ISSN: 0973-7510
E-ISSN: 2581-690X
Despite the growing prevalence of legionellosis in Poland and worldwide, little is known about the extent of public awareness regarding the seriousness of this disease and the appropriate preventive measures. The aim of this work is to assess the Polish adults’ knowledge, perceptions, and beliefs about legionellosis and its causative agents, risk factors, exposure, and other relevant facts. Data for this cross-sectional study were gathered via a questionnaire that was constructed and validated by the study investigators before commencing the survey, which lasted from January to March 2022. Knowledge, attitude and practice towards legionella were measured and quantified. One-way ANOVA and chi square tests were used to compare between demographic variables and the level of knowledge. Regression analysis was conducted to examine the predictors for higher knowledge among study participants. A total of 251 participants with a mean age of 28.26 ± 9.6 were enrolled in the current study. Over two thirds (74%) were females, with higher education (62%). Older age was associated with less knowledge about legionellosis (B = -0.049, p < 0.001), while higher education was associated with more knowledge (B = 1.656, p < 0.001). No significant differences were found between genders (p = 0.066). A knowledge gap was present for diagnostic tests regarding legionella. On the other hand, knowledge about prevention procedures was quite high among study participants. This study showed that overall knowledge about legionellosis in Polish adults was quite low. In particular, older age groups and the less educated are in need of more awareness of legionellosis disease. A knowledge gap was particularly present regarding how the disease is diagnosed. Awareness campaigns containing simple, easy-to-understand information could prove useful in combating the disease.
Legionellosis, Adults, Poland, Cross-sectional Study
Legionellosis is an infectious disease caused by aerobic bacteria of the genus Legionella which were first detected in 1977 in Philadelphia in individuals suffering from pneumonia.1 Although the Legionella spp. natural ecological niche is surface water, it can also be found in a wide variety of settings.2 Consequently, infection can be spread through inhalation of aerosols containing bacteria through the nasopharynx.3 Infection with bacteria from the Legionellaceae family can cause Legionnaires’ disease, Pontiac fever or non-pulmonary legionellosis. As the disease symptoms are similar to those of pneumonia, timely diagnosis is necessary in order to initiate appropriate antibiotic therapy and attain cure.4
Infections with the Legionella spp. are on the rise in Poland and whole Europe as indicated by both the Poviat Sanitary and Epidemiological Stations and the European Centre for Disease Prevention and Control data. However, these statistics are likely to be underestimates, as it is believed that many individuals do not seek medical assistance for this condition.5-7
Education the society about the pathogen’s environments is required to improve risk assessment; discovery of pathogen niches and examination and control of cases and outbreaks. The substantial upsurge in the occurrence of reported cases of legionellosis among inhabitants, geographical variances in the incidence of cases, the pervasiveness of Legionella spp. urge the need to monitor the presence of these bacteria.8
To the best knowledge of the researchers, there are no data about the knowledge and attitude regarding legionellosis, especially among residents in Poland. Hence, Polish adults’ knowledge, perceptions, and beliefs associated with legionellosis were explored through a questionnaire survey, and their responses were analysed with respect to relevant sociodemographic factors. The survey probed into the respondents’ knowledge of the general characteristics of legionellosis, as well as infections, their occurrence, diagnosis, treatment, and prevention, along with their motivation for immediately reporting any symptoms to the Poviat Sanitary and Epidemiological Stations.
Participants and procedure
The data for this study were gathered through a diagnostic survey conducted between January and March 2022 using a proprietary questionnaire constructed before the start of the study. The questionnaire, printed in Polish, was then distributed to the population (n = 251, aged 18-69 years) who agreed to take part in the study and signed the informed consent form were invited to complete the questionnaire.
Reliability and validity of research instrument
The validity was guaranteed via face and content by a group of specialists in nursing, public health and education. The reliability of the instrument was determined through an initial test which involved a diverse population with similar characteristics. A pilot tests were carried out utilizing ten adults in order to assess questionnaire practicability on the basis of the time it took to accomplish the questionnaire, the clarity of the questions, and to ensure accurate result interpretation.9 The instruments generated a Cronbach’s alpha of 0.73, were therefore considered appropriate for use in the present study. Data from these pilot tests were not included in the final study.
