ISSN: 0973-7510
E-ISSN: 2581-690X
A total of 50 root colonising bacterial endophytes were identified from a medicinal plant in this investigation (Barleria lupulina), and characterized based on morphologically selected isolates, BLR41 were Gram-negative motile, rod-shaped and BLR45 was Gram-positive, rod-shaped, and light yellow in color. The BLR41 isolate was motile and the BLR45 was positive for indole. The strains BLR41 and BLR45 were positive for citrate, amylase, protease, and lipase. Among all the isolates, BLR41 and BLR45 exhibited higher enzymatic test positive in citrate, amylase lipase, and protease. Furthermore, PGP efficacy as maximum production of zinc in BLR41 isolate and phosphate solubilization in BLR45. Solubilization of the highest zinc (2.80 μg/mL) was obtained by the isolate BLR41, followed by BLR23 and then BLR35. The highest level of phosphate (2.60 μg/mL) was recorded with the BLR45, followed by the BLR07 and BLR18. A consortium of bacterial strains performed better results than individual inoculation. The isolate BLR41 and BLR45 were identified as Bacillus australimaris and Enterobacter kobei following the 16S rRNA sequencing. Among all, seed germination was induced by 93% in consortium BLR41+BLR45, compared to control (T1). An increase in the shoot length by 30% was recorded with the treatment of BLR41+BRL45, BLR41 increase (54%), and BLR45 (35%), respectively. On the other hand, the consortium of mixed cultures, BLR41+BLR45, showed an increased fresh and the weight of dry 65% and 58%. The findings of this study indicate that the consortium of B. australimaris BLR41 and E. kobei BLR45 significantly enhances plant growth in the Pot experiment by zinc and phosphate solubilization properties.
Endophytic Bacteria, Phosphate Solubilization, Bacillus australimaris, Enterobacter kobei 16S rRNA Sequencing
Endophytic bacteria are beneficial to plants because they colonized the interior tissues of plants without causing damage to the plants. Few endophytes have been described, despite the fact that there are nearly three million different plant species on the earth.1 Plant-related endophytic bacteria may alter the physiochemical environment of microbial populations associated with medicinal plants inside the rhizosphere. Endophytic bacteria have received more attention in recent years in terms of plant health and growth than rhizospheric bacteria due to their close association with the root surface of host plants and increased tolerance to abiotic and biotic stresses.2 There is the potential for the development of positive endophytes and the exploitation of various genera living in various ecosystems. Endophytes promote plant growth by producing phytohormones, the most well-studied of which are auxins and gibberellins from root-associated endophytes.
Acetobacter, Herbaspirillum species, Diazotrophicus, and Azoarcus are root endophytes that play a major part in the formation of 2,3-butanediol and acetoin, which stimulate increase of plant life and fix biological nitrogen compounds, such as ammonium and nitrate, in a host plant.3 Bacterial endophytes have enormous potential because they can reduce the impact of chemical fertilization, particularly nitrogen fertilization, in agriculture. The utilization of naturally occurring symbionts, such as bacterial endophytes, in plant growth potentially lower the use of chemical fertilizers and make it more environmentally sustainable. Bacterial endophytes generate antibiotic chemicals that help the host plant acquire resistance or tolerance by creating siderophores, competing for space and resources, and modifying plant resistance responses.1 Screening root endophytic bacterial species for effective PGPB strains is thus both interesting and necessary. Several PGP-related characteristics were tested together during the initial screening process in the search for effective PGPR strains.
Medicinal plants have long been used in traditional ones medicine in many countries. Traditional medicine is defined as a collection of ideas, beliefs, and experiences-based behaviors from various cultures. Barleria cristata, B. optusa, B. prionitis, B. lupulina, and other Acanthaceae plant species are important. B. lupulina is a fast-growing perennial plant that is widely sold as an ornamental. It is indigenous to India and is widely distributed in the southern and western parts of the country. Furthermore, B. lupulina decoction is used to treat a variety of human ailments due to its antioxidant, antibiotic, and immunomodulatory properties.4
The objective of the research was to identify root-associated endophytic bacteria from medicinal plant of B. lupulina and assess the zinc and phosphate solubilization of individual and endophytic bacterial consortiums, which could improve plant productivity.
