ISSN: 0973-7510
E-ISSN: 2581-690X
The present study concerned with the evaluation of the adding value from the addition of plant extracts, including those from moringa, fennel, sage and green tea, during alginate encapsulation on the viability of probiotic bacteria (L. plantarum DSM 20205 and P. acidilactici DSM 20238) in fruit juice (i.e., kiwi, prickly pear and carrot juice) and drinkable yoghurt throughout storage at 4°C. The results revealed that the survival rates of L. plantarum DSM 20205 and P. acidilactici DSM 20238 cells encapsulated with 0.05% (w/v) moringa extract were significantly higher than those of cells encapsulated with fennel and saga after storage for 30 days. The In vitro digestibility behaviour and survival of the novel capsules were studied in terms of the survival of L. plantarum DSM 20205 and P. acidilactici DSM 20238 based on sequential exposure to simulated salivary, gastric and intestinal fluids. This novel encapsulation additive significantly increased the survival of L. plantarum DSM 20205 and P. acidilactici DSM 20238 compared with the control capsules cells in simulated digestive fluids. Therefore, the appropriate amount of moringa extract for use in culture encapsulation was determined after the addition to fruit juices and drinkable yoghurt, and the effect of this extract was compared with the effect of adding green tea extract (a standard plant extract). Green tea and moringa extracts enhanced the stability of probiotic beads in all products compared to the controls after storage. Encapsulated L. plantarum DSM 20205 and P. acidilactici DSM 20238 showed better survivabilities than the control capsules. The studied strains showed better survival in prickly pear juice and drinkable yoghurt throughout storage at 4°C for 30 days.
Probiotic, Encapsulation, Plant extracts, Gastrointestinal model, Moringa.
The increasing number of innovative foods that promote the consumers health has been taken a priority in the field of food industry over the last decades. The very wide acceptance of functional food products due to their health benefits1. According to FAO/WHO, 20012, “probiotics arel ive microorganisms that when administered in sufficient amounts gives the host a health benefit”. In the early 1900s Elie Metchnikoff connected the longevity of Bulgarian peasants with their high consumption of fermented milk and the probiotics term has been used from this time. This is due to the bacteria present in yogurt which protect the gastrointestinal tract against the damaging effects of harmful bacteria3. In this study, survey have been done on several microorganisms and have revealed the benefits,such as decreasing cholesterol levels, reducing lactose intolerance, stimulating of the immune system, increasing mineral absorption, and relieving constipation as well as anti-hypertensive, anti-mutagenic and anti-carcinogenic effects, available to humans through the use of probiotics4,5,6. The impact of probiotics on human health has been a great advantage for the food industry because these microorganisms describe a significant division within the functional food industry7.
The demand for functional foods has grown considerably in recent years, now accounting for 5% of the international food market3. This increase is correlated with consumer attention, as these products are a source of nutrients and act as a promoters of wellness and health8,9. Fermented milks and yogurts are the most famous food vehicles for the delivery of probiotics due to their high acceptance by consumers and superior nutritional value10,11.
The demand for functional foods has grown considerably in recent years, now accounting for 5% of the international food market3. This increase is correlated with consumer attention, as these products are not only as a source of nutrients but also as promoters of wellness and health8,9. Fermented milks and yogurts are the most famous food vehicles for the delivery of probiotics due to their high acceptance by consumers and superior nutritional value10,11.
Encapsulation has been shown to be an alternative method for the safeguarding of probiotics from detrimental environmental factors12. Sodium alginate iscommonly used for this purpose because of its simplicity, low cost and biocompatibility13. Many researchers have reported that alginate combined with other materials, for example, Hi-maize starch14, inulin, galactooligosaccharides and fluctooligosac- charides15,16, gelatine12, chitosan, pectin, and glucomannan17,18, can be used to improve the survival of different probiotic strains under gastrointestinal environments and in food products during storage.