Instrument content and scoring
The questionnaire, which was developed in the Polish language, began with a short introductory section encouraging eligible individuals to participate in the survey, followed by the explanation of the study aims and assurance of the anonymous and voluntary nature of participation. The next section elicited relevant sociodemographic information (age, gender, and education), while the main section was designed to measure the awareness, knowledge, perceptions, and attitudes about the study topic.
Sample size
Open Epi Info software version 7.2 was used for the sample size calculations. The sample size was calculated using the following parameters: an infinite population size, 95% confidence interval, a power of 0.80, a margin error of 5%, and an anticipated frequency of 50%, resulting in a sample size of 385. A total of 400 forms were given out.
Study ethics
This work was done in accordance with the Declaration of Helsinki.10 Ethical approval was demanded from the Medical University of Warsaw Ethical Committee; however, it was judged as not obligatory. Consent forms were circulated to 400 Polish adults, and active consent was obtained from the participants. The respondents were accordingly informed that participation in the study was voluntary, and that their identity would remain anonymous, and the secrecy of their data was guaranteed.
Statistical analysis
IBM SPSS v28.0 was used for the data analysis. Continuous data were presented as mean ± standard deviation (SD), while categorical data was presented as number and percentage (%). Data normality was visually assessed using histograms and statistically using Shapiro test. Comparisons between categorical data were assessed using chi square test. Regression analysis was conducted to test the associations between the population characteristics and the different outcome variables. The main outcomes were the overall score of the assessment tool. The secondary outcomes were the items with the lowest and the highest score, respectively; as they were identified as the main limitation and strength in knowledge about the infection among study participants. Linear regression was used if the outcome variable was continuous; B coefficients, standard errors (SE) and 95% confidence intervals (95%CI) were obtained. Logistic regression was conducted if the outcome variable was binary; adjusted odds ratio (AOR) and 95% CI were obtained following binary logistic regression. P value less than 0.05 was considered significant for all statistical tests.
Population characteristics
A total of 251 participants were enrolled in the current study. The majority were between 21-30 years (n = 178, 71.2%), females (n = 185, 74%), of tertiary education or higher (n= 155, 62%). Detailed characteristics are present in Table 1.
Table (1):
Population characteristics
| Characteristic | No. | Percentage |
|---|---|---|
| Age (M ± SD) | 28.62 ± 9.61 | |
| Age groups | ||
| >50 | 17 | 6.8 % |
| 41-50 | 13 | 5.2 % |
| 31-40 | 32 | 12.8 % |
| 21-30 | 178 | 71.2 % |
| ≤ 20 | 11 | 4 % |
| Gender | ||
| Male | 65 | 26 % |
| Female | 186 | 74 % |
| Education | ||
| Elementary | 3 | 1.2 % |
| Secondary | 90 | 36 % |
| Higher | 155 | 62 % |
| Vocational | 3 | 1.2 % |
| Total | 251 | 100 % |
Knowledge, attitude and practice towards legionellosis
Table 2 shows the percentage of correct answers for the items assessing the participants’ knowledge among study participants. The percentage of correct answers ranged from 10.4% in item number 11 (What is the Legionnaires’ disease fatality rate without treatment?) to as high as 76.8% in item number 6 (How is Legionella spp. infection is transmitted?). These items were considered the main challenge and strength in participants’ knowledge of legionellosis, respectively. The overall mean score differences were tested using one-way ANOVA. Significant differences were found for age and education (p = 0.01 and p < 0.001, respectively). However, no significant difference was found between genders (Table 3).
