ISSN: 0973-7510
E-ISSN: 2581-690X
Endophytes are the beneficial group of plant symbionts and are potent sources for producing novel metabolites with biotechnological applications. They are efficient sources for the synthesis of plant growth-promoting substances- Indole acetic acid, ammonia, solubilizing phosphates and thus open up sustainable agricultural opportunities. In the present investigation, twenty-two fungal endophytes were isolated from an ethnomedicinal plant-Helicteres isora L., collected from forests of Singbhum, Jhargram, India, and the isolate-Colletotrichum sp. HelS1, exhibited the most potent in vitro plant growth-promoting activities among all. HelS1 synthesized auxin (111.13 µg ml-1), ammonia, and solubilized phosphate (47.22 µg ml-1) in specific culture conditions. A treatment with fungal extract effectively increases the growth of the experimental plant (tomato seedlings) in terms of improvement in relative water contents, dry weight, nodal length, and pigment profiles compared to the untreated ones. There was an increase in root length by 27% compared to the control group. The isolate produced the maximum IAA after 7 days of incubation on Czepak Dox Broth supplemented with tryptophan (5 mg mL-1), sucrose (30 g L-1), and NaNO3 (2.2 g L-1) at pH 6.2. Fourier Transform Infrared Spectroscopic analysis of the crude fungal extract further confirmed the production of indole-like compounds. This investigation suggested that secondary metabolites of endophytes act as a potent plant growth inducer and can be utilized in bio-based crop management techniques.
Helicteres isora, Colletotrichum sp., Growth-promoting Activities
Endophytes are plant-associated beneficial microbiota that colonise internal tissues and form symbiotic associations with their host plants. They have been identified as an enormous source of unexplored bioactive compounds with potential applications in medicine and agriculture. They do so through various mechanisms, such as phytohormone production, the enhancement of nutrient uptake, and the induction of systemic resistance to pathogens. These are potential groups of microorganisms and promote the hosts’ growth in stressful environmental conditions.1 Endophytes are ubiquitous in occurrence in almost every group of plants studied till date. They significantly enrich the life cycle of their respective host plant through their plant growth-promoting properties and additionally assist in eliminating biotic/ or abiotic stress factors. This beneficial property of the fungal endophytes is usually attributed to their metabolic pathways that produce useful secondary metabolites.2 They predominantly belong to the class Ascomycota, with a wide range of habitat distribution occupying tropical to temperate forests. Millions of fungal endophytes represent significant genetic resource for biotechnological alliances. Therefore, in the face of various environmental challenges; endophytes play a critical role in improving growth attributes and releasing trace minerals from their inaccessible, soil-bound form. Applying fungal endophytes and their metabolites as plant growth promoters is a new sustainable way to counteract the long-term hazardous effects of chemicals in agricultural fields.
Many researchers have proposed that endophytes’ early colonization of host root tissues is the best method to enhance agricultural production.3 However, a dual application was also established by the combined effect of both exogenously supplied plant growth hormones and fungal endophyte originated secondary metabolites.4 In recent years, researchers have reported that several fungal strains like Epichloe sp., Piriformospora indica, Trichoderma asperellum, and Colletotrichum sp. can synthesize auxins, which enhance plant development in important crops and help alleviate the negative impacts of environmental challenges.5,6 Endophytic Penicillium commune EP-5 isolated from Ephedra pachyclada, significantly increasing root lengths and improving vegetative growth of maize plants.7 The isolated Acremonium sp. Ld-03 demonstrated the potential of promoting disease resistance and plant growth in Allium tuberosum seedlings.8 As scientists delve into the intricate relationships between plants and fungal endophytes, a growing body of research highlights the diverse mechanisms through which these symbiotic partners positively influence plant growth. These interactions assisted to develop and utilize endophytic resources for the well-being of nature and human beings. Researchers have tried identifying complex bioactive elements from endophytes to formulate compounds with agricultural utility from untapped natural resources. These studies collectively demonstrate the potential of endophytic fungi to enhance the growth and health of agricultural crop species.
Endophytic fungal assemblages harbored inside Indian medicinal plants have been reported from India during the last decade. Helicteres isora L., or “Avartani,” is a significant ethnomedicinal plant of the Malvaceae family. It is widely distributed as indigenous to the southern part of Asia. It is a large shrub with a wide range of pharmacological properties, such as antimicrobial, antioxidant, and antispasmodic activities.9,10 The antimicrobial potential of H. isora silver nanoparticles against extensively drug-resistant clinical isolates of Pseudomonas aeruginosa has been demonstrated in several studies.11 In the present investigation, fungal endophytes were isolated from H. isora and their potential to promote plant growth was screened. Using partial purification and FT-IR spectrum analysis, the current work examined the function of a single isolate by assessing its capacity to produce ammonia, phosphate solubilization, and auxin production. The potential isolate HelS1 was examined to improve growth characteristics in tomato plants following different screening assays. After applying HelS1 cell-free culture extract directly to the seedlings, key plant growth indicators were assessed and statistically compared with the control. The cultural conditions, such as media pH, temperature, carbon, nitrogen, salt sources, and incubation time, significantly affect the growth dynamics of desired microbes. These components determine the optimum production of phytohormones and secondary metabolites from endophytic fungi. Quantifying the ideal growing conditions for large-scale microbial production is important to generate the highest production of bioactive metabolites.12 The findings of this study focused on the active interaction of microbial endophytes to sustain crop growth by the external administration of fungal phytohormones that can stimulate plant growth in various routine conditions.