Currently, the influence of alginate blended with plant extracts on the survival of probiotics in different beverages and foods is not well understood. There are many evidence in the literature regarding, the potential of some antioxidant extract from natural plants containing high contents of phenolic and flavonoids compounds (Maisuthisakul, et al.19, Siriwatanametanon, et al.20, Abdel-Razek, et al. 21 (2017), Badr et al.22 and Shehata et al. 23). Consequently, the objective of this investigation was to determine the impact of alginate encapsulation with certain plant extracts on the stability of probiotic bacteria, including L. plantarum DSM 20205 and P. acidilactici DSM 20238, in fruit juices and drinkable yogurt during storage at 4°C for 28 days.
Preparation of plant extracts
Herbal plants, such as fennel (Foeniculum vulgare), moringa (Moringa oleifera), sage (Salvia officinalis) and green tea (Camellia sinensis L.), were purchased from a market in Alexandria, Egypt, in 2017. All plants were dehydrated in an oven at 55°C for 2 days before powdering. Water extracts were made (1:5) ( w/v) then extracts were filtered through Whatman No. 1 filter paper (Whatman,Spain). All filtrates were then lyophilized using a freezedrier (Dura-Dry MP freezedrier FTS System, USA) at -50 °C for 15–20 h.
Probiotic cultures
Probiotic bacteria, including Lactobacillus plantarum DSM 20205and Pediococcus acidilactici DSM 20238, were purchased from Egypt Microbial Culture Collection (EMCC), Ain shams, Egypt. Cell pellets of L. plantarum DSM 20205 and P. acidilactici DSM 20238, lyophilized cells were subculture in autoclaved MRS broth at 37 °C for 20h. Activated cells were centrifuged at 3000 xg for 20 min, washed twice with 0.1% (w/v) autoclaved water peptone. Cells log were adjusted to 1010 cfu/ml prior encapsulation.
Encapsulation of probiotics
Probiotic capsules were produced with some modifications in accordance with the procedure described by Chaikham, et al.12. Cells were mixed with Sodium alginate sterile solution (Sigma-Aldrich, UK) and plant extracts were added in different concentrations (0.05–0.2%, w/v). Capsules were formed using 0.5 mm sterile needle by injecting the previous mixture in to 0.5 M sterilized calcium chloride solution and then kept for gelation for 30 min. After gelation the beads were washed with sterile saline at 0.85 percent (w / v) and kept at 4 ° C.
Enumeration of immobilized probiotics
Briefly, one gram of probiotic capsules was diluted with 99 ml 0.1M autoclaved phosphate buffer (pH7) (Merck, Germany) and grinded for 10 min, decimal dilutions were done then plating on MRS agar. Plates were anaerobically incubated at 37 °C for 24–72 h.
Viability of probiotic encapsulated with herbal extracts during storage
Ten grams of L. plantarum DSM 20205 and P. acidilactici DSM 20238 immobilized with plant extracts were kept in a glass bottle and stored at 4 °C for 30 days. Survival rates were monitored weekly.
Preparation of simulated digestive fluids and in-vitro digestion of encapsulated probiotics
Simulated salivary fluid (SSF), simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were prepared according to the method proposed by Minekus et al.25, and the details of the solutions including the stock solutions are presented in Table 1.
Table (1):
Details of the composition of stock solutions used to prepare simulated digestive fluids. The final volume of each simulated fluid was adjusted to 500 ml with distilled water.
Components |
Stock Concentration (M) |
SSF (pH 7.0)
Concentration (mM) |
SGF (pH 3.0) Concentration (mM) |
SIF (pH 7.0) Concentration (mM) |
|---|---|---|---|---|
KCl |
0.5 |
20.1 |
6.9 |
6.8 |
KH2PO4 |
0.5 |
8.7 |
0.9 |
0.8 |
NaHCO3 |
1 |
23.6 |
25 |
91 |
NaCl |
2 |
– |
47.2 |
38.4 |
MgCl2(H2O)6 |
0.15 |
0.3 |
0.12 |
0.33 |
SSF = Simulated Salivary Fluid, SGF = Simulated Gastric Fluid, SIF = Simulated Intestinal Fluid.
