ISSN: 0973-7510
E-ISSN: 2581-690X
Actinobacteria are mainly involved in decomposition of organic materials, promotion of plant growth, nutrient mineralization and antagonism against plant pathogens. In the present study forty one Actinobacterial isolates obtained from the different agro ecological (arid, semi arid and humid) regions of India were characterized. Thirty-five out of the 41 isolates produced indole acetic acid (IAA) and 16 isolates produced gibberellic acid (GA) in vitro; Thirty isolates were able to solubilize tri-calcium phosphate (TCP) and 15 were able to solubilize potassium from muscovite mica. Thirty-two isolates showed alkaline phosphatase activity. Glass house screening identified 9 isolates that were highly effective in promoting plant dry weight (>35%) when inoculated on maize and on chickpea (>30%). The principal component analysis showed that isolate A6 was found to be effective in increasing the plant growth parameters both in 30 and 45 DAS, followed by A1 in Maize and A10 was effective at 30 DAS and A17 in 60 DAS on Chick pea. Molecular confirmation using 16S rRNA gene sequence of the strain showed that A1 had 98% similarity with S. enissocaesilis (NCBI Accession no.: MF070481) A6 had only 84% similarity with S. djakartensis which may be a novel organism in terms of activity and A10 had 98% similarity with S. mutabilis (Accession no.: MF070483). A16 had 97% similarity with S. enissocaesilis (Accession no.: MH591469) and A25 had 97% similarity with S. rochei, (Accession no.: MH633722) A28 had 98% similarity with S. rochei (Accession no.: MH633727) and A36 had 97% similarity with Acinetobacter johnsonii (Accession no.: MH636837). The identified strains from our study have a good potential for use as bioinoculants for dryland crops to obtain enhanced crop growth and yield.
Actinobacteria, PGPR, Arid, Semi-arid, Maize, Chickpea
Soil microorganisms have greater role in low-input agriculture which results in the enhancement of plant nutrition and health, and improvement of overall soil quality. Amongst prokaryotes, the Actinobacteria are the second dominant group of microbes in soil and have made a revolution in industrial microbiology, but they have not been exploited much in agriculture. Actinobacteria like Streptomyces are dominant colonizers in the crop rhizosphere involved in organic matter decomposition and can tolerate adverse growth conditions due to the production spores.1 Actinobacteria promote plant growth by producing plant growth-promoting hormones such as auxins or gibberellin.2,3 Contribution of Actinobacteria to the production of plant growth promoting harmones has been well established.4,5 Solubilization of phosphorus biologically, helps in improving the efficiency of P utilization by plants. Most of the available phosphorus in soil get bound with calcium under alkaline condition and with aluminium or iron under acidic conditions and get deposited in soil in fixed form and become unavailable to plants. The solubilization of phosphorus by Actinobacteria has been well documented.5,6 They also possess several properties such as production of antibiotics, synthesis of particular extracellular enzymes, hydrogen cyanide production and siderophore production which help in acting as biocontrol agent in combating several plant diseases.7-9 Actinobacteria can be exploited as potential tools for agriculture and as beneficial inoculants for the future agriculture.10 Plant growth promotion potential of Streptomyces was reported in bean,11 tomato,12 pea,7 wheat,13 rice,14 Chickpea3 and Soybean.2 They also promote mycorrhizal colonization rate at different stages of actinomycete-fungus-plant interactions, including spore germination.15
Dryland agriculture constitutes a very large part of agriculture in India. More than 90% of coarse cereals, 80% of groundnut and 85% of pulses come from dryland cultivation. Low productivity in dryland is due to unpredictable climatic changes and also the application of low dosage of chemical fertilizers. Plant growth promoting Actinobacteria, could be a valuable resource to the poor farmers to meet the nutrients requirement of the plants. Actinobacterial benefits can be maximized by applying as inoculants in dry to semi-dry soils. In the present study isolated forty one Actinobacteria from the rhizosphere soils of different crops growing on red and black soils of different agro ecological (arid, semi arid and humid) regions of Karnataka, Andhra Pradesh; Rajasthan and from pristine forest soils of Karnataka were identified by conventional and molecular methods, tested for plant growth promotional traits and plant bioassay under in vitro conditions on Maize and Chickpea.
