ISSN: 0973-7510
E-ISSN: 2581-690X
Glioblastoma multiforme (GBM) is a highly aggressive brain tumor with a dismal prognosis and limited treatment options. This study explores the anticancer potential of bioactive compounds isolated from Fernandoa adenophylla, a plant renowned for its medicinal properties. We isolated and characterized several phytochemicals from the heartwood roots of Fernandoa adenophylla and evaluated their efficacy against the U87 glioblastoma cell line using both experimental and in silico approaches. The isolated compounds included naphthoquinones and triterpenoids. Among them, Alpha-lapachone (DD) demonstrated the highest anticancer activity, achieving a maximum tyrosinase inhibition of 63.97% at 75 µg/mL after 48 hours. Other compounds, such as Lapachol (AA), Peshawaraquinone (EE), Dehydro-α-lapachone (BB), and Indanone derivatives (CC), also showed significant inhibition but were less effective. Notably, all tested compounds were non-toxic at the concentrations studied, unlike cycloheximide, which had a cytotoxic IC50 value of 0.8 ± 0.5 µg/mL. Furthermore, molecular docking studies on the 3D crystallographic structure of EFGRWT tyrosine kinase revealed notable interactions with key amino acid residues located within the active site. These results highlight the potential of Fernandoa adenophylla compounds as promising therapeutic agents for GBM, suggesting further research into their mechanisms and therapeutic applications.
Fernandoa adenophylla, Bioactive Compounds, U87 Cell Line, Cancer, Illness, Disease, Cytotoxicity Test
Cancer perpetually stands as one of the world’s major mortality factors while glioblastoma multiforme (GBM) represents among the most dangerous and difficult-to-treat types of brain tumors. Throughout history, plant-based remedies served as the main treatment methods for a wide range of illnesses including cancer. Research in drug discovery demonstrates that numerous anticancer medications derived from natural substances include paclitaxel, vincristine, and camptothecin which proves the importance of phytochemicals. Scientific research continues to focus on herbal medicines for treating brain tumours because their multiple treatment methods make them suitable alternatives to traditional treatments while reducing detrimental side effects.
Glioblastoma multiforme (GBM) is a particularly aggressive grade IV glioma characterized by extensive proliferation of glial cells.1 This type of cancer poses a significant clinical challenge, with only around 5% of patients surviving beyond five years.2 According to the Central Brain Tumor Registry of the United States (CBTRUSt), the incidence of GBM ranges from 0.59 to 3.69 per 100,000 people annually.3 GBM is classified into four categories based on histopathological features: IDH-wild type, IDH-mutant, Glioblastoma not otherwise specified, and not-elsewhere-classified. The IDH-wild type is the most prevalent, accounting for approximately 90% of cases.4
Current treatment strategies, including craniotomy, chemotherapy, and medications like temozolomide and bevacizumab, have yielded a median overall survival of less than 15 months.1,5 The development of resistance to these treatments is often linked to factors such as Methylguanine methyltransferase (MGMT) regulation and various proangiogenic factors.6,7 This situation underscores the urgent need for novel therapeutic approaches to improve the management of GBM and enhance patients’ quality of life.
The medical complexity of GBM stems from its molecular characteristics that include intense cellular growth as well as blood vessel formation and cell damage followed by apoptosis resistance. GBM remains difficult to treat because of its multiple features joined with the blood-brain barrier presence dual intratumoral heterogeneity and intense immunosuppressive microenvironment. The available standard medical treatments deliver limited therapeutic value because they commonly cause severe adverse reactions.1,6 An urgent need exists to develop better therapeutic approaches that both defeat drug resistance and offer improved effectiveness together with reduced toxicity levels.
Natural substances are now the subject of expanding cancer research by the medical community for their role as independent treatments or supportive agents in traditional therapies.8,9 Daily treatment of five herbal combinations used as supportive therapy for glioblastoma patients showed better survival outcomes and limited tumour growth according to Trogrlic et al.10 Bioactive phytochemical chemicals found in plants serve as main sources of medical substances while providing cost-efficient advantages through accessibility and posing reduced toxicity than synthetic pharmaceuticals that carry risks of adverse side effects and drug resistance.11,12
Herbal medicines earn special recognition for their dual ability to activate multiple signaling pathways simultaneously while controlling gene expression patterns and enhancing conventional treatment approaches. Anti-cancer actions against GBM models have become evident through studies of pro-apoptotic and anti-angiogenic mechanisms which use flavonoids along with alkaloids and naphthoquinones and triterpenoids phytochemicals. The increasing number of available evidence demonstrates that plant-based compounds have important applications in contemporary oncology research for glioblastoma treatment development.