Study site and sample collection
Helicteres isora L., commonly known as Indian screw tree was collected from the Dalma forest range (3.3731°S, 29.9189°E) near Ghatshila, East Singbhum District, India in August 2019. The disease-free, mature plant sample was procured randomly and placed within a sterile ziplock plastic pack to resist dehydration. Collected branches of screw trees were brought to the Microbiology and Microbial Biotechnology laboratory of Vidyasagar University for the study of fungal endophytes.
Isolation of endophytic fungi
Fresh plant materials, such as stems, leaves, and flowers, were cleaned under running water before being processed to be chopped into 5 mm-wide segments. Explants were sequentially dipped into 70% ethanol to sterilize their surfaces (45 seconds). To remove undesirable bacteria, combine 70% ethanol with 0.5% NaOCl for two minutes.13 60 plant segments in total were individually placed in 2% water agar medium and incubated at 30°C to look for endophytes. The developing hyphal tips were moved to Potato Dextrose Agar Medium (PDA) that had been amended with 0.5 mg/ml streptomycin after 3 to 5 days of inoculation.
Identification of the isolates
The different hyphal morphology and sporangial structures of fungal isolates were seen, and they were then placed under a Carl ZEISS Axio A1 microscope for identification. The recorded morphological data were then compared with the standard literature. Sterile endophytic isolates were subjected to 5.8S rDNA-based sequence analysis of the internal transcribed region (ITS). Obtained sequences were aligned using Clustal W and compared with the Genbank database using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) search program. The phylogenetic relationship was constructed using MEGA 11 after aligning the most similar sequences from nucleotide BLAST result.14
Screening for Plant-Growth promoting activities
Quantitative estimation of Indole-3-acetic acid
The colorimetric assay of IAA production was determined following the method of Patten and Glick with slight modification.15 Briefly, each isolate was inoculated in 25 ml of Czepak Dox Broth supplemented with L-tryptophan (1 mg ml-1) and without tryptophan. The culture media was incubated at 30°C on a rotary shaker at 150 rpm. After centrifugation, 1 ml of culture supernatant was mixed vigorously with 2 ml of salkowaski reagent (mixture of 0.5M FeCl3 and 35% of HClO4), and the absorbance was read at 530 nm. To calculate the IAA concentrations of fungal isolates, the standard calibration plot was built using various concentrations of standard indole.
Phosphate Solubilisation
The phosphate solubilizing capacity was assessed using Jasim et al. (2014)’s recommended methodology.16 The isolates were grown on 2% (w/v) tricalcium phosphate-added Pikovskaya agar medium and incubated at 28°C for 4-5 days. Positive solubilizers were thought to have developed surrounding the colonies in the form of a clear halo zone. Following Nautiyal’s method from 1999, the quantitative assessment of soluble phosphate was further determined using the NBRIP (National Botanical Research Institute’s Phosphate growth) medium.17 A comparison was made between the concentrations of soluble P (gml-1) in the fungal extract by the calibration plot of KH2PO4.
Ammonia Production
Ammonia producing assay was carried out by the inoculation of fungal isolates within peptone water broth culture at 30°±2 for 3 days. After the appropriate incubation period, Nessler’s reagent was added to each test tube and a slight yellow to brown color was detected as a positive indicator.18
Optimizing the parameters for IAA production
The optimization of IAA production was done based on different growth parameters, i.e., concentration of L-tryptophan (0.1, 0.3, 0.5, 0.7, and 0.9 mg ml-1), carbon sources (Sucrose, fructose, dextrose, lactose, and maltose), nitrogen sources (KNO3, Peptone, NH4Cl, and yeast extract), medium pH (5.8, 6.0, 6.2, 6.4, and 6.6) and incubation time (1, 3, 5, 7, and 9 days) for the broth culture. The differences in IAA production were graphically demonstrated by the OVAT method (One variable at a time). All experiments were performed in triplicate to observe the fungal growth rate by calculating the amount of dry weight and average values were observed.