Isolation and identification
Rhizosphere soil samples were collected from Sorghum (Sorghum bicolor), Pearl millet (Pennisetum glaucum), Pigeon pea (Cajanus cajan), Finger millet (Eleusine coracana) and Groundnut (Arachis hypogaea) grown in different agro ecological regions (arid, semi arid and humid) of Karnataka – Belgaum, Hubli, Bijapur and Tumkur districts; Andhra Pradesh – Anantapur district and in Rajasthan – Jaisalmer and 2 soil samples from pristine forest Karnataka (Kegdal, Vibuthi). Five different media were used for isolation of Actinobacteria viz., actinomycetes isolation agar, starch casein agar, arginine glycerol salts medium, humic acid vitamin agar and Kuster’s agar. Isolates were characterized by Holt et al.16
Growth promoting characteristics
Forty-one isolates were characterized for plant growth promotional traits like Indole acetic acid (IAA); and Gibberellic acid (GA) production17,18; qualitative Phosphate solubilisation19 and spectrophotometric P solubilization quanti cation after 10 days of growth (28 ± 2 °C, 125 rpm); Potassium solubilization using Aleksandrov agar20 and quantification by flame photometric method after 10 days (28 ± 2 °C, 125 rpm); Production of enzyme Alkaline phosphatase.21
Plant bioassay
The isolates were screened under glass house conditions to test their ability to promote plant growth. The experiment had 41 treatments (Actinobacterial isolates) with 5 replications and followed a Completely Randomized Design. 300 g of black cotton soil (Vertic Ustochrept) was filled in 330 ml paper cups. Farm yard manure (FYM) was air dried for 3-4 days and passed through 0.2 mm sieve and sterilized intermittently for three times by steam sterilization (121 °C for 20 min) on successive days and then two times dry heat sterilization (160 °C for 3 h each time). The Forty milliliter of the each Actinobacterial isolates grown in starch casein broth was mixed to 100 gm FYM separately and sealed. The Carboxy methyl cellulose (CMC) suspension (one gram of each FYM based inoculant + 10 ml of 1% CMC) was prepared. Maize Seeds (var. JM-216) was sterilized by treating with 5 minutes in 95% ethanol and sodium hypochlorite (NaClO3) and washed four to five times in sterile distilled water. The required seeds along with CMC culture suspension was then left overnight. Next day the seeds were air dried in a laminar air flow work station. Seeds coated inoculants were sown in each cup, the plants were thinned after germination and three plants maintained in each cup. Urea solution was prepared and applied at the rate of 40 µg N g-1 soil. The boiled (30 min) and cooled tap water used for watering the plants. The plant growth observations like height of the plant, leaves number were taken both at 30 and 45 DAS (days after sowing) and recorded dry mass was at 45 days. For Chickpea (JG-16) we followed the same treatment methodology which used for Maize seed treatment. The plant growth observations viz., plant height at 30 and 60 days, nodule parameters and recorded plant dry weight at 60 DAS (days after sowing).
Molecular characterization
Potential Actinobacterial Genomic DNA extracted by the standard C-TAB method. The near full length 16S rRNA gene sequence of the isolates were custom sequenced on ABI 3730 × 1 Genetic Analyzer at Xcelris Labs Ltd., (Ahmedabad, Gujarat, India). The sequences were deposited in the NCBI Genbank database. The strains nearest identifiers were obtained in GenBank (http://www.ncbi.nlm.nih.gov/).
Accession numbers of the Actinobacterial isolates
No. |
Isolates |
Accession numbers |
1 |
A1 |
MF070481 |
2 |
A10 |
MF070483 |
3 |
A16 |
MH591469 |
4 |
A25 |
MH633722 |
5 |
A28 |
MH633727 |
6 |
A36 |
MH636837 |
Soil samples were collected from the rhizosphere of Sorghum, Pearl millet, Pigeon pea, Finger millet, Groundnut (grown at Belgaum, Hubli, Bijapur, Tumkur districts of Karnataka; Anantapur district in Andhra Pradesh and Jaisalmer district of Rajasthan) from arid and semi arid regions. Two soil samples were collected from pristine forest (humid region) in Karnataka (Kegdal, Vibuthi) and a desert soil from Sam, Rajasthan were analysed for physico chemical properties (data not shown). Based on colony morphology and microscopic observations 41 isolates were short listed for further studies.
The soils studied harbored mainly four different genera of Actinobacteria22,23 pre-dominantly Streptomyces and Nocardia followed by Micromonospora and Saccharopolyspora. Arid soils had all four genera; semi arid soils consisted only Streptomyces and Nocardia whereas humid soils consisted mainly Streptomyces, Nocardia and Saccharopolyspora. Forty-one different morphotypes of Actinobacteria (18 isolates from arid soils, 16 from semi arid and 7 from humid regions) were shortlisted for plant growth promotion studies. The isolates were maintained on starch casein agar-slants and stored at 4 °C in the culture bank of All India Network Project on Soil Biodiversity-Biofertilizers at IISS, Bhopal.
Growth promoting characteristics
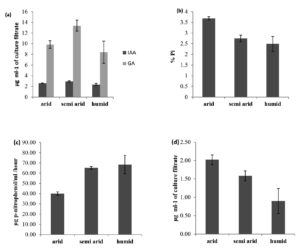
Actinobacterial isolates (41) of all the regions were equally effective in IAA production. The highest IAA production was observed in A6 (5.74 µg ml-1) and A10 (5.62 µg ml-1) of arid zone isolates (Table 1). These isolates were obtained from pearl millet crop rhizosphere grown on arid soil. The mean production of IAA was 2.6, 2.9 and 2.4 µg ml-1 in arid, semi-arid and humid region isolates (Figure 1a).