Among the plants under scientific scrutiny is Fernandoa adenophylla (FA), a member of the Bignoniaceae family native to Africa and Southeast Asia.13 This genus includes 15 species predominantly found in tropical regions, with FA being the only species in India, where it grows in forests across Maharashtra, Gujarat, Rajasthan, and Assam.14
Fernandoa adenophylla, also known as Heterophragma adenophyllum and locally referred to as Ziron, Lotum-poh, Mostan-phul, Dhopa-phali, and Karen wood, has a wide range of traditional uses. In Assam’s Morigaon district, it is used for treating snakebites. Thai traditional medicine employs it for skin conditions, while the Chakma tribe in Bangladesh uses it for haemorrhoids and constipation.15
Traditionally, the aerial parts of Heterophragma adenophyllum are utilized for their therapeutic properties. The leaves are applied topically for skin issues, the fruits are cooked before consumption, and the flowers are eaten fresh. Additionally, the plant is used in massage oils to relieve muscle stress and is cultivated for its flexible wood, used in Burma for making bows and furniture. The roots are used in folk medicine for treating piles, constipation, and snake bites.16-18
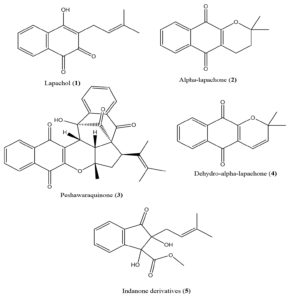
Fernandoa adenophylla is notable for its rich array of potent phytochemicals, including unique naphthoquinones (such as peshwaraquinone, dilapachone, adenophyllone, indadone, and lapachol) and triterpenoids (like ursolic acid, sitosterol, amyrin, and oleanolic acid). These compounds have been studied for their significant pharmacological activities, including leishmanicidal, anti-tuberculosis, antihypertensive, antifungal, and antibacterial effects.15,19-21
This investigation examines Fernandoa adenophylla compounds which demonstrate anticancer potential aimed at the glioblastoma (U87) cell line from a rich ethnopharmacological standpoint due to their potent bioactive constituents. Experimental cytotoxicity assays unite with molecular docking studies which explore two GBM pathogenic proteins namely epidermal growth factor receptor (EGFR) and Contactin-2 (CNTN2) to evaluate their binding interactions and mechanisms.
Collection of plant
The heartwood roots of Fernandoa adenophylla were meticulously gathered from several distinct locations at the University of Peshawar in Khyber Pakhtunkhwa (KPK), Pakistan. The plant was accurately identified by Dr. M. Ilyas, a prominent botanist at the University of Swabi (UOS), KPK. The identification was confirmed, and the voucher specimen, labelled UOS/Bot761, was carefully catalogued and preserved in the Herbarium of the University of Swabi for future reference and research.
The experimental procedures involving the plant material were thoroughly reviewed and received ethical approval from the Experimentation Ethical Committee of the Department of Chemistry at the University of Swabi. This approval affirmed that the study adhered to established ethical standards. Furthermore, the research was conducted in full compliance with the National Research Council’s guidelines on the care and use of plant materials, ensuring that all aspects of the study were performed with the utmost respect for ethical and scientific principles.
Extraction, fractionation and isolation
The heartwood roots of Fernandoa adenophylla were meticulously harvested and shade-dried for 40 to 45 days to maintain their bioactive properties. After drying, the roots were finely powdered and extracted with methanol using a Soxhlet apparatus over six hours to maximize the extraction of the active compounds.
Following extraction, the methanolic extract was fractionated sequentially based on solvent polarity, resulting in fractions of 1 g from n-hexane, 2 g from dichloromethane, 1.5 g from ethyl acetate, and 10 g from methanol. The methanol fraction was subjected to further purification through column chromatography on silica gel (70-230 mesh) with a gradient solvent system of chloroform and methanol, leading to the isolation of a compound identified as “methyl, 1,2-dihydroxy-2-(3-methylbut-2-enyl)-3-oxo-2,3-dihydro-1H-indene-1-carboxylate (3)” with a yield of 73 mg.
Further analysis of the reaction mixture via column chromatography produced 114 distinct fractions. These fractions were categorized into three primary groups based on their thin-layer chromatography (TLC) profiles: fractions 1, 2, and 3. Fraction 1 was purified using column chromatography with a chloroform and methanol solvent system, resulting in the isolation of “2,2-dimethyl-3,4-dihydro-2H-benzo[g]chromene-5,10-dione (4)” with a yield of 7 mg.