Table (2):
Respondents’ knowledge and attitudes about legionellosis
Question item |
No. |
% |
|---|---|---|
1. What is the meaning of the term legionellosis? |
87 |
34.8 |
2. To which systematic group legionellosis belongs? |
163 |
65.2 |
3. In what water sources do you think there is a higher risk of Legionella spp.? |
146 |
58.4 |
4. In what months of the year is there an increase in Legionella spp. infections? |
101 |
40.4 |
5. What sources do we use to obtain information on the incidence of legionellosis in Poland? |
149 |
59.6 |
6. How is Legionella spp. infection is transmitted? * |
192 |
76.8 |
7. To which group of diseases does legionellosis belong? |
109 |
43.6 |
8. How long is the incubation period for legionellosis? |
86 |
34.4 |
9. What is the best treatment for Legionella spp. Infection? |
142 |
56.8 |
10. What are the main illnesses caused by Legionella spp.? |
76 |
30.4 |
11. What is the Legionnaires’ disease fatality rate without treatment? ** |
26 |
10.4 |
12. Does Legionnaires’ disease have to be reported to the Poviat Sanitary and Epidemiological Stations |
119 |
47.6 |
*Item 6 shows the highest score and is considered the main strength among participants’ knowledge
**Item 11 shows the lowest score and is considered the main challenge among participants’ knowledge
Table (3):
Mean overall score of participants’ responses with respect to the demographic characteristics
| Characteristic | Mean ± SD | P- value | |
|---|---|---|---|
| Age | < 20 | 5.40 ± 0.97 | 0.01 |
| 21-30 | 6.41 ± 2.06 | ||
| 31-40 | 5.94 ± 2.21 | ||
| 41-50 | 4.62 ± 1.45 | ||
| > 50 | 5.53 ± 2.27 | ||
| Gender | Male | 5.98 ± 2.02 | 0.432 |
| Female | 6.22 ± 2.10 | ||
| Education | Elementary | 5.69 ± 1.82 | <0.001 |
| Secondary | 6.96 ± 2.28 | ||
| Higher | 7.00 ± 2.65 | ||
| Vocational | 5.67 ± 0.58 | ||
When the level of knowledge about legionellosis was examined against different characteristics of the study population (Table 4), no significant differences were found between different ages, genders or education levels. However, differences in knowledge about the sources of, diagnosis and treatment of legionellosis infection were identified. Participant’s knowledge about prevention of infection was relatively high compared to other knowledge areas (X2= 202.95, p <0.001).
Table (4):
The level of knowledge based on gender, age, and education
| Characteristic | Knowledge level | Chi 2 | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Low (Score 0-7) | Moderate (Score 8-9) | High (Score 10-12) | ||||||
| No. | % | No. | % | No. | % | |||
| Age groups | ||||||||
| >50 | 10 | 58.8 % | 7 | 41.2 % | 0 | 0.0 % | 11.2 | 0.189 |
| 41-50 | 12 | 92.3 % | 1 | 7.7 % | 0 | 0.0 % | ||
| 31-40 | 26 | 81.3 % | 5 | 15.6 % | 1 | 3.1 % | ||
| 21-30 | 126 | 70.4 % | 36 | 20.1 % | 17 | 9.5 % | ||
| ≤ 20 | 6 | 60.0 % | 3 | 30.0 % | 1 | 10.0 % | ||
| Gender | ||||||||
| Male | 51 | 78.5 % | 9 | 13.8 % | 5 | 7.7 % | 2.55 | 0.278 |
| Female | 129 | 69.4 % | 43 | 23.1 % | 14 | 7.5 % | ||
| Education | ||||||||
| Elementary | 2 | 66.7 % | 1 | 33.3 % | 0 | 0.0 % | 3.01 | 0.807 |
| Secondary | 61 | 67.8 % | 20 | 22.2 % | 9 | 10.0 % | ||
| Higher | 114 | 73.5 % | 31 | 20.0 % | 10 | 6.5 % | ||
| Vocational | 3 | 100 % | 0.0 | 0.0 % | 0 | 0.0 % | ||
| Knowledge about different aspects of legionella infection | ||||||||
| Source of infections | 171 | 68.1 % | 54 | 21.5 % | 26 | 10.4 % | 203 | < 0.001 |
| Diagnosis and treatment | 193 | 76.9 % | 50 | 19.9 % | 8 | 3.2 % | ||
| Prevention | 132 | 52.6 % | 0 | 0 % | 119 | 47.4 % | ||
The percentages were given as percent of rows.
Association between population characteristics and knowledge about legionellosis
A linear regression analysis was carried out to assess the association between population characteristics and the overall score as a continuous variable. The overall regression model was significant (F = 13.15, p <0.001, R2 = 0.127). Age was found to be negatively associated with knowledge about legionellosis, with each 1-year increase in age correlating a decrease of 0.049 points in the overall score (p <0.001). Higher education was a significant predictor for a better understanding of the disease compared to other education levels (B= 1.65, p <0.001). However, gender was found to be a non-significant factor (Table 5).