Significant difference in production of GA was observed among the Actinbacterial isolates. Among the 41 isolates tested for GA production 16 isolates produced GA which ranged from 21.00-41.35 µg ml-1 of culture filtrate. The results are presented in Table 1, 2 and 3. The maximum GA production of 41.35 µg ml-1 of culture filtrate was observed in Actinobacterial isolate A17 (Streptomyces) followed by isolate A6 (Streptomyces), which recorded 35.32 mg ml-1. These two isolates were from pearlmillet crop rhizosphere (arid soil).
Table (1):
Production of plant growth promoting hormones, P and K solubilization, Alkaline phosphatase by the arid region isolates of Actinobacteria under in vitro conditions
No. |
Isolates |
IAA (µg ml-1) |
GA (µg ml-1) |
% Pi released at 10 days |
K solubilization (µg ml-1) at 10 days |
Alkaline Phosphatase (µg p-nitrophenol/ml/h) |
|---|---|---|---|---|---|---|
1 |
A3-Streptomyces |
NP |
23.35 |
3.38 |
4.30 |
55.00 |
2 |
A5-Streptomyces |
4.43 |
22.18 |
1.89 |
4.10 |
46.00 |
3 |
A6-Streptomyces |
5.74 |
35.32 |
6.52 |
6.30 |
141.60 |
4 |
A7-Streptomyces |
4.17 |
31.45 |
1.52 |
4.00 |
61.50 |
5 |
A10-Streptomyces |
5.62 |
32.99 |
6.02 |
5.90 |
106.90 |
6 |
A11-Streptomyces |
3.90 |
24.08 |
4.93 |
4.00 |
78.60 |
7 |
A14-Streptomyces |
2.82 |
NP |
5.89 |
NP |
64.50 |
8 |
A17-Streptomyces |
5.42 |
41.35 |
6.76 |
6.30 |
160.90 |
9 |
A20-Micromonospora |
2.02 |
NP |
2.61 |
NP |
68.20 |
10 |
A29-Nocardia |
2.79 |
NP |
NP |
NP |
56.20 |
11 |
A31-Nocardia |
4.33 |
NP |
2.86 |
NP |
NP |
12 |
A32-Streptomyces |
NP |
NP |
2.93 |
NP |
49.10 |
13 |
A37-Nocardia |
2.69 |
NP |
3.35 |
NP |
NP |
14 |
A38-Streptomyces |
2.99 |
NP |
4.16 |
NP |
57.60 |
15 |
A40-Streptomyces |
2.53 |
NP |
5.58 |
NP |
NP |
16 |
A41-Saccharopolyspora |
2.25 |
NP |
3.01 |
NP |
NP |
17 |
A42-Streptomyces |
NP |
NP |
2.00 |
3.80 |
NP |
18 |
A43-Nocardia |
2.13 |
NP |
4.19 |
NP |
NP |
S.Em ± |
0.12 |
0.25 |
0.08 |
0.06 |
0.20 |
|
CD@1% |
0.34 |
0.72 |
0.27 |
0.10 |
0.58 |
NP: Not produced
Table (2):
Production of plant growth promoting hormones, P and K solubilization, alkaline phosphatase by the semi arid region isolates of Actinobacteria under in vitro conditions
No. |
Isolates |
IAA (µg ml-1) |
GA (µg ml-1) |
% Pi released at 10 days |
K solubilization (µg ml-1) at 10 days |
Alkaline Phosphatase (µg p-nitrophenol/ml/h) |
|---|---|---|---|---|---|---|
1 |
A2-Streptomyces |
1.93 |
24.33 |
4.84 |
3.70 |
60.00 |
2 |
A4-Saccharopolyspora |
2.54 |
NP |
NP |
NP |
NP |
3 |
A9-Streptomyces |
NP |
22.79 |
5.99 |
NP |
67.90 |
4 |
A15-Streptomyces |
2.95 |
NP |
2.63 |
NP |
66.80 |
5 |
A16-Streptomyces |
3.72 |
NP |
0.00 |
3.80 |
60.30 |
6 |
A18-Streptomyces |
NP |
NP |
3.18 |
3.90 |
79.20 |
7 |
A19-Streptomyces |
1.17 |
NP |
– |
NP |
57.80 |
8 |
A22-Nocardia |
3.66 |
NP |
– |
NP |
70.80 |
9 |
A23-Nocardia |
1.77 |
NP |
3.72 |
NP |
60.70 |
10 |
A25-Nocardia |
4.09 |
26.11 |
1.16 |
4.00 |
60.70 |
11 |
A27-Streptomyces |
4.28 |
24.45 |
0.15 |
NP |
71.20 |
12 |
A28-Nocardia |
3.17 |
21.00 |
4.18 |
NP |
62.30 |
13 |
A30-Streptomyces |
3.27 |
29.49 |
– |
NP |
74.40 |
14 |
A34-Nocardia |
2.34 |
NP |
– |
NP |
NP |
15 |
A35-Nocardia |
1.67 |
25.97 |
– |
3.80 |
64.30 |
16 |
A36-Streptomyces |
3.25 |
NP |
3.19 |
NP |
61.00 |
S.Em ± |
0.12 |
0.25 |
0.08 |
0.06 |
0.20 |
|
CD@1% |
0.34 |
0.72 |
0.27 |
0.10 |
0.58 |
NP: Not produced
Table (3):
Production of plant growth promoting hormones, P and K solubilization, alkaline phosphatase production by the humid region isolates of Actinobacteria under in vitro conditions