Fraction 2 underwent additional purification using a chloroform and methanol solvent mixture with varying ratios from 8:2 to 7:1, leading to the isolation of “2-hydroxy-3-(3-methylbut-2-enyl) naphthalene-1,4-dione” with a yield of 1.5 mg. Fraction 3 was divided into three sub-fractions, which were further purified using column chromatography with a chloroform and methanol mixture (7:3). Notably, sub-fraction 3.2, processed with a chloroform and methanol mixture (6:2), yielded a stereochemically complex compound identified as “15-hydroxy-7a-methyl-6-(2-methylprop-1-en-1-yl)-7,7a,14b,15-tetrahydro-5H-t-5a,” featuring intricate benzo[5,6] and azuleno[1,8-bc] structures. The final compound, “chromene-5,9,14,16(5a1H,6H)-tetraone,” was isolated with a yield of 2.4 mg (Figure 1).
Further refinement of these compounds was achieved through washing with n-hexane, as outlined in the reported methodology.22
Anti-cancer Activity glioblastoma (U87) cell line
The U87 cell viability assay was conducted using the MTT protocol to evaluate the cytotoxic effects of test compounds (AA, DD, EE, BB, and CC).23 First, U87 cells were seeded into 96-well plates and allowed to grow for 24 hours under standard culture conditions. After the initial incubation, the cells were treated with the test agents, prepared in DMSO, and further incubated for 24 and 48 hours to assess time-dependent effects. Following treatment, MTT dye was added to each well and incubated for 3 hours, allowing metabolically active cells to reduce the dye into purple formazan crystals. The dye was then carefully removed, and DMSO was added to solubilize the formazan crystals, ensuring a colorimetric signal proportional to cell viability. The absorbance of the solubilized formazan was measured at 490 nm using a Multiscan spectrophotometer (Thermo Scientific, USA). Finally, the percentage of growth inhibition was calculated using the formula:
% growth inhibition = [100-(At-Ab) / (Ac-Ab)] x100
where,
At = Absorbance value of test compound (cells + media + drug)
Ac = Absorbance value of control (cells + media + vehicle)
Molecular docking studies
Given the promising in vitro anticancer activity exhibited by compounds isolated from Fernandoa adenophylla, molecular docking studies were undertaken to elucidate their potential binding interactions with two key oncogenic targets: the epidermal growth factor receptor (EGFR) and Contactin-2 (CNTN2). For EGFR, docking simulations were performed within the ATP-binding cleft of the wild-type EGFR (EGFRWT), a receptor frequently implicated in oncogenic signaling. In addition, CNTN2 was selected as a docking target due to its role in glioblastoma (GBM) progression. Using the Molecular Operating Environment (MOE 2016) software, these docking simulations aimed to reveal how these compounds might inhibit the kinase activity of EGFRWT, a target frequently implicated in oncogenic signaling as well as their binding interactions with CNTN2. The 3D crystal structures of the wild-type EGFR protein (PDB ID: 4HJO) and CNTN2 (PDB ID: 2OM5) were sourced from the Protein Data Bank, providing a reliable framework for detailed interaction analyses.
The Amber10EHT forcefield was used for the protonation and energy minimization of protein’s 3D structures. Molecular builder Moule tool inbuilt in the MOE was used to prepare the structures of potent compounds and these structures were further minimized using same forcefield mentioned earlier at a gradient of 0.00001. For PDB ID: 4HJO, First of all docking protocol was validated by using redock method. The native ligand was redocked into the binding site of the downloaded enzyme. The protocol with RMSD value ≈ 1 Å was used for further docking studies. Potential pocket in case of CNTN2, was determined by using the SiteFinder tool inbuilt in MOE and dummy atoms were created at alpha sphere centers on the selected site to perform docking analyses. Docking was performed via the Triangular Matcher method to generate diverse protein-ligand complex conformations. The resulting complexes were ranked according to their GBVI/WSA scoring values, a critical metric for estimating ligand binding free energy within a defined binding pocket where lower values (expressed in kcal/mol) correspond to more favorable binding interactions.
In the protein preparation phase, the QuickPrep module of MOE was employed to refine the target protein by addressing structural irregularities, such as missing residues or improper orientations. The process also included protonation to physiological pH, essential for mimicking in vivo conditions, and the removal of extraneous water molecules that could interfere with docking accuracy. Additionally, atomic constraints were applied where necessary, followed by energy minimization to stabilize the protein in its most favorable conformation for ligand interaction.