Statistical validation of Response surface methodology
After the optimization using OVAT method, the most significant variables (incubation time, pH, sucrose concentration, and L-tryptophan concentration) were additionally analyzed using response surface methodology which was adopted by Box-Behnken design to standardize the obtained results with the help of Minitab 21.2.0 version,19 a set of 29 experiments were performed through assessing each factor at three levels (+1, 0 and -1) where +1 is represented as high values, 0 is encoded by central coded value and -1 encoded for low values (Table). The average IAA concentration was calculated as the actual response of RSM. Predicted response values were calculated using regression equation additionally.
Table:
Experimental design and results of the BBD for the optimization of IAA producing activity
| No. of experiments | Independent variables | IAA Concen. (µg/ml) | ||||
|---|---|---|---|---|---|---|
| Incubation time (Day) | pH | Sucrose Concentration (g/l) | L-tryptophan Concentration (mg/ml) | Measured | Predicted | |
| 1 | -1(5) | -1(6.0) | 0(30) | 0(0.5) | 33.87 | 32.84 |
| 2 | 1(9) | -1 | 0 | 0 | 52.2 | 59.71 |
| 3 | -1 | 1(6.4) | 0 | 0 | 62.3 | 55.48 |
| 4 | 1 | 1 | 0 | 0 | 16.85 | 18.56 |
| 5 | 0(7) | 0(6.2) | -1(28) | -1(0.3) | 6.98 | 7.47 |
| 6 | 0 | 0 | 1(32) | -1 | 5.52 | 5.85 |
| 7 | 0 | 0 | -1 | 1(0.7) | 35.98 | 36.34 |
| 8 | 0 | 0 | 1 | 1 | 14.52 | 14.71 |
| 9 | -1 | 0 | 0 | -1 | 11.58 | 13.16 |
| 10 | 1 | 0 | 0 | -1 | 23.55 | 17.97 |
| 11 | -1 | 0 | 0 | 1 | 36.82 | 41.85 |
| 12 | 1 | 0 | 0 | 1 | 29.12 | 27.00 |
| 13 | 0 | -1 | -1 | 0 | 55.55 | 54.55 |
| 14 | 0 | 1 | -1 | 0 | 22.03 | 22.58 |
| 15 | 0 | -1 | 1 | 0 | 21.3 | 20.21 |
| 16 | 0 | 1 | 1 | 0 | 33.21 | 33.66 |
| 17 | -1 | 0 | -1 | 0 | 33.56 | 34.04 |
| 18 | 1 | 0 | -1 | 0 | 22.3 | 21.40 |
| 19 | -1 | 0 | 1 | 0 | 14.06 | 14.80 |
| 20 | 1 | 0 | 1 | 0 | 18.03 | 17.39 |
| 21 | 0 | -1 | 0 | -1 | 29.52 | 28.97 |
| 22 | 0 | 1 | 0 | -1 | 20.13 | 23.83 |
| 23 | 0 | -1 | 0 | 1 | 55.82 | 51.95 |
| 24 | 0 | 1 | 0 | 1 | 38.2 | 38.58 |
| 25 | 0 | 0 | 0 | 0 | 111.94 | 111.80 |
| 26 | 0 | 0 | 0 | 0 | 112.05 | 111.80 |
| 27 | 0 | 0 | 0 | 0 | 111.18 | 111.80 |
| 28 | 0 | 0 | 0 | 0 | 111.85 | 111.80 |
| 29 | 0 | 0 | 0 | 0 | 111.98 | 111.80 |
Detection and Characterization of IAA
With slight modifications, the solvent extraction approach of Harikrishnan et al. (2014) was employed to obtain IAA from Colletotrichum sp. HelS1.20 The extracted crude compound was eluted with n-Hexene: Ethyl acetate: Isopropyl alcohol (7:2:1) solvent system using pre-coated silica gel TLC plates and compared with commercial IAA. To observe the spots, TLC plates were examined under UV light at 254nm. The partially purified IAA was analyzed using an FTIR spectrophotometer (PerkinElmer FT-IR C98747) in the 400-4000 cm-1 wave number range.
Pot experiment assay
Based on auxin production and other PGP properties, one fungal isolate was chosen to evaluate plant growth promotion of Solanum lycopersicum L. Tomato seeds that were healthy and free of disease were first surface sterilized using 90% ethyl alcohol for 30 seconds, 2% NaOCl for 2 minutes, and then de-ionized double-distilled water. Three seeds were planted in each of the experimental soil mixtures of garden soil and coco peat in a 1:1 ratio in plastic containers. The experimental strategy includes the following treatments: foliar spray of HelS1 liquid extract (50% and 75%) on the plant, treatment with water, and Czepak Dox Broth (CDB) as control. Tomato seedlings were watered twice a day with sterile distilled water and, for the treated variety, a fungal extract. Different growth metrics, including plant fresh and dry weight, shoot and root length, relative water content, and pigment content were assessed after 42 days of treatment.