Isolation of Endophytic Bacteria from Roots of B. lupulina
Young and healthy roots of B. lupulina plants were collected from the Botanical Garden, Department of Botany and Microbiology, Gurukul Kangri (Deemed to be University), Haridwar (29°55′18′′N 78°7′39′′E/29.92167°N 78.12750°E), Uttarakhand (India) (Figure 1). Root samples were washed multiple times with tap water after drying at room temperature to eliminate any remaining dirt on the surface of them. The root samples were then disinfected with 70% ethanol for 3-5 minutes, followed by 4% NaOCl for 2 minutes, to remove any surface bacteria. Finally, the roots were rinsed 5-6 times with double distilled water for 2-5 minutes to eliminate any leftover contaminants from the plant. Surface sterilized root samples were cut into small pieces and serially diluted in sterile distilled water before being spread on the surface of nutrient agar medium described a standard protocol for isolating endophytic bacteria. For two days, incubation was place at 37°C.5 colonies of bacteria with distinct physiological characteristics were selected and purified on nutrient agar plates.
Evaluation of Phosphate and Zinc Solubilizing Properties of Endophytic Bacteria
On the basis of the respective growth in the following medium ranges, the zinc and phosphate solubilization properties of bacterial isolates were studied.6
Phosphate Solubilization
The isolates were evaluated for phosphate solubilization using the Pikovskaya’s medium. The isolates of bacteria cultured on the Pikovskaya medium showing a clear halo zone around the colonies were evaluated qualitatively for phosphate solubilization activity by opting for the yellow colour method.7 For quantitative analysis, Bacterial strains that had developed for 24 to 36 hours in fresh cultures were inoculated into 50 mL of Pikovskaya’s broth, cultured for 48 hours, and centrifuged. Supernatant 5 mL was collected with 5 mL of tri-calcium orthophosphate for solubilization as well as incubated for colour development. The culture supernatant was taken to determine the soluble zinc content by using a spectrophotometer (UV-1601 Shimadzu).
Zinc Solubilization
Root endophyte cultures were inoculated spot on sterile Petri dishes containing sterilized Bunt and Rovira medium followed by incubation at 37°C for 2-4 days. The formation of halo zones surrounding the colonies suggested the capacity of bacterial cultures to solubilize zinc. The size of the developed clear halo zones was calculated and recorded. Cultures forming halo zones were then quantified in basal broth medium according to the method as defined by Bunt and Rovira 1955). The Bacterial cultures were transferred into flasks containing sterilized broth in triplicate and then incubated at 37°C for seven days. After that, the medium was centrifuged for 10 minutes at 10,000 rpm. The supernatant of culture was taken to determine of the soluble zinc content by using a spectrophotometer (UV-1601 Shimadzu). The quantity of solubilized zinc in the inoculated samples was compared to that of the uninoculated control and was expressed as μg/mL.8
Biochemical and Morphological Characterization of Selected Endophytic Bacteria
Bergey’s Manual of the Determinative Bacteriology was used to identify the morphological characteristics of selected bacterial isolates.9 The selected root endophytes were characterized for the motility of the individual isolates by measuring in 0.4% sulphide indole motility agar medium. The amount of ammonia that was produced was calculated with the use of Nessler’s reagent and peptone water, according to the procedure that was provided. Tryptone broth was inoculated with bacterial cultures and incubated for the indole test for 72 hours at 30°C. During the incubation period, addition and mixing of Kovac’s reagent was done to confirm the synthesis of indole. For the citrate utilization test, the bacterial isolates were inoculated on Simmon’s citrate agar medium and then incubated for 96 h at 30°C.10 Activity with urease was evaluated as a result of the method.11 The amylase production test uses iodine solution with starch agar medium.12 Tween 20 peptone agar was used to test lipase activity, while glucose yeast peptone agar with gelatin was used to measure protease activity.13 The catalase test was carried out by putting a freshly developed (24-hour-old) bacterial culture on (3 %) H2O2.14 Gram staining was performed with 48 h grown culture and shape of the bacterial cell was observed.15