Molecular docking simulations were carried out under default MOE parameters, ensuring a robust and reproducible evaluation of the binding affinities and poses of the compounds. Following the docking process, a thorough visual analysis was performed using the Discovery Studio Visualizer, allowing for an in depth examination of key interactions between the isolated compounds and critical amino acid residues within the receptor’s active site. This analysis not only highlighted the binding efficiency but also provided insight into the potential mechanisms through which these compounds could exert their anti-cancer effects
Anti-cancer activity against glioblastoma (U87) cell line
The anti-cancer activity of compounds isolated from Fernandoa adenophylla was evaluated using the glioblastoma (U87) cell line, and the results demonstrated varied efficacy among the compounds tested. The tyrosinase inhibition assay indicated that the inhibitory effects of the compounds-AA, DD, EE, BB, and CC-were both concentration- and time-dependent.
Among the compounds, DD emerged as the most effective tyrosinase inhibitor. At a concentration of 75 µg/mL, DD achieved a maximum inhibition of 63.97% after 48 hours, indicating its strong potential as a therapeutic agent. Compound AA also exhibited notable inhibition, with its effectiveness increasing progressively with concentration and time, reaching 58.01% inhibition at the highest concentration after 48 hours (Table 1).
Table (1):
Anti-cancer Activity glioblastoma (U87) cell line of compounds isolated from Fernandoa adenophylla
| Sample | Doses | 24 Hrs | 48 Hrs |
|---|---|---|---|
| Lapachol | 25 µg/mL | 9.48 | 54.87 |
| 50 µg/mL | 13.36 | 57.38 | |
| 75 µg/mL | 13.4 | 58.01 | |
| Alpha-lapachone | 25 µg/mL | 8.33 | 52.51 |
| 50 µg/mL | 15 | 57.82 | |
| 75 µg/mL | 28.66 | 63.97 | |
| Peshawaraquinone | 25 µg/mL | 43.9 | 15.45 |
| 50 µg/mL | 48.45 | 19.2 | |
| 75 µg/mL | 57.5 | 53.88 | |
| Dehydro-α-lapachone | 25 µg/mL | 39.2 | 46.9 |
| 50 µg/mL | 40.13 | 51.91 | |
| 75 µg/mL | 57.5 | 53.88 | |
| Indanone derivatives | 25 µg/mL | 39.2 | 46.9 |
| 50 µg/mL | 40.13 | 51.91 | |
| 75 µg/mL | 57.5 | 53.88 | |
| Temozolomide | 250 µg/mL | 45.75 | – |
| 500 µg/mL | – | 45.70 |
In contrast, EE displayed potent inhibition at higher concentrations, although its performance was inconsistent at lower concentrations and longer incubation periods. Notably, EE achieved 53.88% inhibition at 75 µg/mL after 48 hours, but showed a decrease in effectiveness at lower concentrations and shorter times.
Both BB and CC demonstrated similar inhibitory profiles, showing significant inhibition at higher concentrations and extended incubation times. The maximum inhibition for both compounds reached 57.5% at 75 µg/mL after 48 hours.
Overall, the data suggest that all tested compounds possess potential as tyrosinase inhibitors, with DD standing out as the most promising candidate for further development and investigation.
Cytotoxicity activity
The cytotoxicity assessment of the isolated compounds (AA, DD, EE, BB, and CC) from Fernandoa adenophylla revealed promising safety profiles, as none of the compounds exhibited cytotoxic effects at the tested concentration of 0.5 µg/mL (Table 2). All compounds were classified as non-toxic under the experimental conditions, suggesting their potential for further development as safe therapeutic agents. This lack of cytotoxicity at a biologically relevant concentration underscores their suitability for applications where safety is a critical consideration. In contrast, the positive control, cycloheximide, demonstrated significant cytotoxicity with an IC50 value of 0.8 ± 0.5 µg/mL, confirming its potent cell-killing activity. This comparison highlights the unique safety advantage of the Fernandoa adenophylla compounds, which, even at a concentration of 0.5 µg/mL, did not compromise cell viability. The pronounced cytotoxic effect of cycloheximide further validates the robustness of our experimental system and provides a clear benchmark for evaluating the non-toxic nature of the tested compounds.
Table (2):
Cytotoxicity Activity of compounds isolated from Fernandoa adenophylla
Compounds |
IC50 ± SEM (µg/mL) |
|---|---|
Lapachol |
Not toxic |
Alpha-lapachone |
Not toxic |
Peshawaraquinone |
Not toxic |
Dehydro-α-lapachone |
Not toxic |
Indanone derivatives |
Not toxic |
Cyclohexamide |
0.8 ± 0.5 |
These findings are particularly encouraging, as they suggest that the compounds from Fernandoa adenophylla possess a favorable therapeutic window, combining biological activity with an absence of cytotoxicity. This makes them attractive candidates for further pharmacological exploration, especially in contexts where minimizing off-target toxicity is paramount. Future studies will focus on elucidating the mechanisms underlying their biological activity while maintaining their non-toxic profile.