Data Analysis
Data obtained from different studies and in vivo plant growth bioassay was analyzed in triplicate. Differences of existing results were derived by mean ± standard deviation of calculation using one-way analysis of variance (ANOVA) by GraphPad Prism (Ver 8.4.2; GraphPad Software, La Jolla, CA, USA). Duncan’s multiple range tests were conducted for subsequent comparisons at P < 0.05 (SAS, Cary, NC, USA).
The extent of colonization of various endophytic fungi in different plants was assessed by considering different parameters. The colonization rate of endophytic fungi was calculated as the total number of tissue segments infected by the fungi divided by total number of infected segments incubated.21 The relative abundance (%) of each endophytic fungal species was calculated as number of isolates of each endophytic fungal species divided by total number of endophytic fungal isolates x100.22
Isolation and Identification of fungal isolates
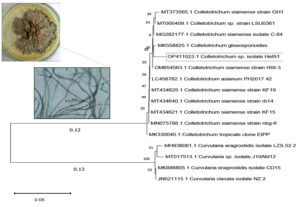
A total of twenty-two endophytic fungal endophytes were isolated from sixty different tissue segments of H. isora plant (9 from the stem part, 8 from leaf part, and 5 from the flower segments) and grouped into several identified genera, of which Colletotrichum sp. (44%) was the dominant genera occupying stem segments of the plant. There were more fungal endophytes discovered in association with the stems compared to leaf and floral components. However, it was also noted that certain fungal genera were associated with particular tissues. For example, Fusarium sp. is a global endophytic genus that inhabits all sections of the plant, whereas Cochliobolus sp. prefers to colonize both stems and leaves (Supplementary Table 1 & 2). In comparison to leaves and flowers, the colonization rate of the fungal isolates was much greater (75%) in the case of stems. 5.8s rDNA sequencing was used to determine the precise position in the phylogeny of the most potent isolates. The sequencing of the endophytic isolate HelS1 was found to be more than 98% identical to that of Colletotrichum sp. (Gen Bank accession: OP411023) (Figure 1 and (Supplementary Table 3).
Figure 1. Morphological and microscopic characteristic of endophytic fungal isolate HelS1 (Colletotrichum sp.); the phylogenetic tree of isolate HelS1 constructed according to 16s rDNA sequence. The number at branch points was the significant bootstrap values. The scale bar represents 0.05 substitutions per nucleotide position
Growth promoting activities of endophytic isolate
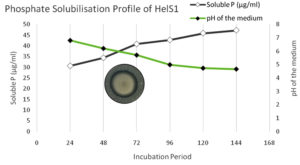
Out of all the isolates, twelve were able to synthesize IAA in Czepak Dox broth at various concentrations. In a medium enriched with 1% tryptophan, the quantitative IAA synthesis assay varied from 6.06 to 111.13 µg ml-1, whereas in a medium devoid of tryptophan, it ranged from 3.82 to 80.06 µg ml-1. Only a few numbers of isolates that were further chosen for the quantitative assessment of soluble P showed phosphate solubilizing capacity. The fungus isolate HelS1 produced a maximum concentration of IAA (111.13 µg ml-1) and dissolved phosphate (47.22 µgml-1), followed by HelL2 (64.70 µg ml-1; 42.10 µg ml-1) and HelF2 (64.26 µg ml-1; 32.68 µg ml-1), in that order. On the sixth day of the incubation period, with a pH of 4.6, the maximum solubilization of the HelS1 isolate was observed (Figure 2). The color intensity of the peptone broth showed that the majority of the endophytic fungal isolates could create ammonia, ranging from yellow to brown, respectively. The one isolate that met the criteria was chosen to evaluate its impact on plant growth characteristics under control conditions (Supplementary Table 4).