Table (5):
Linear regression showing the association between population characteristics and the overall knowledge score
Characteristic |
B |
SE |
95% |
CI |
P value |
|---|---|---|---|---|---|
Age |
-0.049 |
0.013 |
-0.075 |
-0.023 |
<0.001 |
Gender |
|||||
Male |
Ref |
||||
Female |
-0.577 |
0.312 |
-1.193 |
0.038 |
0.066 |
Education |
|||||
Others* |
Ref |
||||
Tertiary education |
1.656 |
0.288 |
1.088 |
2.223 |
<0.001 |
B: unstandardized coefficient, SE: standard error, CI: confidence interval
*Others include: elementary, secondary and vocational education
Binary logistic regression was conducted to identify the predictors of correct answers for the previously identified strength and limitation in the participants’ knowledge of the disease. For item number 11 (What is the Legionnaires’ disease fatality rate without treatment?), age was found to be a significant predictor for more correct answers (AOR = 1.04, 95% CI 1.01 – 1.08). No effect was found for gender and education level. On the other hand, age showed a negative association (AOR = 0.95, 95% CI 0.92 – 0.98) with item no.6 (How is Legionella spp. infection is transmitted?), while higher education was associated with better knowledge (AOR = 3.75, 95%CI 1.79 – 7.86) (Table 6).
Table (6):
Binary logistic regression showing the association between population characteristics and the main challenge and strength among participants’ knowledge of legionellosis
| Characteristic | 11. What is the Legionnaires’ disease fatality rate without treatment? (R2 = 0.08) | 6. How is Legionella spp. infection is transmitted? (R2 = 0.21) | ||||||
|---|---|---|---|---|---|---|---|---|
| AOR | 95% | CI | P value | AOR | 95% | CI | P value | |
| Age | 1.04 | 1.01 | 1.08 | 0.022 | 0.95 | 0.92 | 0.98 | 0.001 |
| Gender | ||||||||
| Male | Ref | |||||||
| Female | 1.09 | 0.33 | 3.56 | 0.890 | 0.73 | 0.28 | 1.93 | 0.528 |
| Education | ||||||||
| Others | Ref | |||||||
| Tertiary education | 2.31 | 0.89 | 6.00 | 0.085 | 3.75 | 1.79 | 7.86 | <0.001 |
AOR: adjusted odds ratio, CI: confidence interval
*Others include: elementary, secondary and vocational education
Perceived knowledge about different aspects of legionellosis infection
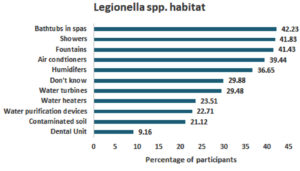
When queried about potential for legionella, most participants identified that legionella habitat included bathtubs in spas (n = 106, 42.2%), showers (n = 105, 41.83%) and fountains (n = 104, 41.4%) while dental units were the least recognized (n = 23, 9.16%) (Figure 1).
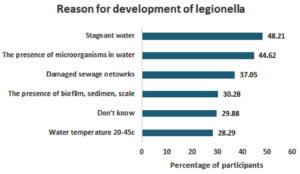
In terms of understanding the main conditions facilitating the development of legionellosis, stagnant water was the most common response (n = 121, 48.21%), whereas a water temperature between 20-45 °C (n = 71, 28.29%) was the least acknowledged (Figure 2).
Figure 2. Participants’ Perceptions, Attitudes and Beliefs Regarding the Reasons for the Development of Legionellosis
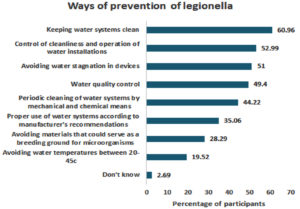
Most participants believed that maintaining cleanliness in water systems and consistent control of cleanliness were the primary prevention methods for legionellosis infection (60.69% and 52.99%, respectively) (Figure 3).
Figure 3. Participants’ Perceptions, Attitudes and Beliefs Regarding the Ways to Prevent Legionellosis
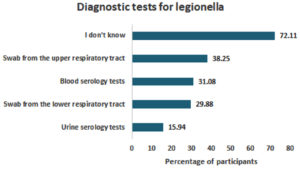
Interestingly, the majority of participants (n = 180, 72.11%) subjects were not aware of the correct diagnostic tests for legionellosis. A nasal swab was the second most common choice (n = 96, 38.25%), while urine tests were the least recognized (n = 40, 15.94%) (Figure 4).