Molecular docking studies
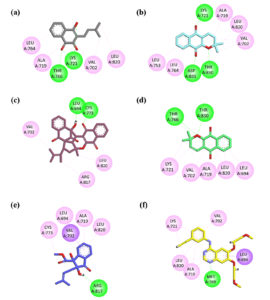
Molecular docking studies were conducted to predict the binding interactions between isolated compounds and the epidermal growth factor receptor (EGFR) tyrosine kinase. The results indicated that these compounds interact with EGFR through a combination of hydrophilic and hydrophobic interactions, underscoring their potential as inhibitors of EGFR activity. The 2D interaction plots of the isolated compounds alongside the native ligand are illustrated in Figure 2.
Figure 2. 2D interaction plots illustrating the binding interactions of isolated compounds within the active site of EGFRWT (PDB ID: 4HJO). (a) Lapachol; (b) Alpha-lapachone; (c) Peshawaraquinone; (d) Dehydro-alpha-lapachone; (e) Indanone Derivative; (f) 2D interaction plot of the native ligand erlotinib
Compound 1 (Lapachol) displayed three significant hydrogen bonding interactions with the target protein. Two of these bonds were formed with the Lys721 residue: one involved the carbonyl oxygen of the compound, while the other was associated with its hydroxyl (-OH) group. The third hydrogen bond was established between the second carbonyl oxygen, located within the central ring of the compound, and the Thr766 residue. In addition to these hydrogen bonds, notable hydrophobic interactions were observed, including p-alkyl interactions with Leu764, Ala719, and Val702, as well as alkyl interactions with Leu820 and Val720 (Figure 2a).
Similarly, Compound 2 (Alpha-lapachone) also exhibited three hydrogen bonding interactions with EGFR. The first interaction was observed between one of the carbonyl oxygen atoms and the Lys271 residue. The second carbonyl oxygen formed a hydrogen bond with the Asp831 residue, while the ether oxygen (-O-) within the ring structure engaged in a hydrogen bond with the Thr830 residue. Furthermore, hydrophobic interactions were identified with Leu820, Ala719, Val702, Leu764, and Leu753, reinforcing the compound’s binding affinity (Figure 2b).
Compound 3 (Peshawaraqiunone) formed two conventional hydrogen bonds with active site residues, specifically with Cys773 and Leu694. Additionally, this compound demonstrated a p-alkyl interaction and an alkyl interaction with Leu820, along with further alkyl interactions with Arg817 and Val702, contributing to its overall binding stability (Figure 2c).
These findings highlight the multifaceted nature of the interactions between the isolated compounds and EGFR, suggesting their potential as candidates for further development in targeted cancer therapy.
The plots highlight key hydrogen bonds and hydrophobic interactions between the compounds and essential amino acid residues, demonstrating their potential as EGFR inhibitors.
Compound 4 (Dehydro-alpha-lapachone) demonstrated significant binding interactions through hydrogen bonding with key amino acid residues, specifically Thr766 and Thr830. Additionally, hydrophobic interactions with amino acids Leu820, Val702, Ala719, Leu694, and Lys721 were observed, indicating a robust interaction profile that may contribute to its potential efficacy as an EGFR inhibitor (Figure 2d).
Compound 5 (Indanone Derivative) exhibited a single hydrogen bonding interaction with the Arg817 residue. Furthermore, a p-s interaction between the aromatic ring of the compound and Val702 was noted, enhancing its binding affinity. Other hydrophobic interactions with Leu820, Ala719, Leu694, and Cys773 further support its potential interaction with the EGFR active site (Figure 2e).
In contrast, the co-crystallized native ligand erlotinib formed conventional hydrogen bonds with Met769 and established a p-s interaction with Leu694. It also exhibited hydrophobic interactions with key amino acid residues, including Lys721, Val702, Ala719, and Leu820. These interactions underline erlotinib’s mechanism of action as a potent EGFR inhibitor (Figure 2f).
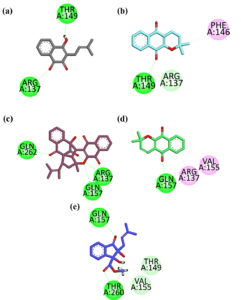
In parallel, the compounds were also docked against CNTN2, a brain-enriched protein implicated in glioblastoma progression. The CNTN2 docking results of all isolated compounds are summarized below:
Compound 1 – Lapachol exhibited two hydrogen bonding interactions with CNTN2. One bond was formed between its hydroxyl (-OH) group and the Thr149 residue, while the second bond was observed between the carbonyl oxygen of its central ring and the Arg137 residue (Figure 3a).