Figure 2. Phosphate solubilisation profiling of HelS1 in response of medium pH and incubation time period
Optimization of variable cultural conditions
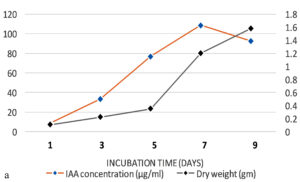
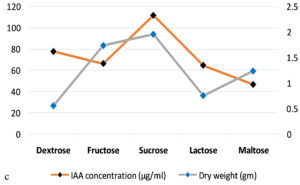
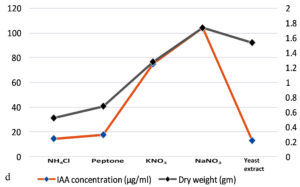
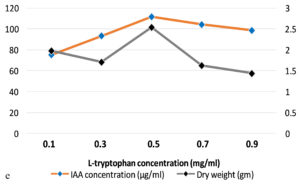
The production of IAA started to increase after 1st day which reached in maximum (i.e., 108.59µg ml-1) on the 7th day of incubation. Whereas on further incubation, a sharp decline in IAA production was observed on the 9th day (Figure 3a). Similarly, while optimizing the effect of medium pH conditions, it was revealed that maximum production occurred at 6.2 (i.e., 109.32 µg ml-1) and further decline the fungal growth due to increase in pH (Figure 3b). Among different carbon sources, the production of auxin was recorded maximum (111.94 µg ml-1) in the medium supplemented with sucrose (Figure 3c). The minimum fungal growth and IAA production has occurred when medium was supplemented with maltose. On the other hand, in case of nitrogen sources, NaNO3 (104.36 µg ml-1) followed by KNO3 was proved better in comparison to others (Figure 3d). Similarly, while optimizing the effect of L-tryptophan concentration showed a gradual increase between 0.1-0.5 mg ml-1, and a little decline of IAA production observed at medium supplemented with 0.7mg ml-1 of L-tryptophan (Figure 3e). Finally, the optimum conditions for the highest production of auxin were observed, i.e. 111.94 µg ml-1 along with the growth rate on the 7th day of incubation, medium pH 6.2 amended with sucrose and 0.5mg ml-1 of L-tryptophan. To get significant values of optimized data; we adopted the response surface method using a central composite design.
Figure 3. Production of IAA under different growth parameter of ; a) incubation time (days), b) medium pH, c) carbon sources, d) nitrogen sources and e) L-tryptophan concentration (mg/ml)
Response Surface Methodology (RSM) analysis
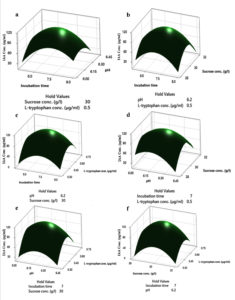
Based on OVAT analysis, the most significant four cultural parameters were recognized to use as an effective factor in response surface analysis. To compare the actual and predicted response value, the Box-Benkhen design (BBD) was adopted to perform 29 sets of experiments through central composite design (Table). In this design, 0.5mg ml-1 tryptophan, 7th days of incubation time, 6.2 pH and 30gl-1 of sucrose were observed optimum for the maximum production, which reached 111.98 µg ml-1. Media supplemented with 0.3 mgml-1 of tryptophan, 32gl-1 sucrose, 6.2 pH, and the 7th day of incubation period showed the least amount of IAA production i.e., 5.52 µg ml-1. The significant model F-value 165.04 suggests that the analysis of variance data significantly fit the experimental model, the p-value (p<0.001) indicates high model accuracy, while values greater than 0.001 were not significant. A predicted R2 value of 96.54% agreed with an Adj R2 value of 98.80% and relationships between different independent variables and optimum values affecting IAA production was evaluated using response surface plots (Figure 4) (Supplementary Table 5).
Figure 4. Response surface plots of the effects of media compositions on IAA production by HelS1. a) Interaction of pH and incubation time, b) Interaction of sucrose concentration and incubation time, c) Interaction of L-tryptophan concentration and incubation time, d) Interaction of L-tryptophan concentration and pH, e) Interaction of L-tryptophan concentration and sucrose concentration and f) Interaction of sucrose concentration and pH
Chromatographic and Biophysical Analysis of IAA
The Rf value of extracted IAA was 0.89, which was similar to that of standard IAA (Figure 5). These findings are consistent with those of Harikrishnan et al.20 The IR spectrum of HelS1 IAA showed peaks at 2972 and 2229 cm-1 indicating the presence of C-H alkanes. The absorption band found at 1713 cm-1 is due to the C=O bond and that at 1633 cm-1 indicated the presence of N-H stretch. The occurrence of the C-N stretch was attributed to the peaks at 1223 and 1041 cm-1. This is in accordance with the findings of Jagannath et al.23
Figure 5. a) Thin layer chromatography of Standard IAA (I) and extracted IAA (II) which is visible under UV light; b) Fourier Transform Infra-Red (FTIR) spectrum of HelS1 IAA
Effect on growth attributes
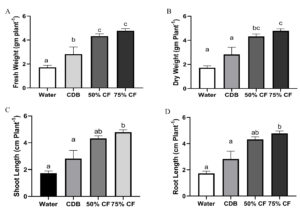
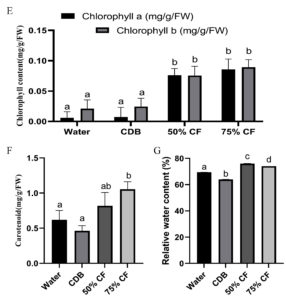
In the presence of endophytic strain HelS1 (Colletotrichum sp.), it was observed that all of the endophytic fungal extract-treated seedlings responded favorably according to the different experimental setups. Conversely, the control seedlings displayed poorly formed plant characteristics. It was found that a significant difference in shoot and root length was observed between the seedlings treated with foliar spray of 50% and 75% fungal extract of the selected isolate. This indicated better PGP effects in HelS1 treated tomato seedlings group compared with the control group after 6 weeks of treatment. Foliar spray of HelS1 culture filtrate (75%) on tomato plants showed an improvement in every growth parameter, like fresh weight (4.78±0.18c), dry weight (1.35±0.30b), shoot length (15.10±0.4c), and root length (3.86±0.20b) (Figure 6) (Different letters signify characteristics of plant growth that differ from the control at a P<0.05 significance level). The water-treated tomato seedlings outperformed CDB-treated ones the least among the four groups of tomato seedlings. There was a statistically significant variation in the relative water content between various tomato seedling groups. Each experiment was examined in triplicate manners.