Figure 4. Participants’ Perceptions, Attitudes and Beliefs Regarding the Tests Performed to Diagnose Legionellosis
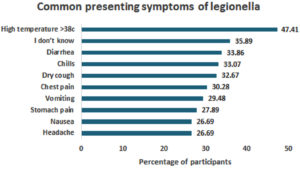
When it came to symptoms, fever was considered the most common When it came to symptoms, fever was considered symptom of legionellosis infection (n = 119, 47.41%), while nausea and headache were deemed the least common symptoms (n= 67, 26.69%) (Figure 5).
Figure 5. Participants’ Perceptions, Attitudes and Beliefs Regarding the Symptoms of Legionella sp. infection.
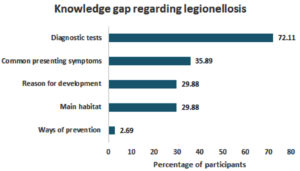
Knowledge gap among study participants
Notably, the study revealed a significant knowledge gap regarding diagnostic tests among participants; over two thirds (72.11%) selected “I don’t know” as their response to this question. On the other hand, awareness about prevention methods was quite high, as only a small percentage (2.69%) reported not knowing how to prevent legionellosis infection (Figure 6).
Legionellosis is caused by a bacterium that has been living in our environment at least since the second half of the 20th century when it was discovered in Philadelphia.11 Every year, we notice an increase in the number of cases of diseases caused by Legionella spp. in the world.12 Consequently, mandatory case reporting is in place in many countries. However, as this is not a common practice, actual statistics are not known, and the lack of official cases of Legionnaires’ disease and Pontiac fever in some regions may not be indicative of the infection rates in the population.13
Our survey demonstrated that the Polish adults’ level of knowledge about Legionnaires’ disease is low. This finding can be potentially attributed to the fact that Poland has one of the lowest rates of both diseases. Still, due to the lack of reports, it is not possible to ascertain the true figures.14 The study participants seem to be aware of this fact, as they largely concurred that all cases of diseases caused by Legionella spp. should be reported to the Sanitary and Epidemiological Station.
Furthermore, our study illustrated that advanced age was negatively associated with knowledge about Legionnaires’ disease, demonstrating the special need for awareness campaigns in these older populations. Our study also revealed that people with tertiary education (or above) showed better knowledge than others, and thus information about these bacteria should be presented in a simple manner suitable for people with less education. In contrast to the prevention method, we found that there was a high knowledge gap regarding diagnostic tests for Legionnaires’ disease. This is probably because prevention measures are overlapping and generalizable for most diseases.
Noteworthy, as far as we know, our current work is the first to assess the awareness and knowledge of population towards these bacteria. However, a Japanese study attributed an outbreak of Legionella pneumonia to insufficient knowledge and understanding about Legionella and Legionnaires’ disease.15
This topic is important because these bacteria can be found in any setting, especially in public buildings, where a large percentage of hot water installations are contaminated by microbiological microorganisms, including Legionella spp.16 The highest percentages of bacteria occurrence are recorded in multi-family buildings and medical facilities.17 Consuming drinking water contaminated with Legionella also carries a high risk of infection.18 While some studies indicate that about 30% of buildings can be contaminated, the quantity of bacteria is likely to vary considerably.19 With polluted taps, even a clean central water supply promotes bacterial colonisation.20 Carrying out preventive measures and monitoring facilities will thus contribute to minimising the risk of infection.20 Currently, we also have records of procedures for prophylaxis and prevention of the occurrence of legionella infections.21 Extant research also shows that hospitals are the most likely sources of bacteria, which are least present in hotels.22 On the other hand, social housing units and dormitories pose a greater risk of bacterial contamination.22
Members of the Legionella family have been found in the water systems of hospitals in Poland as well as worldwide (in the water supply, as well as air-conditioning units, swimming pools, and balneotherapy facilities).23 Thus, greater control of the water supply networks is essential, given that nosocomial infections can pose threat to already ill individuals that are hospitalised for other reasons, as well as medical staff.23 However, as legionellosis symptoms are similar to those of pneumonia, detailed urine and blood tests are needed, thus potentially delaying diagnosis and treatment.24 Consequently, regular internal water quality control must be implemented. However, disinfection and destruction of microbes can be challenging, especially in low flow systems and in dead branches.25 Nevertheless, when carried out accurately, chemical disinfection will eliminate the accumulation of bacteria in water systems.26
It is well known that delayed diagnosis and treatment contribute to extending the hospitalisation time of patients infected with Legionella spp.27 While some of these patients may be treated in internal or pulmonary wards, the average hospital stay must last up to 14 days.28,29 As a result, during summer months, when rising temperatures contribute to the growth of bacteria in water systems, patient admissions for legionellosis treatment may cause overcrowding.29
Sewage network inspection and decontamination programs should also be applied not only in the facilities that have been inspected in the past, but also to newly opened facilities. A particular focus should be given to dental units, where microbes can be present not only in water systems but also on infrequently used instruments.30 Thus, in addition to implementing appropriate sanitary measures, dental staff should wear face and eye protection during any treatment, because inhalation of contaminated aerosols carries the greatest risk of infection.