Compound 2 – Alpha-lapachone demonstrated three interactions with CNTN2. It formed a hydrogen bond between one of its carbonyl oxygen atoms and the Thr149 residue, engaged in a p-alkyl interaction with Phe146, and its ether oxygen (-O-) established a carbon-hydrogen bond with the Arg137 residue (Figure 3b).
Compound 3 – Peshawaraqiunone formed four conventional hydrogen bonds with CNTN2. Specifically, one hydrogen bond was established via its carbonyl oxygen with Gln262; two hydrogen bonds were formed with Gln157 (one from the carbonyl oxygen and one from the ether oxygen within the ring structure); and a fourth bond was observed with Arg137 through the carbonyl oxygen. Additionally, a p-alkyl interaction with Arg137 was noted (Figure 3c).
Compound 4 – Dehydro-alpha-lapachone interacted with CNTN2 by forming one hydrogen bond with the Gln157 residue via its carbonyl oxygen, along with two p-alkyl interactions involving Arg137 and Val155 (Figure 3d).
Compound 5 – Indanone Derivative showed three hydrogen bonds with CNTN2-one between its carbonyl oxygen and Gln157 and two involving Thr260 (one via the hydrogen of the -OH group and one via the carbonyl oxygen). In addition, carbon-hydrogen bond interactions were observed with Thr149 and Val155, attributed to the hydrogens of the methoxy group in the ester functionality (Figure 3e).
The exploration of Fernandoa adenophylla as a potential therapeutic agent against glioblastoma multiforme (GBM) is crucial, given the aggressive nature of this brain tumor and its resistance to conventional therapies. GBM remains a formidable clinical challenge due to its high recurrence rates, often leading to poor patient outcomes despite current advancements. Natural products, recognized for their extensive pharmacological benefits, continue to inspire novel therapeutic approaches, and within this context, Fernandoa adenophylla holds promise.
In recent studies, key bioactive compounds identified within Fernandoa adenophylla, designated as DD and AA, have demonstrated considerable cytotoxic effects against glioblastoma cell lines, particularly the U87 line. These findings are consistent with earlier research on natural compounds, which showed potential in enhancing glioblastoma treatment outcomes.24 Importantly, these compounds exhibited significant growth inhibition at higher concentrations and longer exposure durations, while also demonstrating a favorable safety profile as related to a previously published study.25 The absence of cytotoxicity at tested concentrations highlights their potential as more tolerable therapeutic agents compared to conventional chemotherapies, which are often associated with debilitating side effects that profoundly affect patients’ quality of life.26
The molecular docking studies provide deeper insights into the mechanisms underlying these compounds’ actions, specifically their interactions with key oncogenic drivers such as the epidermal growth factor receptor (EGFR) and Contactin 2 (CNTN2). EGFR, frequently implicated in GBM pathogenesis, is critical in driving tumor proliferation, survival, and invasiveness. The strong hydrogen bonding observed between compounds DD and AA and the receptor’s kinase domain suggests a potential to inhibit EGFR’s activity effectively. Additionally, EE and CC showed strong hydrogen bonding interactions with CNTN2. This inhibition could subsequently disrupt downstream signaling pathways known to be dysregulated in GBM, thereby attenuating the aggressive behavior of these tumors.27,28 These results are further substantiated by comparative studies on natural inhibitors of EGFR, which demonstrated similar mechanisms of action and therapeutic potential in mitigating tumor progression and resistance.29
From a broader perspective, these findings underscore the importance of advancing research on Fernandoa adenophylla. While in vitro and in silico studies lay a promising foundation, future research must prioritize in vivo investigations to evaluate the bioavailability, pharmacokinetics, and pharmacodynamics of these compounds. Additionally, exploring the synergistic effects of these compounds with established GBM therapies could provide new avenues for optimizing treatment protocols. Given the increasing recognition of the tumor microenvironment’s role in immune evasion and therapy resistance,25 understanding how compounds DD and AA interact with immune cells and influence the microenvironment will be essential. Such insights could potentially expand the therapeutic applications of these compounds to combination regimens, including immunotherapy.