No. |
Isolates |
IAA (µg ml-1) |
GA (µg ml-1) |
% Pi released at 10 days |
K solubilization (µg ml-1) at 10 days |
Alkaline Phosphatase (µg p-nitrophenol/ml/h) |
|---|---|---|---|---|---|---|
1 |
A1-Streptomyces |
5.02 |
34.77 |
6.00 |
6.10 |
83.60 |
2 |
A12-Streptomyces |
1.80 |
NP |
– |
NP |
60.40 |
3 |
A13-Streptomyces |
1.88 |
NP |
– |
NP |
62.60 |
4 |
A24-Nocardia |
NP |
NP |
0.15 |
4.00 |
54.20 |
5 |
A33-Nocardia |
1.72 |
NP |
– |
NP |
58.50 |
6 |
A44-Streptomyces |
3.04 |
NP |
3.35 |
NP |
NP |
7 |
A45-Streptomyces |
3.43 |
28.63 |
4.26 |
NP |
62.30 |
S.Em ± |
0.12 |
0.25 |
0.08 |
0.06 |
0.20 |
|
CD@1% |
0.34 |
0.72 |
0.27 |
0.10 |
0.58 |
NP: Not produced
Semi-arid region isolates were more effective in GA production than the isolates from the arid and humid region, the mean production of GA from the isolates of the arid, semi-arid and humid region was 9.8, 13.4, and 8.4 µg ml-1 (Figure 1a).
All the 41 isolates were examined for their ability to solubilize tricalcium phosphate (TCP) on Pikovskaya’s medium. All the isolates were able to show clear zone of solubilization. The solubilization zones ranged from 5.25 mm to 16 mm after four days of incubation.22 Among the 41 isolates, 30 isolates showed more than 10 mm solubilization zone and were considered promising and selected for quantification. The pH of the Pikovskaya’s broth was dropped to 4.1 from an initial pH of 6.2. There was good correlation between the extent of the pH drop of the culture medium and the amount of P solubilization (R2 = 0.96). The highest % Pi solubilized was recorded in isolate A17 (Streptomyces) (6.76%) followed by A6 (Streptomyces) (6.52%). These two isolates were obtained from pearl millet rhizosphere in arid soil. Isolates from the arid region were more effective in P solubilisation than the isolates from semi-arid and humid regions. The mean of % P solubilization from TCP was 3.7%, 2.8%, and 2.5%, respectively, in arid, semi-arid and humid region isolates (Figure 1b).
All the isolates were tested for the production of alkaline phosphatase and the results are presented in Table 1, 2 and 3. Actinobaterial isolates differed significantly in production of alkaline phosphatase. Out of 41 isolates, 32 isolates produced alkaline phosphatase (12 from arid 14 semi arid and 6 from humid regions) which ranged from 46.0 to 160.9 µg p-nitrophenol/ml culture filtrate/hour. The highest was recorded in Actinoacterial isolate A17 (Streptomyces) (160.0 mg ml-1) which was isolated from pearl millet rhizosphere (arid).
The mean production of phosphatase was 40.1, 65.2 and 68.5 µg p-nitrophenol/ml/hr in arid, semi-arid and humid region isolates (Figure 1c).
Potassium involves in many physiological processes and imparts resistance against pests and diseases to plants. In our study, among 41 isolates tested 15 isolates were able to solubilize potassium on Alkesandrov medium containing muscovite mica as insoluble potassium source. Potassium soluilization ranged from 3.70 to 6.30 µg K ml-1 of culture filtrate at 10 days. The results are presented in Table 1, 2 and 3. The highest potassium was released by Actinobacterial isolate A6 and A17 (Streptomyces) (6.30 mg ml-1) which was isolated from pearl millet rhizosphere in arid soil. The mean K solubilization production was 2.0, 1.6, and 1.0 mg K ml-1 in arid, semi-arid and humid region isolates (Figure 1d).