Figure 6. changes in growth parameters after 42 days treatment on tomato seedlings; A-D. Differences in fresh weight, dry weight, shoot length and root length of endophyte treated seedling relative to control; E-G. changes in pigment content and relative water content of the plants. Error bar represents the standard deviation of means from replicates. Different letters indicate differences on plant growth parameters compared to the control at P <0.05
Global agriculture faces challenges to ensure food security for the rapidly growing population. Balancing the need for increased food production with environmental conservation is crucial for sustainability.24 The agricultural sector has been significantly making changes to farming strategies, including the use of precision farming and the implementation of genetically modified crops. Besides this, integrating endophytes into sustainable agriculture practices is a promising tool for enhancing crop production.25-27 Numerous research reports confirm that endophytic fungi play a crucial role in lessening the harmful impacts of both pests and environmental stress on key crops like rice, maize, wheat, sorghum, and barley. These fungi achieve this by influencing the plants’ internal hormone levels (specifically IAA, Gibberellins, and abscisic acid), which, in turn, triggers various physiological, biochemical, and defense-related mechanisms. This interaction demonstrates the potential for using endophytic fungi to bolster the resilience of crops against both biotic and abiotic challenges.28,29
Fungal endophytes can be used as bio-formulations to strengthen plants’ defense and growth. An experimental work by Bader et al. demonstrated the ability of an endophytic Trichoderma sp. to act as an indole-3-acetic acid producer and promote the growth of tomatoes against wilt disease, which again supports the beneficial role of endophytic fungal colonizers.30 There are numerous reports on the bioactivity of endophytes isolated from H. isora, but to our knowledge, no thorough analysis of plant growth promoting endophytes has been carried out till date.
The present experimental work looked at the auxin-producing abilities of fungal endophytes in H. isora. The growth data revealed the growth-inducing potential of isolate HelS1 to varied degrees. The endophytic isolate Colletotrichum sp. improved the seedling growth of S. lycopersicum L. through the foliar application of culture extract due to its phosphate solubilizing ability.31 Phosphate availability has also been found to influence lateral root formation and the rate of photosynthesis. The treated group showed higher chlorophyll content (0.174±0.01b), which is a clear indication of adequate carbon fixation with increasing nutrient accessibility from the soil. It was found that stem tissues (60%) of H. isora harbors more endophytic fungi than the leaf tissues (52%). Fusarium sp. was found to be the most dominant one. The occurrence of various fungal genera as endophytes of the test plant confirms the tissue specificity of fungal endophytes.
Endophytic Colletotrichum sp. has been earlier isolated as an endophyte of Dendrobium moniliform and exhibited a suitable amount of auxin production and acted as a plant growth elicitor.32 A recent study on Colletotrichum gloeosporioides, isolated from leaves of Cymbidium aloifolium L. showed promising antimicrobial and IAA-producing activity.33 Endophytic Colletotrichum siamense JB224.g1 has been assessed against a potent phytopathogen of tomato- Fusarium oxysporum, as a strong antifungal agent.34 Comparing this, our work proved the colonization of another effective endophytic strain of Colletotrichum sp., isolate HelS1 which not only manifests its auxin-producing ability (111.13 µg ml-1) but also 75% of culture extract improves the growth of the treated tomato seedlings. The culture filtrate of the fungal isolate contains auxin-like compounds, which can act as inducers of cell- or tissue-specific auxin responses. The exogenously applied auxin can rapidly induce the expression of transcriptional reprogramming of IAA-responsive genes.35 Endophytic fungal extract of Pacilomyces variotii, has shown to enhanced the IAA related gene expression and improved overall immunity in potato and rice seedlings.36,37 Extracts of endophytic fungi- Bipolaris, an isolate of Cannabis sativa, when applied in-vivo exhibits responses similar to our present study. Actually, the change in growth occurs due to the up-and-down regulation of several growth-promoting genes named as Auxin induced protein AUX22, IAA3, IAA4, 22A, auxin responsive protein SAUR50, SAUR78, after the foliar application of fungal extracts on different seedlings, with huge yields and enhancements of growth parameters.38,39
These results suggest that endophytes could be inoculated onto plants to naturally enhance auxin production or their culture extract could be applied directly for growth enhancement. Thus plants are naturally ready to compete against stresses. Due to a significant commercial need for IAA worldwide, plants with endophytes that can produce auxin can be planted in arid locations with non- or semi-fertile soil and limited water supply to properly utilize the wasteland there. Endophytes can also be infected to promote adequate plant growth.