Affinity of Phosphate Solubilizing Bacteria (PSB) and Zinc Solubilizing Bacteria (ZSB) Isolated from Roots of B. lupulina
This test was carried out to determine the compatibility of PSB and ZSB isolates. For non-compatible combinations, the solubilizing zinc was simultaneous to the phosphate-solubilizing isolates, and growth suppression was seen at the site of conjunction after 48 hours of incubation at 37°C with the phosphate-solubilizing isolates streaked on general-purpose Pikovskaya’s Agar Medium plates.16
Molecular Identification of Selected Endophytic Bacterial Isolates
The bacterial isolates that showed potent outcomes in pot trial studies were identified by 16S rRNA sequencing by following Liu et al. 17 Muscle alignment was done by using MEGAX software, as previously adopted by Kumar et al.18 The neighbour-joining method was followed for the construction of the phylogenetic tree.19
Scanning Electron Microscope Analysis
A scanning electron microscope was used to perform morphological and elemental characterization on endophytic bacteria (Zeiss, SEM, India). A multi-mineral sample was used to calibrate the SEM. The flesh from endophytic bacteria were scraped using a sterile blade and forceps and immersed in 10X PBS (pH 7) for sample preparation (phosphate buffer saline). Thin transverse sections were cut after the piece was put on a clean glass slide. The material was 3.5% glutaraldehyde treated and kept at 4°C overnight. Using ethanol gradients, the sample was dehydrated by 60%, and then by 80%.20
Screening of Potential PSB and ZSB Isolates and Effect of their Consortium on Growth of B. lupulina in Pot Assay
The stimulation was compared between the efficiency of the PSB and ZSB in promoting growth of B. lupulina seedlings in pot trials by inoculating the individual bacterial isolates as well as their consortium. The B. lupulina seeds were procured from mature plants and surface sterilization was done by 95% ethanol and NaOCl (4%) and finally washed with sterilized distilled water. The seeds were grown in four groups of five seeds each in a randomized sequence design: T1- B. lupulina seeds, untreated as control, T2-seeds + BLR41, T3-seeds + BLR45, and T4-seeds + BLR41 + BLR45. All of the T1 sets were not treated by any of the bacterial isolates. Seed germination was recorded every day after the seed germinated till complete germination was achieved. The assessment of early vegetative parameters viz., root length, stem length, and the wet and dry weights of the seedling are all measured was carried out after 30, 60, 90 days of sowing.21 Based on the findings of the pot trial research, the best prospective isolates were chosen and described in the laboratory.
Evaluation of Zinc and Phosphate-solubilizing Endophytic Bacteria
The B. lupulina plant was used to screen a total of fifty endophytic bacterial isolates, which were given the codes BLR01–BLR50. Five isolates (BLR03, BLR23, BLR35, BLR41, and BLR48) were zinc solubilizes while five isolates (BLR07, BLR18, BLR38, BLR45, and BLR50) were phosphate solubilizes as concise halo zones formed around their respective colonies (Figure 2). In addition, the zinc-solubilizing isolates (BLR03, BLR23, BLR35, BLR41, and BLR48) successfully dissolved the insoluble zinc complex by generating halo zones ranging from 6.48 to 12.46 mm in diameter. BLR41 formed the most halo zones, followed by BLR35. Both isolates exceeded the other isolates statistically significantly. All inoculated tubes had significantly higher solubilized zinc concentrations than the uninoculated control, and that was no effect on the results in the control. However, the isolate BLR41 solubilized the most zinc (2.80 μg/mL), followed by BLR23 and BLR35 (Table 2). Moreover, the isolates BLR07, BLR18, BLR38, BLR45, and BLR50 solubilized the phosphate, forming a halo zone with a diameter of 6.62-11.24 mm. The maximum phosphate solubilization was done by BLR45, which was similar to BLR23 and BLR38. The data of phosphate solubilization index (PSI) showed the highest index with BLR45, followed by BLR23 and BLR38. The selected isolates were then assessed for phosphate solubilization. In the broth assay, all of the isolates exhibited phosphate solubilization. The highest level of solubilization (2.60 μg/mL) was recorded with the BLR45, followed by the BLR07 and BLR18 (Table 1).