Figure 1. (a) IAA and GA production (b) Phosphate solubilization (c) Alkaline phosphatase (d) Potassium solubilization by Actinobacterial isolates of different agro ecological regions of India
Plant bioassay
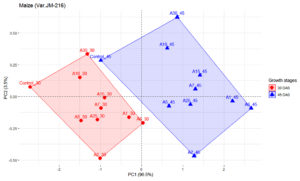
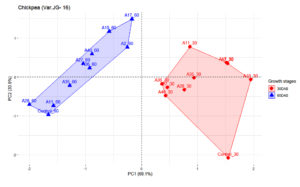
Figures 2 and 3 represents the principal component analysis for data obtained from observations taken in maize and chickpea plants. The dataset for maize contains 9 individuals (isolates), 1 control and 2 variables (Plant height and number of leaves). Similarly, for chickpea the dataset contains 9 individuals (isolates), 1 control and 2 variables (plant height and nodule number). Prior to analysis of PCA, another analysis was conducted to determine any outliers present, which in this present analysis detected no outliers. Since PC1 had higher inertia, it was used for analyzing the individuals that had higher effectiveness (96.5%) on maize plant compared to PC2 (3.5%). As illustrated in Figure 2, observations from two growth stages, i.e. 30 DAS and 45 DAS were distributed across the PC1 axis. Isolate A6 was found to be effective both in 30 and 45 DAS, followed by A1. The isolate A5 had lower efficiency as compared to other treatments. In Figure 3, the first two dimensions of PCA explains 100% of the total dataset inertia; which express that 100% of the individuals and variables cloud total variability is explained by the plane, wherein the inertia related to the first dimension (PC1) represents 69.1% and the second dimension represents (30.9%) respectively. Based on PCA on chickpea data, the inference that could be drawn is that A10 was effective in 30 DAS and A17 in 60 DAS.
Figure 2. Influence of highly effective actinobacterial isolates on growth of Maize (Var. JM-216) at two growth stages under greenhouse conditions
Figure 3. Influence of highly effective actinobacterial isolates on growth of Chickpea var (JG-16) at two growth stages under greenhouse conditions
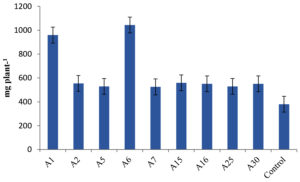
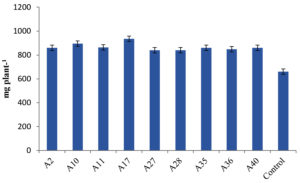
Differences in plant growth promotion and dry weight by Actinobacterial isolates was observed in our experiment. 41 Actinobacterial isolates were screened in polyacrylic paper cups on Maize and Chickpea. We have categorized the isolates based on per cent increase in dry matter production of Maize. Out of 41 isolates, 9 isolates were found highly effective (>35% plant DW increase), 12 isolates were moderately effective (25%-35% plant DW increase), 7 isolates were effective (15%-25% plant DW increase) and 13 strains were ineffective (<15%) in increasing the inoculated plants dry weight (Table 4, Figure 4). Similarly in Chickpea, 9 were found highly effective (>30% increase in plant DW), 17 isolates were moderately effective (20%-30%) 12 isolates were effective (10%-20%) and 3 isolates were ineffective (<10%) in increasing the DW of the plant and the results are presented in Table 5 and Figure 5.
Table (4):
Categorization of actinobacterial isolates based on increase in dry weight of inoculated maize (Var. JM-216) plants
Effectiveness* |
No. of isolates from arid region |
No. of isolates from semi-arid region |
No. of isolates from humid region |
|---|---|---|---|
HE |
3 (A5, A7, A6) |
5 (A2, A15, A16, A25, A30) |
1 (A1) |
ME |
8 (A10, A11, A14, A17,
A29, A32, A43, A3) |
3 (A22, A27, A35) |
1 (A4) |
E |
3 (A37, A40, A20) |
3 (A23, A28, A36) |
1 (A44) |
IE |
4 (A31, A41, A38, A42) |
5 (A9, A19, A13, A34, A18) |
4 (A12, A24, A45, A33) |
* Based on increase in dry weight, Highly effective (HE) = >35%, Moderately effective (ME) = 25%-35%, Effective (E) = 15%-25%, Ineffective (IE) = <15%
Table (5):
Categorization of Actinobacterial isolates based on increase in dry weight of inoculated Chickpea (JG- 16) plants
Effectiveness* |
No. of isolates from arid region |
No. of isolates from semi-arid region |
No. of isolates from humid region |
|---|---|---|---|
HE |
4 (A10, A11, A17, A40) |
5 (A28, A27, A2, A35, A36) |
0 |
ME |
7 (A5, A6, A29, A38,
A3, A43, A41) |
7 (A30, A34, A9, A15,
A16, A18, A19) |
3 (A4, A44, A1) |
E |
6 (A7, A14, A32,
A42, A20, A31) |
2 (A23, A25) |
4 (A24, A33, A45, A12) |
IE |
1 (A37) |
2 (A22, A13) |
0 |
*Based on increase in dry weight Highly effective (HE): >30%, Moderately effective (ME): 20%-30%, Effective (E): 10%-20%, Ineffective (IE): <10%
Figure 4. Influence of highly effective strains of actinobacterial isolates on dry weight of Maize at 45 DAS
Figure 5. Influence of highly effective strains of actinobacteria on dry weight of Chickpea at 45 DAS
Molecular characterization and phylogenetic analysis of the efficient Actinobacterial isolates
Based on colony morphology efficient Actinobacterial isolates of Maize, viz. A1, A6, A16, A25 and Chickpea A10, A28 and A36 were confirmed as Streptomyces. Molecular confirmation showed that A1 had 98% similarity with S. enissocaesilis (Accession no.: MF070481) A6 had only 84% similarity with S. djakartensis which may be a novel organism in terms of activity and A10 had 98% similarity with S. mutabilis (Accession no.: MF070483). A16 had 97% similarity with S. enissocaesilis (Accession no.: MH591469) and A25 had 97% similarity with S. rochei, (Accession no.: MH633722) A28 had also 98% similarity with S. rochei (Accession no.: MH633727) and A36 had 97% similarity with Acinetobacter johnsonii (Accession no.: MH636837).