Over the past few decades, the utilization of microbes and their derivatives has become increasingly prevalent in drug development and agricultural processes. In the present study, the isolate Colletotrichum sp. HelS1 exhibited potent in vitro plant growth-promoting activities, synthesized auxin, ammonia, and solubilised phosphate. Foliar application of HelS1 extract significantly enhanced the growth of tomato seedlings by improving different physiological parameters. Also, the production of plant growth promotors has been optimized, adopting response surface methodology techniques. The isolate HelS1 exhibited a remarkable increase in the production of auxin/indole-like compounds upon growing on the optimized media. In this context; this study highlighted the pivotal role of endophytic fungi and their immense applicability in key areas of agriculture for both qualitative and quantitative improvement of cash crops.
Additional file: Additional Table S1-S5.
ACKNOWLEDGMENTS
The authors acknowledge USIC (University Sophisticated Instrument Center) of Vidyasagar University for support with instrumental facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RD and DB designed the study. RD performed the experiments and analyzed the data. DB supervised the study. RD and DB wrote the manuscript. DB edited the manuscript. Both authors read and approved the manuscript for publication.
FUNDING
This research work was supported by the Swami Vivekananda fellowship from Government of West Bengal with Funding Grant ID- WBP211641292288.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript and/or in the supplementary files.
ETHICS STATEMENT
Not applicable.
- Ballesteros GI, Newsham KK, Acuna-Rodriguez IS, Atala C, Torres-Diaz C, Molina-Montenegro MA. Extreme environments as sources of fungal endophytes mitigating climate change impacts on crops in Mediterranean-type ecosystems. Plants, People, Planet. 2023;6(1):148-161.
Crossref - Kumar A, Verma JP. Does plant-microbe interaction confer stress tolerance in plants: a review? Microbiol Res. 2018;207:41-52.
Crossref - Santra HK, Banerjee D. Natural products as fungicide and their role in crop protection. Natural Bioactive Products in Sustainable Agriculture. 2020:131-219.
Crossref - Ismail MA, Amin MA, Eid AM, et al. Comparative Study between exogenously applied plant growth hormones versus metabolites of microbial endophytes as plant growth-promoting for Phaseolus vulgaris L. Cells. 2021;10(5):1059.
Crossref - Abo Nouh FA. Endophytic fungi for sustainable agriculture. Microbial Biosystems. 2019;4(1):31-44.
Crossref - Rauf M, Awais M, Ud-Din A, et al. Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Front Plant Sci. 2021;11:614971.
Crossref - Khalil AM, Hassan SE, Alsharif SM, et al. Isolation and characterization of fungal endophytes isolated from medicinal plant Ephedra pachyclada as plant growth-promoting. Biomolecules. 2021;11(2):140.
Crossref - Khan MS, Gao J, Munir I, et al. Characterization of endophytic fungi, Acremonium sp., from Lilium davidii and analysis of its antifungal and plant growth-promoting effects. BioMed Res Int. 2021;9930210.
Crossref - Deshpande HA, Bhalsing SR. Helicteres isora L: regeneration through meristem culture. Acta Biologica Szegediensis. 2015;59(1):55-58. https://abs.bibl.u-szeged.hu/index.php/abs/article/view/2868
- Kumar S, Jena PK, Kumari M, et al. Validation of tribal claims through pharmacological studies of Helicteres isora L. leaf extracts: an empirical research. Int J Drug Dev. Res. 2013;5(1):279-85.
- Mapara N, Sharma M, Shriram V, Bharadwaj R, Mohite KC, Kumar V. Antimicrobial potentials of Helicteres isora silver nanoparticles against extensively drug-resistant (XDR) clinical isolates of Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2015;99:10655-67.
Crossref - Grahovac J, Grahovac M, Dodic J, Bajic B, Balaz J. Optimization of cultivation medium for enhanced production of antifungal metabolites by Streptomyces hygroscopicus. Crop Protection. 2014;65:143-52.
Crossref - Li HY, Shen M, Zhou ZP, Li T, Wei YL, Lin LB. Diversity and cold adaptation of endophytic fungi from five dominant plant species collected from the Baima Snow Mountain, Southwest China. Fungal Diversity. 2012;54:79-86.
Crossref - Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biol Evol. 2007;24(8):1596-1599.
Crossref - Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol. 2002;68(8):3795-801.
Crossref - Jasim B, Joseph AA, John CJ, Mathew J, Radhakrishnan EK. Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3 Biotech. 2014;4:197-204.