Table (1):
Zinc solubilization by plant promoting root endophytic bacteria.
| No. | Isolates | Zinc solubilization (Qualitative) | Zinc solubilization (Quantitative) | ||
|---|---|---|---|---|---|
| Zinc halo zone (mm) | ZSI | Z Conc. (μg/mL) | |||
| 1 | Control | N.A | N.A | N.A | N.A |
| 2 | BLR03 | + | 9.24 | 10.24 | 1.8 |
| 3 | BLR11 | + | 6.48 | 7.48 | 1.2 |
| 4 | BLR23 | + | 7.24 | 8.24 | 1.4 |
| 5 | BLR35 | + | 8.35 | 9.35 | 1.6 |
| 6 | BLR41 | + | 12.46 | 13.46 | 2.8 |
Table (2):
Phosphate solubilization by plant promoting root endophytic bacteria.
| No. | Isolates | Phosphate solubilization (Qualitative) | Phosphate solubilization (Quantitative) | ||
|---|---|---|---|---|---|
| Phosphate halo zone (mm) | PSI | P Conc. (μg/mL) | |||
| 1 | Control | N.A | N.A | N.A | N.A |
| 2 | BLR07 | + | 8.15 | 9.15 | 2.1 |
| 3 | BLR18 | + | 9.34 | 10.34 | 1.7 |
| 4 | BLR27 | + | 6.62 | 7.62 | 2.3 |
| 5 | BLR38 | + | 7.42 | 8.42 | 1.8 |
| 6 | BLR45 | + | 11.24 | 12.42 | 2.6 |
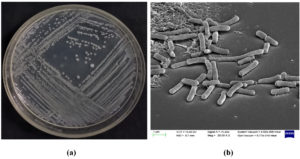
Figure 2. BLR41 strain Bacillus australimaris (a) Streaking plate and (b) Scanning electron microscope image
Biochemical and Morphological Characterization of Endophytic Bacteria
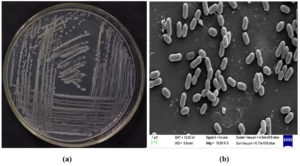
The morphologically selected isolates, BLR41 were gram-negative motile, and rod-shaped (Figure 2). BLR45 was Gram-positive, rod-shaped and lightly yellow in color (Figure 3 and 4). The BLR41 isolate was motile and the BLR45 was positive for indole. Catalase and ammonia production were found to be positive in the isolates. Urease production was observed with BLR41. The strains BLR41 and BLR45 were positive for citrate, amylase, protease, and lipase (Table 3).
Table (3):
Biochemical characterization of root endophytic bacteria.
| Biochemical Characteristics | Strain | ||
|---|---|---|---|
| BLR41 | BLR45 | ||
| Gram staining | + | – | |
| Cell shape | Circular | Rod | |
| Motility test | + | – | |
| Ammonia test | + | – | |
| Indole test | + | – | |
| Citrate production | + | + | |
| Urease production | + | – | |
| Amylase production | + | + | |
| Lipase production | + | + | |
| Protease production | + | + | |
| Catalase production | + | – | |
Figure 3. BLR45 strain Enterobacter kobei (a) Streaking plate and (b) Scanning electron microscope image
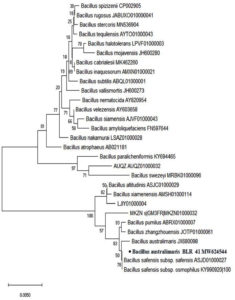
Figure 4. Isolate BLR41 exhibits 99% similarity with Bacillus australimaris (accession number MW624544)
Molecular Identification of Selected Endophytes
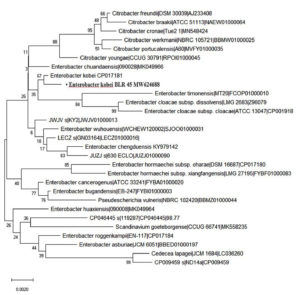
Using 16S rRNA sequencing, the isolates that promote plant growth were identified. The gene sequences revealed that isolate BLR41 resembled Bacillus australimaris 99% of the time and isolate BLR45 resembled Enterobacter kobei 98.55% of the time. The sequences were submitted to GenBank and assigned the accession numbers MW624544 and MW624688 (Figure 4 and 5).
