf C. Newcastle disease in free-living and pet birds. In Newcastle Disease. Edited by Alexander, D.J. Boston. Kluwer Academic Publishers. 1988:197–246.
Crossref - Pedersen JC, Senne DA, Woolcock PR, et al. Phylogenetic relationships among virulent Newcastle disease virus isolates from the 2002–2003 outbreak in California and other recent outbreaks in North America. J Clin Microbiol. 2004;42:2329–2334.
Crossref - Stegniy BT, Muzyka D., Tkachenko SV, Khartikh Abdulla A. Newcastle disease: modern classification of the pathogen, diagnosis and prevention of the disease (literature review). Veterinary medicine. 2012;96:120–122. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/96/47.pdf
- Stegniy BT, Muzyka DV, Stegniy AB, et al. Serological monitoring of orthomyxoviruses and paramyxoviruses among wild birds of southeastern black sea in 2014. Veterinary medicine. 2015;101:24–27. Accessed February 23, 2022. http://jvm.kharkov.ua/sbornik/101/1_6.pdf
- Stegniy BT, Muzyka DV, Rula OM, Biloyvan OV. Study of wild birds near the sea of Azov for availability of antibodies to orthomyxovirus and paramyxovirus infections. Veterinary medicine. 2015;101:92–94. Accessed February 23, 2022. http://jvm.kharkov.ua/sbornik/101/3_26.pdf
- Bolotin VI. Molecular-genetic studies of a new unknown paramyxovirus isolated from wild birds in Ukraine. Veterynarna medytsyna. 2015;101:9–12. Accessed February 23, 2022 http://www.jvm.kharkov.ua/sbornik/101/1_2.pdf
- Litvinova ZA. Ensuring the eupizootic and veterinary and sanitary well-being of the livestock industries of the Amur Region. Far Eastern Agrarian Bulletin. 2018;2(46):77–84. Accessed February 23, 2022. https://www.vestnik.dalgau.ru/upload/iblock/7f3/Vestnik46.zip
- Silko NYu, Shestopalov AM, A case of Newcastle disease virus isolation in Russia. Bulletin of Novosibirsk State University. Series: Biology, Clinical Medicine. 2010;8(1):57–61. Accessed February 23, 2022. https://nsu.ru/xmlui/handle/nsu/4382
- Marchenko VYu, Susloparov IM, Shipovalov, AV, et al. Circulation of Highly Pathogenic Avian Flu Virus in the Russian Federation in 2014-2015. Problems of Particularly Dangerous Infections. 20161:48–54.
Crossref - Onishhenko GG, Shestopalov AM, Ternovoj VA, et al. Detection in Western Siberia of highly pathogenic H5N1- influenza viruses, genetic and related viruses, circulating in Southeast Asia in 2003–2005. Advances in agribusiness science and technology. 2006;2(406):1–3.
- Muzyka DV, Chaplyhina AB. Results of immunological studies on the presence of antibodies to ortho- and paramyxoviruses in a number of common bird species of the North-Eastern Ukraine. Branta: 2015;18:133–140. Accessed February 23, 2022. https://branta.org.ua/branta-pdf/18/12_ks_muzyka.pdf
- Baryshnikov PI, Bondarev AYu, Novikov BV. Infection of wild birds with pathogens of viral diseases in the forest-steppe zone of the Altai Territory. Bulletin of the Altai State Agricultural University. 2008;4(42):40–41.
- Baryshnikov PI. Viral infections of wild birds in the steppe region of the Altai Territory. Bulletin of the Altai State Agricultural University. 2017;3:129–132.
- Bary`shnikov PI, Novikov NA. Mapping the spread of viral infectious diseases in wild birds in the steppe zone of the Altai Territory. Bulletin of the Altai State Agricultural University. 2018; 4(162):99–102.
- Yashkulov KB, Shhelkanov MYu, Lvov SS, et al. Isolation of influenza A viruses (Orthomychoviridae, Influenza A virus), Dchori (Orthomychoviridae, Thogotovirus) and Newcastle disease (Paramychoviridae, Avulavirus) on Maliy Zhemchuzhny Island in the northwestern part of the Caspian Sea. Virology Issues. 2008;3:34–38.
- Dimitrov KM, Bolotin V, Muzyka D, et al. Repeated isolation of virulent Newcastle disease viruses of sub-genotype VIId from backyard chickens in Bulgaria and Ukraine between 2002 and 2013. Arch Virol. 2016;161(12):3345–3353.