Legionellosis is a severe pneumonia caused by Legionella spp. bacteria. Nearly 95% of patients necessitate hospitalization, and 10% die. However, there is no study showing the awareness and knowledge of population towards these bacteria. Thus, this cross-sectional study was conducted to evaluate the knowledge, attitude and perception of the Polish population towards legionellosis, and to detect factors affecting the knowledge and perception scores among the residents. Given the low level of knowledge about legionellosis among Polish adults, it is necessary to implement population education initiatives in this country. Specifically, it is essential to educate the population about Legionella infections, diagnosis, treatment and infection prevention, as well as the presence of Legionella spp. in the environment, including the water environment of residential houses (bathrooms). Moreover, attention should be paid to bacterial colonisation in the healthcare environment. However, given that the study sample was relatively small, these assertions should be corroborated through further studies involving larger cohorts.
ACKNOWLEDGMENTS
The authors would like to thank Al-Ahliyya Amman University, Amman, Jordan, for the financial support granted to cover the publication fee of this research article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
NS, ZL, IK, KK and MA conceptualized the study. NS, ZL, IK, KK and MA applied methodology. ZL and IK performed investigation. NS and IK performed software analysis. NS, ZL and IK performed formal analysis. MA validated the data. NS, ZL, IK, KK and MA wrote original draft. NS, ZL, IK and MA wrote, reviewed and edited the manuscript. IK and MA performed supervision. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Crespi S, Drasar V, Salva-Serra F, et al. Legionella maioricensis sp. nov., a new species isolated from the hot water distribution systems of a hospital and a shopping center during routine sampling. Int J Syst Evol Microbiol. 2023;73(1):005686.
Crossref - Haupt TE, Heffernan RT, Kazmierczak JJ, et al. An outbreak of Legionnaires’ disease associated with a decorative water wall fountain in a hospital. Infect Control Hosp Epidemiol. 2012;33(2):185-191.
Crossref - Khajezadeh M, Mohseni F, Khaledi A, Firoozeh A. Contamination of dental unit water lines (DUWL) with Legionella pneumophila and Pseudomonas aeruginosa; A Middle East systematic review and meta-analysis. Eur J Microbiol Immunol. 2023;12(4):93-99.
Crossref - Burke AC, Gina W, Muhammed R. Clinical Diagnosis of Legionnaire’sDisease: Six Characteristic Clinical Predictors. Am J Med. 2015;128(7):e21-22.
Crossref - ECDC Legionnaires’ disease Annual Epidemiological Report for 2019. European Centre for Disease Prevention and Control, Stockholm. 2019.
- Pancer K, Stypułkowska-Misiurewicz H. Epidemiology of diseases caused by Legionella spp. Nowa Medycyna. 2009;1:61-65.
- Guzek A. Legionellosis – a disease rarely diagnosed in Poland. Polish pharmacist; 2020. https://www.aptekarzpolski.pl/wiedza/legionellosis-choroba-rzadko-rozpoznawana-w- polsce/ [accessed January 18, 2022].
- Schwake DO, Alum A, Abbaszadegan M. Legionella occurrence beyond cooling towers and premise plumbing. Microorganisms, 2021;9:2543.
Crossref - Prous DY, Garcםa MJ, Salvanes FR, Ortells LC. Validation of questionnaires. Reumatologia Clinica (English Edition), 2009; 5(4):171-177.
Crossref - World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA, 2013;310(20):2191-2194.
Crossref - Zayed AR, Dina MB, Steinert M, et al. Comparative Genomics of Legionella pneumophila Isolates fromthe West Bank and Germany Support Molecular Epidemiology of Legionnaires’ Disease. Microorganisms. 2023;11(2):449.