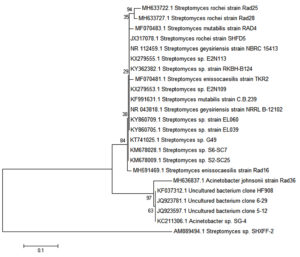
The phylogenetic tree of the isolates (Figure 6) was constructed based on UPGMA algorithm using MEGA 6.06 with a bootstrap value (n = 1000). No direct common ancestor were observed among the studied six Streptomyces sp., even the strains from the same species of Streptomyces rochei Rad 25 do not share the immediate ancestor as Streptomyces rochei Rad 28 strain and the strain have a separate new clade. Common node (bootstrap value of 84%) was observed in S. mutabilis strain Rad 4, S. enissocaesilis strain TKR2 S. enissocaesilis strain Rad 16 and Acinetobacter johnsonii strain Rad 36.
The microbial activity in crop rhizosphere is greatly influenced by root activities such as exudation of organic substrates like amino acids, sugars/carbohydrates, enzymes and vitamins. These substances as well as microbial interactions help in releasing crop nutrients and make it available to plant growth.24
Actinobacteria exhibit various properties that are useful for promotion of plant growth and in improving soil health.14 These organisms can be exploited as potential tools for agriculture and as beneficial inoculants for the future agriculture.10 In our study, Streptomyces were found to be the dominant genus among all the isolated Actinobacteria. Most of the Actinobacteria in soils belong to the genus Streptomyces.25 The Streptomyces dominance in soil was reported earlier by Alexander26 and Gesheva & Geshava27 The phylogenetic analysis of 38 actinomycetes isolates as associated with the rhizosphere of cacao showed that they are genetically diverse and belong to the genus Streptomyces.28 The predominant genus of 108 Actinomycete isolates from arid areas of Egypt was found to be Streptomyces, followed by Nocardia.29
Among the 41 isolates tested 35 isolates produced indole acetic acid (IAA) and sixteen isolates produced GA. Similar results also reported by Patten and Glick30 who reported that 80 percent of rhizosphere microorganisms can produce secondary metabolites containing auxins which helps to promote root elongation and plant growth. Other researchers reported the production of IAA, GA3, and zeatine by Actinobacteria.31 Similarly, highest amount of IAA 10.1 µg ml-1 and GA3 12.0 µg ml-1 production was recorded from the Streptomyces canus isolated from mycorrhiza Glomus mosseae spores.32 Out of 41 isolates tested 30 isolates were able to solublize phosphorus. It was observed that there was a drop in pH up to 4.1 from an initial pH of 6.2 in the medium. There was a good correlation between the extent of pH drop of the culture medium and P solubilization (R2 = 0.96). Organic acids production is the main mechanism of insoluble phosphate solubilization,33 increase in the P solubilization with pH decline of the culture filtrate was also observed by Gupta et al.34 Out of 35 endophytic actinomycetes from wheat plants, 17 isolates solubilized phosphate from 5 to 42 mg/100 ml.35
In this study, out of 41 isolates tested 15 isolates solubilized potassium; these isolates also solubilized P along with K, since all K solubilizers are showing PO4 solubilizing activity the mechanism of K solubilization may also be attributed to the organic acids production. Actinomycete species are capable of mobilizing bound potassium from the agro wastes of cocoa and addition of actinomycetes to the mixture of sterile cocoa pods (crushed to small granules) enhanced K mobilization in onion crop.36 30 potassium mobilizing bacterial isolates (mica as insoluble K source) from the soils of Belgaum and Dharwad districts of Karnataka mobilized K.37
In our study, thirty-two isolates out of 41 were able to produce alkaline phosphatase. Similar results were reported38 on the production of alkaline phosphatase by Actinobacterial isolates. The plant growth promoing traits viz., production of IAA, phophate solubilization and several enzyme activities by Actinobacteria of saline soils was reported by Djebaili et al.5
Actinobacterial isolates differed significantly in improving plant growth attributes. The isolates which are highly effective in improving the growth of maize were isolate A6 followed by isolate A1 obtained from the arid and humid region. These isolates were effective in improving plant growth parameters like height of the plant, number of leaves and dry weight of maize. Such increased plant growth due to actinobacterial inoculation was also earlier reported in rye grass by tomato. Franco & Valencia9 Franco-Correa38 and Stamenov et al39 reported such enhancement of plant growth in wheat under water stress due to Streptomyces inoculation. Significant enhancement of plant growth parameters on Sorghum under glass house conditions was observed with the inoculation of Streptomyces strains.40
In chickpea, the significant increase in plant growth parameters and nodulation were recorded with isolates A10 followed by A17 (Streptomyces obtained from arid soils). This enhanced plant growth parameters may be due to the synthesis of IAA, GA and cytokinins by Streptomyces sp., These results support the view of Gopalakrishnan et al.41 who reported that actinomycetes produced auxins, gibberellins and cytokinins which help in plant growth promotion. Similar results were reported on increased nodule, and nodulation size due to inoculation of Streptomyces lyndicus WYEC 108 in pea plant and in chickpea.3 Thus, the results from our study indicated that all the ecological regions (arid, semi-arid and humid) studied for the experiments are a rich reservoir for the plant growth promoting Actinobacteria isolation.