Figure 5. Isolate BLR45 exhibits 98.55% similarity with Enterobacter kobei (accession number MW624688).
Effect of ZSB and PSB Isolates on Growth of B. lupulina in Pot Experiment
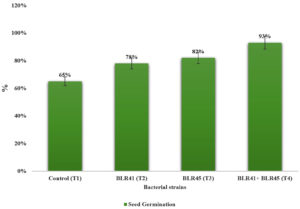
The results showed that most of the PSB and ZSB isolates, both alone and as a consortium, substantially (p<0.05) improved the growth of B. lupulina seedlings. (Table 4). All individual and combined applications as a consortium increased the shoot length in comparison to un-inoculated control plants. A consortium of bacterial strains performed better than individual inoculation, according to the findings. Improvement in maximum seed bacterization (93%) was resulted by the BLR41+BLR45 consortium. Among all, seed germination was induced by 93% in consortium BLR41+BLR45, followed by (T2) BLR41 and (T3) BLR45 as compared to (T1) control (Figure 6).
Table (4):
Effect of bacterial isolates on seedling germination and vegetative growth parameters of B. lupulina.
| Growth measurements* | |||||||
|---|---|---|---|---|---|---|---|
| Isolates | Seed Germination | Root length (cm) | Shoot length (cm) | Fresh root weight (g) | Fresh Shoot weight (g) | Dry root weight (g) | Dry shoot weight (g) |
| Control (T1) | 65% | 0.42±1.6 | 4.8±4.9 | 0.43±2.9 | 3.45±0.34 | 0.32±0.10 | 0.90±0.45 |
| BLR41 (T2) Bacillus australimaris | 78% | 0.61 ±1.8** | 6.6 ±4.4** | 0.64±0.20** | 4.42 ±3.73* | 0.52±0.023** | 0.23±0.36* |
| BLR45 (T3) Enterobacter kobei | 82% | 0.78 ±2.3* | 7.2 ±4.8** | 0.91±0.28** | 6.87 ±2.8** | 0.61±0.029* | 0.27±1.7ns |
| BLR41+BLR45 (T4) | 93% | 0.92 ±2.4** | 8.2 ±4.2** | 0.94±4.4** | 6.98±1.4** | 0.78±0.061** | 0.29±0.35** |
| SEM | 1.89 | 1.75 | 1.56 | 1.62 | 0.9 | 0.75 | 0.67 |
| CD at 1% | 3.56 | 1.52 | 1.62 | 1.26 | 0.69 | 0.38 | 0.82 |
| CD at 5% | 2.58 | 1.25 | 0.94 | 1.15 | 0.54 | 0.58 | 0.72 |
SEM = standard error mean; CD = Critical Difference, Control (Non-bacterized seeds) Values are mean of ten plant samples from each treatment; significance at 0.01 and 0.05 levels of analysis of variance (ANOVA) ** = significant at 0.01 level of LSD as compared to control; * = Significant at 0.05 level of LSD as compared to control; ns = Not Significant at 0.05 level of LSD as compared to control.
In pot trial studies, an increase in the parameters of seedling growth was recorded with plants where seeds were treated with BLR41 and BLR45 individually as compared to non-bacterized seeds. The consortium of BLR41+BLR45 significantly increased all the vegetative parameters. An increase in the shoot length by 30% was recorded with the treatment of BLR41+BRL45, BLR45 followed by BLR41 (54%) and BLR45 (35%), respectively. On the other hand, the consortium of mixed culture, BLR41+BLR45, showed an increased fresh and dry weight of 65% and 58% over control, inoculated individually, resulted in fewer BLR41 and BLR45, respectively, in the fresh and dry weight consortium as compared to its (Table 4).