Crossref - Wan HQ, Chen LG, Wu LL, Liu XF. Newcastle disease in geese: natural occurrence and experimental infection. Avian Pathol. 2004;33(2):216–221
Crossref - Wang JY, Liu WH, Ren JJ, et al. Characterization of emerging Newcastle disease virus isolates in China. Virology Journal. 2015;12:119.
Crossref - Dortmans JC, Peeters BP, Koch G. Newcastle disease virus outbreaks: vaccine mismatch or inadequate application? Vet. Microbiol. 2012;160(1–2):17–22.
Crossref - Bwala DG, Abolnik C, van Wyk A, et al. Efficacy of a genotype 2 Newcastle disease vaccine (Avinew) against challenge with highly virulent genotypes 5d and 3d. J. S. Afr. Vet. Assoc. 2009;80:174–178.
Crossref - Kapczynski DR, King DJ. Protection of chickens against overt clinical disease and determination of viral shedding following vaccination with commercially available Newcastle disease virus vaccines upon challenge with highly virulent virus from the California 2002 exotic Newcastle disease outbreak. Vaccine, 2005;23:3424–3433.
Crossref - Yu L, Wang Z, Jiang Y, et al. Characterization of newly emerging Newcastle disease virus isolates from the People’s Republic of China and Taiwan. J. Clin. Microbiol. 2001;39:3512–3519.
Crossref - Miller PJ, King DJ, Afonso CL, Suarez DL. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine. 2007;25:7238–7246.
Crossref - Hu S, Ma H, Wu Y, et al. A vaccine candidate of attenuated genotype VII Newcastle disease virus generated by reverse genetics. Vaccine. 2009;27:904–910.
Crossref - Miller PJ. Estevez C, Yu Q, et al. Comparison of viral shedding following vaccination with inactivated and live Newcastle disease vaccines formulated with wild-type and recombinant viruses. Avian Dis. 2009;53:39–49.
Crossref - Ban-bo BA. Modern methods of combating Newcastle disease in the world in the period 2005-2007. Veterinary patology. 2008;4:15–16 Accessed February 23, 2022. https://vetpat.ru/wp-content/uploads/2014/03/04-27-2008.pdf#page=16
- Orynbaev MB, Sultankulova KT, Kerimbaev AA, et al. Molecular and biological properties of pathogenic Newcastle disease viruses isolated in Kazakhstan. Agricultural Biology. 2016;51(2):255–263.
Crossref - Krasikov AP, Zuev AV. Dominant infectious diseases of animals and birds in the Omsk region and the role of disinfection in the system of prevention measures. Bulletin of the Omsk GAU. 2019; 1(33):105–120. Accessed February 23, 2022. https://readera.org/vestnik-omgau/2019-1-33
- Djavadov ED, Dmitrieva ME. Effective vaccinal prevention as epizootic pledge of wellbeing industrial enterprise of poultry farming. Russian Veterinary Journal. 2012;3: 6–7.
- Zhuravel NA. Economic justification for preventive vaccination of broilers against viral diseases. Scientific Notes of the Kazan State Academy of Veterinary Medicine named after N.E. Bauman. 2015:57–60.
- Elnikov VV. Diagnosis and vaccine prevention of Newcastle disease. Efficient poultry farming. 2008;6:44–45.
- Belyavtseva EA. Study of the dynamics of the intensity of immunity in Newcastle disease. Scientific works of the Southern branch of the National University of Life and Environmental Sciences of Ukraine «Crimean Agrotechnological University». 2008; 111:18–23.
- Bilan AM, Skidan AV. Production of veterinary and sanitary control during broiler breeding. Current Issues of Science, Technology and Production, Troitsk: South Ural State Agrarian University. 2016:20–22. Accessed February 23, 2022. http://sursau.ru/upload/iblock/43b/stud_2015.pdf#page=20
- Dmitrieva ME. Veterinary well-being – the key to profitable work of poultry. Poultry and poultry products. 2014;1:23–25.
- Ravikumar R, Chan J, Prabakaran M. Vaccines against Major Poultry Viral Diseases: Strategies to Improve the Breadth and Protective Efficacy. Viruses. 2022;14(6): 1195.