Crossref - Samuelsson J, Hallstrom LP, Marrone G, Dias JG. Legionnaires’ disease in the EU/EEA*: increasing trend from 2017 to 2019. Euro Surveill. 2023;28(11):2200114.
Crossref - Agrawal A, Bhattacharyya S. Microorganisms in Pathogenesis and Management of Acute Disseminated Encephalomyelitis (ADEM). In: Dwivedi, M.K., Sankaranarayanan, A., Kemp, E.H., Shoenfeld, Y. (eds) Role of Microorganisms in Pathogenesis and Management of Autoimmune Diseases. 2023 (pp. 211-237). Springer, Singapore.
Crossref - Stypulkowska-Mirusiewicz H, Czerwinski M. Legionellosis in Poland in 2017. Epidemiolog Rev. 2019;73(2):151-155.
Crossref - Yabuuchi E, Agata K. An outbreak of legionellosis in a new facility of hot spring bath in Hiuga City. Journal of the Japanese Association for Infectious Diseases, 2004;78(2):90-98.
Crossref - Brune M. Legionnaires’ Disease and Water Systems: History and Prevention. Lebensmittelchemie. 2023;77(3):65-77.
- Marcham, CL. White Paper on Engineering Controls for Bioaerosols in Non-Industrial/Non-Healthcare Settings. American Conference of Governmental Industrial Hygienists (ACGIH®) Bioaerosols Committee. 2021.
- Mentula S, Kaariainen S, Jaakola S, et al. Tap water as the source of a Legionnaires’ disease outbreak spread to several residential buildings and one hospital, Finland, 2020 to 2021. Euro Surveill. 2023;28(11):2200673.
Crossref - Kruse E-B, Wehner A, Wisplingoghoff H. Prevalence and distribution of Legionella spp in portable water system in Germany, risk factors, associated with contamination, and effectiveness of thermal disinfection. Am J Infect Control. 2016;44(4):470-474.
Crossref - Zimoch I, Paciej J. Spatial analysis of the health risk caused by the presence of Legionella bacteria in hot water systems in the Silesian Voivodeship. Environmental Protection, 2014;4(36):23-28.
- Kaiser K. Legionella in internal water supply systems. Market Installer, 2013; 1-2: 79-83.
- Kokkinos K, Gogos P, Alamanos CY, Vantarakis A. Prevalence of Legionella spp. in water systems of hospitalsand hotels in South Western Greece. Int J Environ Health Res. 2012;22(4):340-354.
Crossref - Bongiovanni A, Colazingari V, Messineo A, Del Cimmuto A, De Giusti M, La Torre G. Can legionellosis be considered an occupational risk in the healthcare sector? A systematic review and meta-analysis. Public Health. 2023;214:31-37
Crossref - Juda T. Legionella – experiences of the Provincial Specialist Hospital in Jastrzךbie Zdrףj -clinical cases. Borgis-New Medicine 2009; 1:68. http://www.nowamedycyna.pl
- Cai Y, Sun, T, Li G, An T. Traditional and emerging water disinfection technologies challenging the control of antibiotic-resistant bacteria and antibiotic resistance genes. Acs Es&T Engineering. 2021;1(7):1046-1064.
Crossref - Torato M, Valentini P, Costa AL, Giorgi S, Casini B, Baggiani A. Rate of Legionella pneumophilia colonizationin hospital hot water Network after time flow taps installation. J Hosp Infect. 2018;98(1):60-63.
Crossref - Viasus D, Gaia V, Manzur-Barbur C, Carratala J. Legionnaires’ disease: update on diagnosis and treatment. Infect Dis Ther. 2022;11(3):973-986.
Crossref - Schweitzer VA, van Heijl I, Boersma WG, et al. Narrow-spectrum antibiotics for community-acquired pneumonia in Dutch adults (CAP-PACT): a cross-sectional, stepped-wedge, cluster-randomised, non-inferiority, antimicrobial stewardship intervention trial. Lancet Infect Dis. 2022;22(2):274-283.
Crossref - Wojtyła-Buciora P, Chrzanowska E, Marcinkowski JT. Occurrence of Legionella sp. in hot water installations incare facilities healthcare and public buildings. Hygeia Public Health. 2013;48(3):327-332.
- Bzdęga W, Trykowski J, Bzdęga J. System disinfection. water and compressed air of the dental unit. Nowiny Lekarskie. 2007;76(5):436-439.
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.