In this study, the selected bacterial isolates were found to have distinct morphological characteristics. The capacity of the bacterial isolates to solubilize zinc and phosphate was used to describe them. Out of 50 isolates, five were able to solubilize phosphate, while five others were marked as zinc solubilizers. In addition to performing a quantitative phosphate solubilization experiment, these isolates were further treated, showing that the isolates BLR07, BLR18, BLR38, BLR45, and BLR50 solubilized the phosphate, forming a halo zone of 6.62-11.24 mm in diameter. Further, the zinc-solubilizing isolates (BLR03, BLR23, BLR35, BLR41, and BLR48) effectively solubilized by creating halo zones ranging from 6.48 to 12.46 mm around the insoluble zinc complex. The maximum zone was formed by BLR41, followed by BLR3522 have also reported a higher rate of Zn and P solubilization by Bacillus aryabhattai and Bacillus subtilis. Similarly, 81 isolates have also been reported to solubilize phosphate at a high rate.23 Rhizobacteria may be employed as bio-fertilizers if they have a wide range of plant growth-promoting qualities. A good example of a feature that helps plants thrive under harsh conditions is catalase activity.24 This study indicated that all of the isolates tested have catalase activity25. Five PSB isolates were tested for their catalase activity by observing the formation of gas bubbles when H2O2 was added to bacterial cells. Furthermore,26 it is observed the catalase activity of all seven phosphorus-solubilizing strains. The presence of bacteria in the rhizosphere that can produce ammonia indicates the ammonification process.27 All the selected isolates produced ammonia as well, which is a significant trait of PGPR in producing enzymes such as hydrolytic enzymes such as chitinase and proteases that contribute significantly to the biological control of plant diseases.28 All of the isolates tested positive for protease, whereas BLR41 and BLR45 tested positive for chitinase activity. Two selected isolates, B. australimaris and E. kobei, showed positive results for siderophore production. The selected PSB and ZSB isolates BLR41 and BLR45 were identified as Bacillus australimaris BLR41 and Enterobacter kobei BLR45, respectively. Catalase-positive bacteria that generate hydrolytic enzymes, exopolysaccharides, and ammonia. These are the strains that have been found Gram-negative rods and Gram-positive that possess phosphate and zinc solubility. All of these features are aimed towards enhancing the growth of B. lupulina seedlings in pot trials. Similar observations have also been reported in previous studies where the strains Enterobacter sp.,29 Kosakonia, Pluralibacter, Phytobacter, Klebsiella, Metakosakonia, Enterobacter, Lelliottia, and Kluyvera species were favourable in numerous plant growth aspects. The usage of Inoculants PSB and ZSB may prove to be effective a viable option for meeting plant nutritional requirements.
The growth data revealed that the isolates improved seedling development to varied degrees. The combination of zinc and phosphorus-solubilizing root bacteria outperformed the separate bacteria. Several findings corroborate these two strains excellent impacts on zinc and phosphorus solubilization suggest that zinc and phosphorus-solubilizers have the potential to improve plant growth., Enterobacter kobei and Bacillus astralimaris, on plant growth30 also reported the maximum plant growth due to ZSB. Similarly, enhancements by using zinc-solubilizing Bacillus sp., improvements in plant growth metrics such as measure the lengths of the shoots and roots, as well as collect the fresh and dry biomass of the shoots and roots were obtained BLR41. 31 E. kobei was found to enhance plant growth through phosphorus solubilizing. Species of Bacillus and Lysinibacillus have also been found to enhance biomass and plant height.32 PSB synthesis of organic compounds such as cytokinin, gibberellin, and IAA found in the root zone. has been associated to increased plant height.33
The root endophytic bacteria BLR41 and BLR45 were isolated from Barleria lupulina. The characterization of BLR41 is a Gram-positive, and BLR45 is a Gram-negative the best enzymatic reaction such as citrate, amylase, lipase and protease and identification with 16S rRNA gene sequencing in the name of BLR41 is Bacillus australimaris and BLR45 is Enterobacter kobei. BLR41 is B. australimaris and BLR45 is E. kobei strain also have an effect on B. lupulina through the synthesis of Indole and ammonia., additionally, PGP effects of zinc and phosphate solubilization. It may be inferred that the twin bacteria consortia with phosphate and zinc-solubilizing capacities promotes the growth of the B. lupulina plant in a pot experiment, and that this consortium has the potential to improve the growth and yield of B. lupulina in the field of agriculture.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the Department of Botany and Microbiology for providing them with the chance to conduct this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
Not applicable.