Crossref - Nuraliev ER, Kochish II. Necessary and obligatory vaccination of birds against Newcastle disease in private farms as a natural reservoir of infection for industrial poultry farming. Proceedings of the Orenburg State Agrarian University. 2017;22(64):119–123. Accessed February 23, 2022. https://orensau.ru/images/stories/docs/izvestia/2017/izvestia_2_64_20170503.pdf
- Zhuravel N., Miftakhutdinov AV. Improving the methodological approach and recommendations for economic evaluation of the effectiveness of vaccination of broiler chickens. Ideas of young scientists to the agroindustrial complex: veterinary sciences. Chelyabinsk: Federal State Budgetary Educational Institution of Higher Professional Education South Ural State Agrarian University. 2020:160–171.
- Galenko SS, Samsonova VS, Gromov IN, Bol`shakova EI. Tension of postvaccinal immunity in birds vaccinated against Newcastle disease, infectious bronchitis and SSY-76. International scientific and practical conference of undergraduate and graduate students. EE «Vitebsk «Badge of Honor» Order State Academy of Veterinary Medicine. 2014:197–198. Accessed February 23, 2022. https://repo.vsavm.by/bitstream/123456789/4693/1/k-2014-7-3-197-198.pdf
- Karamendin KO, Kydyrmanov AI, Asanova SE, et al. Phylogenetic characteristics of a highly pathogenic strain of Newcastle disease virus isolated in 2013 in a poultry farm in southeastern Kazakhstan. Biotekhnology. Theory and practice. 2014;1:43–48. Accessed February 23, 2022. https://www.biotechlink.org/index.php/journal/issue/view/25
- Karamendin KO, Asanova SE, Kydyrmanov AI, et al. Isolation of Newcastle disease virus velogenic pathogen from vaccinated birds in southeastern Kazakhstan. Mikrobiology and virology. 2013;3(2):117–124. Accessed February 23, 2022. http://acagor.kz:8080/xmlui/bitstream/handle/123456789/62/ИЗОЛЯЦИЯ%20ВИРУСА%20БОЛЕЗНИ%20НЬЮКАСЛА%20МЕЗО
ГЕННОГО%20ПАТОТИПА%20ОТ.pdf?sequence=1&isAllowed=y - Popov OV, Gerasimchik VA. On the question of Newcastle disease in chickens. International scientific and practical conference of undergraduate and graduate students. EE «Vitebsk «Badge of Honor» Order State Academy of Veterinary Medicine. 2014:53. Accessed February 23, 2022. https://repo.vsavm.by/bitstream/123456789/4228/1/k-2014-7-3-53.pdf
- Djavadov E. Trends will intensify. Farm animals. 2013;3–4:18–20.
- Borisov AV, Borisov VV, Irza VN, Rahmanov AM. Scientific and legal support of measures to combat highly pathogenic avian influenza. Proceedings of the Federal Animal Health Center. 2007;5:83–93.
- Bisyuk IYu. Antigenic and immunogenic properties of inactivated vaccine against highly pathogenic avian influenza «AviFluVac-IEKVM» in laboratory and industrial conditions. Veterinary medicine, 2010;94:57–60.
- Stehniy AB, Holovko VO. Determination of the optimal ratio of antigens of avian influenza virus and Newcastle disease in the development of technology for the manufacture of inactivated emulsified associated vaccine against highly pathogenic avian influenza and Newcastle disease. Veterinary medicine. 2009;92:448–452.
- Muzyka DV. Peculiarities of formation of specific immunity in agricultural poultry after inoculation of inactivated trivalent vaccine against influenza of poultry subtypes N5, N7 and Newcastle disease. Animal biology. 2013;15(3):70–77. Accessed February 23, 2022. https://aminbiol.com.ua/index.php/ua/arkhiv/93-archive/bt3-15-2013/256-5-7
- Swayne DE, Kapczynski D. Vaccines, vaccination and immunology for again influenza viruses in poultry: In: Swayne D.E., ed. Avian Influenza. Ames, Iowa: Blackwell Publishers. 2008:407–452.
Crossref - Kostina LV, Zaberezhnyy AD, Grebennikova TV, et al. Vaccines against avian influenza in poultry. Problems of virology. 2017;62(2):53–60. https://doi.org/10.18821/0507-4088-2017-62-2-53-60
- Sadovnikov NV, Sharaevskaya IM. Laboratory diagnosis and vaccine prevention of avian influenza. Agrarian Bulletin of the Urals. 2016;11(153):56–61. Accessed February 23, 2022. http://agvu.urgau.ru/ru/19-joomla/537-11-154-2016.html
- Chegrynets, A. I., Saliy, O. O., Sobko, I. A., & Krasinko, V. O. Immunological evaluation of inactivated Newcastle disease vaccine depending on adjuvant composition. Regulatory Mechanisms in Biosystems, 2021;12(3): 490-497.