The implications of this research extend beyond the immediate context of GBM treatment. By focusing on natural products like Fernandoa adenophylla, the study aligns with a patient-centric approach that emphasizes both efficacy and improved quality of life. This holistic perspective is particularly relevant in the context of GBM, where the physical and emotional burden of the disease is profound. Integrating therapies with a lower toxicity profile and potential to enhance patient well-being could significantly impact clinical practice, offering hope for better patient outcomes.
In conclusion, exploring Fernandoa adenophylla as a potential therapeutic agent against GBM presents a compelling narrative. The combination of a promising safety profile, targeted inhibition of key oncogenic pathways, and the potential for synergistic applications underscores its relevance in the evolving landscape of GBM treatment. Moving forward, rigorous preclinical and clinical studies are essential to further elucidate the therapeutic potential of these compounds. This research not only advocates for the clinical application of Fernandoa adenophylla but also reinforces the broader significance of harnessing nature’s pharmacological diversity to address complex and treatment-resistant cancers like GBM.
This investigation assessed the anticancer properties of bioactive compounds extracted from Fernandoa adenophylla against the glioblastoma (U87) cell line, employing both experimental and in silico methodologies. The studied compounds lapachol (AA), alpha-lapachone (DD), peshawaraquinone (EE), dehydro-α-lapachone (BB), and indanone derivatives (CC)-demonstrated varying levels of tyrosinase inhibition, with alpha-lapachone (DD) achieving the highest inhibition rate of 63.97%. Although none of the compounds exhibited cytotoxic effects under the tested conditions, their significant inhibitory activity against glioblastoma cells suggests potential as therapeutic agents. The absence of cytotoxicity at the examined concentrations points to a favorable safety profile for these compounds. These findings imply that bioactive molecules derived from Fernandoa adenophylla could provide valuable insights for the development of novel treatments for glioblastoma. Molecular docking studies indicated that the isolated compounds established favorable interactions with the EGFR tyrosine kinase, demonstrating reasonable binding affinity and forming both hydrophilic and hydrophobic interactions with key amino acid residues, including Cys773, Thr766, Leu694, Ala719, Lys721, and Thr830. The docking analysis against CNTN2 revealed that all these compounds exhibited strong molecular binding through various interaction types (hydrogen bonds, p-alkyl, carbon-hydrogen bonds) with critical CNTN2 residues, particularly Thr149, Arg137, Phe146, Thr260, Gln262 and Gln157. These results suggest that, in addition to targeting EGFR, the compounds may also modulate CNTN2 function. Such interactions suggest that these isolated compounds could serve as promising candidates for developing EGFR inhibitors, further supporting their potential as therapeutic agents for glioblastoma multiforme. Further investigations, particularly in vivo studies and comprehensive molecular assessments, are essential to validate these findings and fully explore the therapeutic capabilities of these compounds in cancer treatment.
ACKNOWLEDGMENTS
The authors are thankful to the Researchers Supporting Project number (RSPD2024R1035), King Saud University, Riyadh, Saudi Arabia.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AR conceptualized and supervised the study. SN and ZA performed extraction and purification. HS and AAI performed molecular docking. MK, UR and AA docking analysis and anticancer activity methodology. AR analyzed the data. NAA performed data interpretation. AR wrote the manuscript. HS and AAI edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee Sh U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3-9.
- Delgado-Lopez PD, Corrales-Garcia EM. Survival in glioblastoma: A review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062-1071.
Crossref - Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. Cbtrus statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2013-2017. Neuro Oncol. 2020;22(Suppl 1):iv1-iv96.
Crossref - Grochans S, Cybulska AM, Siminska D, et al. Epidemiology of glioblastoma multiforme-literature review. Cancers (Basel) 2022;14(10):2412.
Crossref - Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the seer 17 population-based registries. J Neurooncol. 2012;107(1):207-212.
Crossref - Wu W, Klockow JL, Zhang M, et al. Glioblastoma multiforme (gbm): An overview of current therapies and mechanisms of resistance. Pharmacol Res. 2021;171:105780.
Crossref - Lu KV, Bergers G. Mechanisms of evasive resistance to anti-vegf therapy in glioblastoma. CNS Oncol. 2013;2(1):49-65.
Crossref - Chabattula SC, Gupta PK, Govarthanan K, et al. Anti-cancer Activity of Biogenic Nat-ZnO Nanoparticles Synthesized Using Nyctanthes arbor-tristis (Nat) Flower Extract. Appl Biochem Biotechnol. 2004;196(1):382-399.
Crossref - Yang Z, Zhang Q, Yu L, Zhu J, Cao Y, Gao X. The signaling pathways and targets of traditional chinese medicine and natural medicine in triple-negative breast cancer. J Ethnopharmacol. 2021;264:113249.