The findings of this study are of much significance to crop production in utilizing efficient Actinobacterial isolates like A6 and A1 (Streptomyces) for maize and A17 and A10 (Streptomyces) in chickpea cultivation to obtain enhanced crop yield. The identified cultures are of much use in dry land agriculture and can be routinely used as biofertilizer for solubilzation of P and K with the advantage to crop in obtaining plant growth promoting hormonal action for enhancing crop growth and yield. The best actinobacterial isolates identified in this research have to be evaluated on other crops singly or in combination with other beneficial microbial inoculants like Azotobacter, Azospirillum, Rhizobium and Mycorrhiza for obtaining increased crop yields.
ACKNOWLEDGMENTS
The authors are grateful to the Director, ICAR-Indian Institute of Soil Science, Bhopal, India, for providing the necessary facilities for this investigation.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
The research was funded by the Indian Council of Agricultural Research, New Delhi, India, under the aegis of the All-India Network Project on Soil Biodiversity-Biofertilizers.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Alexander M. Introduction to soil microbiology, 2nd Edn. Wiley, New York. 1977.
- Fatmawati U, Meryandini A, Nawangsih AA, Wahyudi AT. Screening and characterization of actinomycetes isolated from soybean rhizosphere for promoting plant growth. Biodiversitas Journal of Biological Diversity. 2019;20(10):2970-2977.
Crossref - Sreevidya M, Gopalakrishnan S, Kudapa H, Varshney RK. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz J Mic. 2016;47(1):85-95.
Crossref - Wahyudi AT, Priyanto JA, Afrista R, Kurniati D, Astuti RI, Akhdiya A. Plant growth promoting activity of actinomycetes isolated from soybean rhizosphere. Online J Biol Sci. 2019;19(1):1-8.
Crossref - Djebaili R, Pellegrini M, Smati M, del Gallo M, Kitouni M. Actinomycete strains isolated from saline soils: plant-growth-promoting traits and inoculation effects on Solanum lycopersicum. Sustainability. 2020;12(11):4617.
Crossref - Soumare A, Boubekri K, Lyamlouli K, Hafidi M, Ouhdouch Y, Kouisni L. Efficacy of phosphate solubilizing Actinobacteria to improve rock phosphate agronomic effectiveness and plant growth promotion. Rhizosphere. 2021;17:100284.
Crossref - Tokala RK, Strap JL, Jung CM, et al. Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl Environ Microbiol. 2002;68(5):2161-2171.
Crossref - Shimizu M. Endophytic actinomycetes: biocontrol agents and growth promoters. In: Maheshwari D. (Eds) Bacteria in agrobiology: Plant Growth Responses Springer, Berlin, 2011:201-220.
Crossref - Franco M, Valencia H. Evaluation of actinomycetes as growth inhibits Fusarium oxysporum f.sp. dianthi in carnations (Dianthus caryophyllus var. rosana). Ascolfinf. 2001;27(6):40-43.
- Pragya R, Yasmin A, Anshula J. An insight into agricultural properties of actinomycetes. Int J Res Bio Sci. 2012;1:7-12.
- Nassar AH, El-Tarabily KA, Sivasithampara K. Growth promotion of bean (Phaseolus vulgaris L.) by a polyamine-producing isolate of Streptomyces griseoluteus. Plant Growth Regulation. 2010;40:97-106.
Crossref - El-Tarabily KA, Sivasithamparamb K. Non-Streptomycete actinomycetes as Bio-control agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biol Biochem. 2008;38(7):1505-1520.
Crossref - Sadeghi E, Nasrabadi RG, Movahedi SA, Etesami H. Actinobacterial and fungal strain-enriched wheat straw as an effective strategy for alleviating the effect of salinity stress on soil chemical and biochemical properties. Chem Biol Technol Agric. 2022;9(1):90.
Crossref - Gopalakrishnan S, Vadlamudi S, Bandikinda P, et al. Evaluation of Streptomyces strains isolated from herbal vermicompost for their plant growth-promotion traits in rice. Microbiol Res. 2014;169(1):40-48.
Crossref - Franco-Correa M, Quintana A, Duque C, Suarez C, Rodriguez MX, Barea JM. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol. 2010;45(3):209-217.