Crossref - Abdyldaeva RT, Akmatova EK, Saadanov IU. Comprehensive diagnosis of Newcastle disease. Bulletin of Altai State Agrarian University. 2016;7(141):149–152.
- Plys VM. Serological monitoring of Newcastle disease in Dnipropetrovsk region. Veterinary science, technologies of animal husbandry and nature management/ 2015;30(2):186–189.
- Stegniy BT, Kosheliev VV, Muzyka DV, et al. Epizootological monitoring of Newcastle disease, infectious bronchitis and chickenpox reduction syndrome among agricultural poultry poultry in the region of Ukraine. Veterinary medicine. 2015;100:31–35. Accessed February 23, 2022. http://www.jvm.kharkov.ua/sbornik/100/1_9.pdf
- Krasochko PA, Krasochko IA, Krasochko PP, et al. Specific prevention of infectious diseases of farm animals and birds in the Republic of Belarus. Actual problems of treatment and prevention of diseases of young animals, Vitebsk. 2019:56–61. Accessed February 23, 2022. https://repo.vsavm.by//handle/123456789/8696
- Silko NYu, Shestopalov AM, Shestopalova LV. Study of oncolytic activity of strains of Newcastle disease virus. Kazan Science. 200;91:13–18. Accessed February 23, 2022. https://www.kazanscience.ru/files/IV_2009.pdf#page=15
- Subbotin AM, Maksimovich VV. Epizootic situation in the Republic of Belarus. Modern Problems of Infectious Pathology in Children and Youth, Vitebsk. 2017:63–74. Accessed February 23, 2022 https://repo.vsavm.by/handle/123456789/3787
- Manukyan VA, Dzhavadov ED, Laptev GYu, et al. The use of enzymatic probiotics in the feeding of broilers. Poultry and poultry products. 2013;5:22–24.
- Shevchenko A. About incubator-poultry farms. Poultry. 2010;1:31.
- Nuraliev ER, Kochish II. Necessity of obligatory vaccination of birds against Newcastle disease in private farms as a natural reservoir of infection for industrial poultry farming. Proceedings of the Orenburg State Agricultural University. 2017;2(64):119–123.
- Avdosieva IK, Basarab OB, Melnychuk VV, Rehenchuk IL. Effective innovations prevention Newcastle disease. Scientific and Technical Bulletin of the State Research Control Institute of Veterinary Preparations and Feed and the Institute of Biological Species of Animals. 2019;20(2):169–174.
Crossref - Dmitrieva ME. Immunosuppression due to infectious anemia in chickens is a post-vaccination response. Farm animals. 2014;1:76–79.
- Cobb SP. The spread of pathogens through trade in poultry hatching eggs: overview and recent developments. Rev. Sci. Tech. OIE. 2011;30(1):165–175.
Crossref - Oganesyan A.S., Varkentin A.V., Baskakova N.Y., Karaulov A.K. Qualitative risk assessment of avian influenza virus transmission through incubation eggs. Veterinary Science Today. 2018;1:11–18.
Crossref - Council direktive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC // Off. J. European Union, 14.01.2006. Accessed February 23, 2022. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32005L0094
- OIE Terrestrial Animal Health Code. 2022; Chap. 10.4. Accessed February 23, 2022. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/
current/chapitre_avian_influenza_viruses.pdf - Marenko N. Optimal microclimate in the poultry house. Animal breeding in Russia. 2008;10:23–29.
- Purswell JL, Dozier WA, Olanrewaju HA, et al. Effect of Temperature-Humidity Index on Live Performancein Broiler Chickens Grown From 49 To 63 Days of Age. ASABE – 9th International Livestock Environment Symposium 2012, ILES 2012, Spain. 2012:41–49.
Crossref - Gadzhiev RM. The main components of heat transfer in poultry houses. Agrarian Science. 2014;8:31–32.
- Asrutdinova RA, Gavrilova KYu. Zoohygienic assessment of growing conditions for broiler chickens. Scientific Notes of the Kazan State Academy of Veterinary Medicine named after N.E. Bauman.. 2017;3(231):8–12.