Crossref - Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170(1):265-270.
Crossref - Kavamura VN, Santos SN, da Silva JL, et al. Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiol Res. 2013;168(4):183-191.
Crossref - Box GE, Behnken DW. Some new three level designs for the study of quantitative variables. Technometrics. 1960;2(4):455-475.
Crossref - Harikrishnan H, Shanmugaiah V, Balasubramanian N, Sharma MP, Kotchoni SO. Antagonistic potential of native strain Streptomyces aurantiogriseus VSMGT1014 against sheath blight of rice disease. World J Microbiol Biotechnol. 2014;30:3149-61.
Crossref - Pandey SS, Singh S, Babu CV, et al. Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep. 2016;6(1):26583.
Crossref - Dastogeer KM, Tumpa FH, Sultana A, Akter MA, Chakraborty A. Plant microbiome-an account of the factors that shape community composition and diversity. Curr Plant Biol. 2020;23:100161.
Crossref - Jagannath S, Konappa NM, Alurappa R, Chowdappa S. Production, characterization of indole acetic acid and its bioactive potential from endophytic fungi of Cymbidium aloifolium L. Journal of Biologically Active Products from Nature. 2019;9(5):387-409.
Crossref - Fasusi OA, Cruz C, Babalola OO. Agricultural sustainability: microbial biofertilizers in rhizosphere management. Agriculture. 2021;11(2):163.
Crossref - Dubey A, Saiyam D, Kumar A, Hashem A, Abd_Allah EF, Khan ML. Bacterial root endophytes: Characterization of their competence and plant growth promotion in soybean (Glycine max (L.) Merr.) under drought stress. Int J Environ Res Public Health. 2021;18(3):931.
Crossref - George P, Gupta A, Gopal M, Thomas L, Thomas GV. Indigenous rhizobacteria possessing abiotic stress tolerant traits promote vigorous growth of coconut seedlings via increased nutrient uptake and positive plant-microbe feedback. Proceedings of the Indian National Science Academy. 2022;88(1):1-16.
Crossref - Brauer VS, Rezende CP, Pessoni AM, et al. Antifungal agents in agriculture: Friends and foes of public health. Biomolecules. 2019;9(10):521.
Crossref - Ali R, Gul H, Hamayun M, et al. Aspergillus awamori ameliorates the physicochemical characteristics and mineral profile of mung bean under salt stress. Chem Biol Technol Agric. 2021;8:1-3.
Crossref - Gonzalez-Teuber M, Contreras RA, Zuniga GE, Barrera D, Bascunan-Godoy L. Synergistic association with root endophytic fungi improves morpho-physiological and biochemical responses of Chenopodium quinoa to salt stress. Front Ecol Evol. 2022;9:787318.
Crossref - Bader AN, Salerno GL, Covacevich F, Consolo VF. Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J King Saud Univ Sci. 2020;32(1):867-873.
Crossref - Perez-Torres CA, Lopez-Bucio J, Cruz-Ramirez A, et al. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. The Plant Cell. 2008;20(12):3258-3272.
Crossref - Shah S, Shrestha R, Maharjan S, Selosse MA, Pant B. Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants. 2018;8(1):5.
Crossref - Wary S, Sarma A, Talukdar R, Tayung K. Leaf endophytic fungi of Cymbidium aloifolium L. produces antimicrobials and indole-3-acetic acid. S Afr J Bot. 2022;149:381-388.
Crossref - Silva Santos SD, Silva AA, Polonio JC, et al. Influence of plant growth-promoting endophytes Colletotrichum siamense and Diaporthe masirevicii on tomato plants (Lycopersicon esculentum Mill.). Mycology. 2022;13(4):257-270.
Crossref - Zhang Y, Yu J, Xu X, et al. Molecular mechanisms of diverse auxin responses during plant growth and development. Int J Mol Sci. 2022;23(20):12495.
Crossref - Cao J, Liu B, Xu X, et al. Plant endophytic fungus extract ZNC improved potato immunity, yield, and quality. Front Plant Sci. 2021;12:707256.
Crossref - Waqas M, Khan AL, Lee IJ. Bioactive chemical constituents produced by endophytes and effects on rice plant growth. Journal of Plant Interactions. 2014;9(1):478-87.
Crossref - Lubna, Asaf S, Khan AL, et al. Growth-promoting bioactivities of Bipolaris sp. CSL-1 isolated from Cannabis sativa suggest a distinctive role in modifying host plant phenotypic plasticity and functions. Acta Physiologiae Plantarum. 2019;41:1-6.
Crossref - Ortiz J, Soto J, Fuentes A, Herrera H, Meneses C, Arriagada C. The endophytic fungus Chaetomium cupreum regulates expression of genes involved in the tolerance to metals and plant growth promotion in Eucalyptus globulus roots. Microorganisms. 2019;7(11):490.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.