Crossref - Holt JG, Krirg NR, Sneat, PH, Standley JT, Williams ST. Bergey’s manual of determinative bacteriology, 9th edn. Williams and Wilkins, Baltimore, USA. 1994.
- Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol.1951;26(1):192-195.
Crossref - Paleg LG. Physiological effects of gibberellins. Ann Rev Plant Physiol. 1965;16:291-322.
Crossref - Pikovskaya RI. Mobilization of phosphates in soil in connection with the vital activities of some microbial species. Mikrobiol. 1948;17:362-370.
- Hu XF, Chen J, Guo J. Two phosphate and potassium solubilizing bacteria isolated from Tiannu mountain, Zhejiang, China. World J Micro Biotech. 2006;22:983-990.
Crossref - Tabatabai MA, Bremner, JM. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Boil Biochem. 1969;1(4):301-307.
Crossref - Radha TK. Diversity of Actinomycetes in crop rhizosphere of arid, semi arid and humid regions (AER 3 and AER 6) and their effect on growth and yield of maize (Zea mays l.) and chickpea (Cicer arietinum). Ph.D (Agri. Microbiology) Thesis, Univ Agric Sci Bangalore, 2016;220.
- Radha TK, Rao DLN, Sree Ramulu KR. Media comparison for enumeration of Actinobacteria and their catabolic diversity in the crop rhizosphere of arid, semi-arid and humid regions (AER 3 and AER 6) of India. Int J Curr Microbiol Appl Sci. 2017;6(10):955-962.
Crossref - Meyer JR, Linderman RG. Selective influence on populations of rhizosphere or rhizoplane bacteria and actinomycetes by mycorrhizas formed by Glomus fasciculatum. Soil Biol Biochem, 1986;18(2):191-196.
Crossref - Goodfellow M, Simpson KE. Ecology of Streptomycetes. Front Appl Microbiol. 1987;2:97-125.
- Alexander M. Introduction to Soil Microbiology, Wiley International Edition, Tropan Company Ltd. Japan. 1961.
- Gesheva V, Geshava R. Physiological and antagonistic potential of actinomycetes from Ioquat rhizosphere. Microbio Res. 2000;155(2):133-135.
Crossref - Barreto TR, de Silva ACM, Soares ACF, de Souza JT. Population densities and genetic diversity of actinomycetes associated to the rhizosphere of Theobroma cacao. Braz J Microbiol. 2008;39(3):464-470.
Crossref - Mansour SR. The occurrence and distribution of soil actinomycetes in Saint Catherine area, South Sinai, Egypt. Pak J Biol Sci. 2003;6(7):721-728.
Crossref - Patten CL, Glick BR. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol. 1996;42(3):207-220.
Crossref - Solans M, Vobis G, Wall LG. Saprophytic actinomycetes promote nodulation in Medicago sativa- Sinorhizobium meliloti symbiosis in the presence of high N. J Plant Growth Reguln. 2009;28(2):106-114.
Crossref - Poovarasan S, Mohandas S, Paneerselvam P, Saritha B, Ajay KM. Mycorrhizae colonizing actinomycetes promote plant growth and control bacterial blight disease of pomegranate (Punica granatum L. cv Bhagwa). Crop Protection. 2013;53:175-181.
Crossref - AtlasRM, Bartha R. Microbial Ecology, Fundamentals and Applications, Addison-Wesley, London. 1981.
- Gupta N, Sahoo D, Basak UC. Evaluation of in vitro solubilization potential of phosphate solubilizing Streptomyces isolated from phyllosphere of Heritiera fomes (mangrove). Afr J Microbiol Res. 2010;4(3):136-142.
- Gangwar M, Rani S, Sharma N. Investigating endophytic actinomycetes diversity from Rice for plant growth promoting and antifungal activity. Int J Adv Life Sci. 2012;1:10-21.
- Narayanasamy P, Arunkumar A, Cyril KA,Titty H. Coco Pods As Source of organic potassium and potassium mobilization by Actinomycetes. The Internet J Nut Wellness. 2008;8(2).
Crossref - Archana DS. Studies on potassium solubilizing bacteria. M.Sc. (Agri.) Thesis, Univ Agric Sci, Dharwad, 2007;64.
- Franco-Correa M, Quintana A, Duque C, Suarez C, Rodriguez MX, Barea JM. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol. 2010;45(3):209-217.
Crossref - Stamenov D, Jarak M, Duric S, Hajnal-Jafari T, Andelkovi S. The effect of Azotobacterand actinomycetes on the growth of English ryegrass and microbiological activity in its rhizosphere. Res J Agric Sci. 2012;44(2):93-99.
- Yandigeri MS, Meena KK, Singh D, et al. Drought-tolerant endophytic Actinobacteria promote growth of wheat (Triticuma estivum) under water stress conditions. Pl Growth Reg. 2012;68(3):411-42.
Crossref - Gopalakrishnan S, Srinivas V, Vidya MS, Rathore A. Plant growth-promoting activities of Streptomyces spp. in sorghum and rice. Springer Plus. 2013;2(1):574.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.