- Egorov IA, Vertiprakhov VG, Grozina AA, et al. Age dynamics of pancreas secretory function and intestinal microbiota in meat broiler chicks and theier parental lines. Agricultural Biology. 2017;52(4):757–865.
Crossref - Savchenko PA. To ecological and epizootological zoning of the south of the Krasnoyarsk Territory on influenza A viruses. Bulletin of the Krasnoyarsk State Agrarian University. 2016;4:46–51.
- Sobolev IA, Kurskaya OG, Sharshov KA, et al. Variability of the Influenza A virus. South of Russia: ecology, development. 2016;411(1):170–177.
Crossref - Muzyka DV. Biological properties of low-pathogenic influenza virus subtype H15N7, first isolated in Eastern Europe. Veterinary medicine. 2014;98: 60–63. Accessed February 23, 2022. http://jvm.kharkov.ua/sbornik/98/2_15.pdf
- Rula OM, Stegniy BT, Muzyka DV, et al. Use of the method of selection of «dry drop of blood» for epizootological monitoring of infectious diseases of agricultural and wild animals. Veterinary medicine. 2016;102:110–113. Accessed February 23, 2022. http://jvm.kharkov.ua/sbornik/102/2_28.pdf
- Zykov SA. Highly pathogenic avian influenza. Effective livestock breeding. 2019;4:78–79.
- Volkov MS, Varkentin AV, Irza VN, et al. Study of avian influenza virus circulation in the territory of the Obsunur migratory focus of the Republic of Tieva. Veterinary Science Today. 2016;4:8–18. Accessed February 23, 2022. https://veterinary.arriah.ru/jour/article/view/267/0
- Bilan AM, Skidan AV. Economic justification of preventive treatments of broilers against infectious diseases. Current Issues of Science, Technology, and Production, Troitsk: South Ural State Agrarian University. 2016:23–25. Accessed February 23, 2022. http://sursau.ru/upload/iblock/43b/stud_2015.pdf#page=23
- Skotnikova TA. Improving the production technology and methods of using vaccines against Newcastle disease: Doctoral thesis. Shhelkovo, 2010; 1-50.
- 229. Zhuravlyova, V.A. Modeling and assessment of the risk of Newcastle disease in the Russian Federation: PhD thesis, Pokrov, 2009; 1-107.
- Derkach IM. Risk analysis as a tool for combating zoonoses. Veterinary medicine. 2013;97:184–186. Accessed February 23, 2022. http://jvm.kharkov.ua/sbornik/97/3_74.pdf
- Bardina NC, Varkentin AV, Karaulov AK. Overview of the epidemic situation on certain infectious animal diseases in the Russian Federation in 2018. Veterinary Science Today. 2019;3:45–50.
Crossref - Dzhupina SI, Ban-bo BA. On the nature of the epizootic process of Newcastle disease in chickens. Veterinary pathology. 2008;4:24–25. Accessed February 23, 2022. https://vetpat.ru/wp-content/uploads/2014/03/04-27-2008.pdf#page=25
- Belyavczeva EA, Polishhuk SV, Gurenko IA. Study of the influence of weather conditions on the spread of avian influenza virus. Proceedings of the Agricultural Science of Taurida. 2015;1(164):146–155.
- Karamendin KO, Kydyrmanov AI, Zhumatov KH, Sayatov MH. Moletiplex PCR for simultaneous differential diagnosis of Influenza A and Newcastle disease. Bulletin of KazNU. Biology Series, 2015;48(2):152–157. Accessed February 23, 2022. https://bb.kaznu.kz/index.php/biology/article/view/811
- Bezuhlyi M, Stegniy B, Bisiuk I, Rublenko M. Current issues of biosafety and biosecurity in the development and production of immunobiological drugs for veterinary medicine. Veterinary medicine. 2011;95:5–10. Accessed February 23, 2022. http://nbuv.gov.ua/UJRN/vetmed_2011_95_3
- Ryu S, Kim BI, Lim JS, Tan CS, Chun BC. One Health Perspectives on Emerging Public Health Threats. J Prev Med Public Health. 2017;Nov;50(6):411-414.
Crossref - Ul‐Rahman, Aziz, et al. Zoonotic potential of Newcastle disease virus: Old and novel perspectives related to public health. Reviews in Medical Virology, 2022, 32.1: e2246.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.