ETHICS STATEMENT
Not applicable.
- Kumar V, Jain L, Jain SK, Chaturvedi S Kaushal P. Bacterial endophytes of rice (Oryza sativa L.) and their potential for plant growth promotion and antagonistic activities. S Afr J Bot. 2020;134:50-63.
Crossref - Majeed A, Muhammad Z, Ahmad H. Plant growth promoting bacteria: role in soil improvement, abiotic and biotic stress management of crops. Plant Cell Rep. 2018;37(12):1599-1609.
Crossref - Soumare A, Diedhiou AG, Thuita M, et al. Exploiting biological nitrogen fixation: a route towards a sustainable agriculture. Plants. 2020;9(8):1011.
Crossref - Kumar V, Jain L, Jain SK, Chaturvedi S Kaushal P. Bacterial endophytes of rice (Oryza sativa L.) and their potential for plant growth promotion and antagonistic activities. S Afr J Bot. 2020;134:50-63.
Crossref - Kumari R, Kumar S, Kumar A, Goel KK, Dubey RC. Antibacterial, antioxidant and Immuno-modulatory properties in extracts of Barleria lupulina Lindl. BMC Complementary Altern Med. 2017;17(11):484.
Crossref - Hameed A, Yeh MW, Hsieh YT, Chung WC, Lo CT, Young LS. Diversity and functional characterization of bacterial endophytes dwelling in various rice (Oryza sativa L.) tissues, and their seed-borne dissemination into rhizosphere under gnotobiotic P-stress. Plant Soil. 2015;394(1):177-197.
Crossref - Hamim A, Boukeskasse A, Ouhdouch Y, et al. Phosphate solubilizing and PGR activities of ericaceous shrubs microorganisms isolated from Mediterranean forest soil. Biocatal Agric Biotechnol. 2019;19:101128.
Crossref - Shaikh S, Saraf M. Biofortification of Triticum aestivum through the inoculation of zinc solubilizing plant growth promoting rhizobacteria in field experiment. Biocatal Agric Biotechnol. 2017;9:120-126.
Crossref - Chen Y, Wang W, Zhou D, et al. Biodegradation of lignocellulosic agricultural residues by a newly isolated Fictibacillus sp. YS-26 improving carbon metabolic properties and functional diversity of the rhizosphere microbial community. Bioresour Technol. 2020;310:123381.
Crossref - Reyes AT. Morpho-biochemical aided identification of bacterial isolates from Philippine native pig. Adv Pharmacol Clin Trials. 2018;3:000148.
Crossref - Feyzi H, Chorom M, Bagheri G. Urease activity and microbial biomass of carbon in hydrocarbon contaminated soils. A case study of cheshmeh-khosh oil field, Iran. Ecotoxicol Environ Saf. 2020;199:110664.
Crossref - Carrasco M, Villarreal P, Barahona S, Alcaino J, Cifuentes V, Baeza M. Screening and characterizatio of amylase and cellulase activities in psychrotolerant yeasts. BMC Microbiol. 2016;16:21.
Crossref - Priyanka P, Kinsella GK, Henehan GT, Ryan BJ. Isolation and characterization of a novel thermo-solvent-stable lipase from Pseudomonas brenneri and its application in biodiesel synthesis. Biocatal Agric Biotechnol. 2020;29:101806.
Crossref - Hayward AC. Characteristics of Pseudomonas solanacearum. J Appl Bacteriol. 1964;27(2):265-277.
Crossref - Durga CSS, Ruben N, Chand MSR, Indira M, Venkatesh C. Comprehensive microbiological studies on screening bacteria for self-healing concrete. Materialia. 2021;15:101051.
Crossref - Ahmad I, Ahmad M, Hussain A, Jamil M . Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: a promising approach for improving cotton growth. Folia Microbiol. 2021;66(1):115-125.