Crossref - Trogrliז I, Trogrlic D, Trogrliז D, Trogrliז AK. Treatment of glioblastoma with herbal medicines. World J Surg Onc. 2018;16(1):28.
Crossref - Alhumaydhi FA, Aljohani ASM, Rashid U, et al. In vivo antinociceptive, muscle relaxant, sedative, and molecular docking studies of peshawaraquinone isolated from Fernandoa adenophylla (Wall. ex G. Don) steenis. ACS Omega. 2021;6(1):996-1002.
Crossref - Teklehaymanot T, Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, northwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3:12.
Crossref - Shah ZA, Khan MR. Peshawaraquinone a novel naphthoquinone and a new indanone from the stem of Heterophragma adenophyllum Seem. Rec Nat Prod. 2015;9(2):169-174.
- Krishna G, Kumar A, Lakshminarasimhan P, Kumar A. Distributional note on Fernandoa adenophylla (Wall. ex G. Don) Steenis (Bignoniaceae). Indian J For. 2016;39(2):173-174.
Crossref - Chorsiya A, Singh MV, Khasimbi S. Fernandoa adenophylla: a review of its phytochemistry, traditional and pharmacology use and future aspects. Curr Tradit Med. 2020;7(3):348-354.
Crossref - Waziri A, Bharti C, Aslam M, et al. Probiotics for the chemoprotective role against the toxic effect of cancer chemotherapy. Anticancer Agents Med Chem. 2022;22(4):654-667.
Crossref - Rahmatullah M, Samarrai W, Jahan R, et al. An ethnomedicinal, pharmacological and phytochemical review of some Bignoniaceae family plants and a description of Bignoniaceae plants in folk medicinal uses in Bangladesh. Adv Nat Appl Sci. 2010;4:236-254.
- Ibrahim AM, Chauhan L, Bhardwaj A, et al. Brain-derived neurotropic factor in neurodegenerative disorders. Biomedicines. 2022;10(5):1143.
Crossref - Rauf A, AlOmar TS, Sarfaraz S, et al. Density functional theory, molecular docking, In vitro and In vivo anti-inflammatory investigation of lapachol isolated from Fernandoa adenophylla. Heliyon. 2023:9(12):e22575.
Crossref - Rauf A, Rashid U, Shah ZA, et al. Anti-inflammatory and anti-diabetic properties of indanone derivative isolated from Fernandoa adenophylla in vitro and in silico studies. Sci Rep. 2024;14(1):9624.
Crossref - AlOmar TS, Rauf A, Rashid U, et al. Molecular docking, DFT studies, and anti-inflammatory evaluation of peshawaraquinone isolated from Fernandoa adenophylla. J Biomol Struct Dyn. 2023;42(20):10604-10616.
Crossref - Shah ZA, Abu-Izneid T, Rauf A, et al. Phosphodiesterase 1 inhibition and molecular docking study of phytochemicals isolated from stem heartwood of Heterophragma adenophyllum Seem. S Afr J Bot. 2020;135:274-279.
Crossref - Sana T, Zehra S, Khan M, et al. Urease and human glioblastoma (U87) cells growth inhibitory iridoid glycoside acetates from Nyctanthes arbor-tristis Linn. Nat Prod Res. 2024;39(12):3486-3494.
Crossref - Giammona A, Commisso M, Bonanomi M, et al. A Novel Strategy for Glioblastoma Treatment by Natural Bioactive Molecules Showed a Highly Effective Anti-Cancer Potential. Nutrients. 2024;16(15):2389.
Crossref - Mall SK, Yadav T, Waziri A, Alam MS. Treatment opportunities with Fernandoa adenophylla and recent novel approaches for natural medicinal phytochemicals as a drug delivery system. Exploration of Medicine. 2022;3(6):516-539.
Crossref - Zhai K, Kubatka P, Busselberg D. Antitumor effects induced by natural molecules in the brain. Natural Molecules in Neuroprotection and Neurotoxicity. 2024:281-323.
Crossref - Ciftci HI, Radwan MO, Sever B, et al. EGFR-targeted pentacyclic triterpene analogues for glioma therapy. Int J Mol Sci. 2021;22(20):10945.
Crossref - Edpuganti S. Glioblastoma Multiforme: A Review of the Genetic Alterations. International Journal of High School Research. 2024;6(7):5-9.
Crossref - Adeniran OY. Identification of Novel Plant-derived Inhibitors of the EGFR Kinase Domain Using vHTS, QSAR and Molecular Docking Approaches. Asian J Biochem Genet Mol Biol. 2024;16(7):69-84
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.