Crossref - Liu Z, Zhou H, Xie W, Yang Z Lv Q. Long-term effects of maize straw return and manure on the microbial community in cinnamon soil in Northern China using 16S rRNA sequencing. PloS One. 2021;16(4):e0249884.
Crossref - Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870-1874.
Crossref - Manganyi MC, Regnier T, Kumar A, Bezuidenhout CC, Ateba CN. Phylogenetic analysis and diversity of novel endophytic fungi isolated from medicinal plant Sceletium tortuosum. Phytochem Lett. 2018;27:36-43.
Crossref - Srivastava V, Goel G, Thakur VK, Singh RP, de Araujo ASF, Singh P. Analysis and advanced characterization of municipal solid waste vermicompost maturity for a green environment. J Environ Manage. 2020;255:109914.
Crossref - Dubey RC, Khare S, Kumar P, Maheshwari DK. Combined effect of chemical fertilisers and rhizosphere-competent Bacillus subtilis BSK17 on yield of Cicer arietinum. Arch Phytopathol Plant Prot. 2014 ;47(19):2305-2318.
Crossref - Suleman M, Yasmin S, Rasul M, Yahya M, Atta BM, Mirza MS. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PloS One. 2018;13(9):e0204408.
Crossref - Marques AR, Resende AA, Gomes FC, et al. Plant growth-promoting traits of yeasts isolated from the tank bromeliad Vriesea minarum LB Smith and the effectiveness of Carlosrosaea vrieseae for promotin bromeliad growth. Braz J Microbiol. 2021;52(3):1417- 1429.
Crossref - Pranaya K, Bhat BN, Devi GU, Triveni S. Colony, morphological and biochemical characteristics of cotton phyllosphere bacteria and its antagonistic activity against the Alternaria leaf spot of cotton. IJCS. 2020;8(6):1103-1107.
Crossref - Seleiman MF, Semida WM, Rady MM, et al. Sequential application of antioxidants rectifies ion imbalance and strengthens antioxidant systems in salt-stressed cucumber. Plants. 2020;9(12):1783.
Crossref - Senousy HH, Abd Ellatif S, Ali S. Assessment of the antioxidant and anticancer potential of different isolated strains of cyanobacteria and microalgae from soil and agriculture drain water. Environ Sci Pollut Res. 2020;27(15).
Crossref - Jung EH, Smich J, Rubino JG, Wood CM. An in vitro study of urea and ammonia production and transport by the intestinal tract of fed and fasted rainbow trout: responses to luminal glutamine and ammonia loading. J Comp Physiol B. 2021;191(2):273-287.
Crossref - Dukare AS, Paul S, Nambi VE, et al. Exploitation
of microbial antagoniss for the control of postharvest diseases of fruits: a review. Crit Rev Food Sci Nutr. 2019;59(9):1498-1513.
Crossref - Kumar N, Dubey RC. Plant growth-promoting attribute of an endophyte Enterobacter roggenkampii BLS02 isolated from Barleria lupulina Lindl. Org Agric. 2022;12(68):137-145.
Crossref - Mekonnen H, Kibret M. The roles of plant growth promoting rhizobacteria in sustainable vegetable production in Ethiopia. Chem Biol Technol Agric. 2021;8(1):1-11.
Crossref - Kaur T, Devi R, Kour D, Yadav, A Yadav AN. Plant growth promotion of barley (Hordeum vulgare L.) by potassium solubilizing bacteria with multifariou plant growth promoting attributes. Plant Sci Today. 2021;8(1):17-24.
Crossref - Prittesh P, Avnika P, Kinjal P, Jinal HN, Sakthivel K Amaresan N. Amelioration effect of salt-tolerant plant growth-promoting bacteria on growth and physiological properties of rice (Oryza sativa) under salt-stressed conditions. Arch Microbiol. 2020;202(9):2419-2428.
Crossref - Ahmad I, Ahmad M, Hussain A, Jamil M . Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: a promising approach for improving cotton growth. Folia Microbiol. 2021;66(1):115-125.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.