Survival of encapsulated probiotics in fruit juices and yogurt during storage
Assessing the viability of encapsulated probiotics in fruit juices and drinkable yogurt, 10 g ofencapsulated probiotics were inoculated aseptically into 90 ml of pasteurized kiwi, prickly pear and carrot juices or 90g of milk before storing at 4 °C. post inoculation, samples were taken at 0, 7, 14, 21 and 28days in order to quantify viable numbers as CFU/mlon MRS agar12. Product pH changes were monitored.
Data analysis
Results presented as standard deviation ± average. Variance analysis (ANOVA) was performed using (SPSS 16. Inc., USA). Duncan’s multiple range tests (P<0.05) determined the significant differences between the means of treatment groups.
Viability of L. plantarum DSM 20205 and P. acidilactici DSM 20238 encapsulated with plant extracts during storage
This study is investigating the potential for using plant extracts to increase the stability of probiotic strains during storage at 4°C. Plant extracts, including fennel, moringa, saga and green tea, typically include high quantity of antioxidant components1,26,27. In this study, encapsulated probiotic cells and free cells were assessed (Table 2). The results showed that 0.05% moringa extract could noticeably increase the survival rates of L. plantarum DSM 20205 and P. acidilactici DSM 20238 cells when compared to control and other plant extract treatments (P<0.05). Fennel and saga extracts had the smallest impacts on the survival of probiotic cells compared to the other extracts. Similar findings were obtained by Chavarri et al.17 for B. bifidum and L. Gasseri encapsulated with quercetin during storage at 4°C for 28 days. The effects of the extracts of different species of green tea on the survival of Bifidobacterium animalis spp. lactis LAFTI-B94, Lactobacillus paracasei LAFTI-L26 and Lactobacillus acidophilus LAFTI-L10were studied by Lopez de Lacey et al.27; they concluded thatthe addition of these extracts during encapsulation had a positive effect on the viability of probiotic cells. This positive effectcanbe attributed to the antioxidant activity of these plant extracts, and this activity is important to the viability and stability of probiotic bacteria.
Table (2):
Stabilityof two probiotic strains encapsulatedwithandwithout plant extractsduringrefrigeratedstorage
| Plant extracts | Concentration (%w/v) | Number of survival cells (CFU/g beads) | Cell loss (log CFUs) | ||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |||
| Lactobacillus plantarumDSM 20205 | |||||||
| Control | 0 | 9.69 ±0.04a | 8.34 ±0.08b | 7.41 ±0.15c | 6.25± 0.18d | 4.58 ± 0.18e | 5.11 ± 0.14A |
| Green Tea | 0.05 | 9.42 ±0.09a | 8.64 ±0.09b | 7.93 ±0.13c | 6.96 ±0.22d | 6.38 ± 0.07e | 3.04 ± 0.14E |
| 0.1 | 9.33 ±0.11a | 8.25 ± 0.2b | 7.68 ±0.24c | 6.51± 0.08d | 5.85 ± 0.14e | 3.48 ± 0.12D | |
| Moringa | 0.05 | 9.32 ±0.13a | 8.87 ±0.23a | 8.06 ±0.28b | 7.50 ±0.07c | 5.65 ± 0.10d | 3.67 ± 0.04D |
| 0.1 | 9.58 ± 0.09a | 8.56 ± 0.15b | 8.04 ± 0.28c | 7.36 ± 0.19d | 5.75 ± 0.25e | 3.83 ± 0.025CD | |
| Fennel | 0.05 | 9.18 ± 0.17a | 8.34 ± 0.27b | 7.47 ± 0.24c | 6.67 ± 0.10d | 4.60 ± 0.34e | 4.58 ± 0.22BC |
| 0.1 | 9.28 ± 0.30a | 8.63 ± 0.10b | 7.30 ± 0.04c | 6.41 ± 0.09d | 4.83 ±0.06e | 4.45 ± 0.32C | |
| Marmaria | 0.05 | 9.38 ±0.19a | 8.34 ± 0.20b | 7.94 ± 0.21c | 6.64 ± 0.12d | 4.76 ±0.1e | 4.61 ± 0.18BC |
| 0.1 | 9.36 ± 0.12a | 8.04 ±0.15b | 7.35 ± 0.13c | 6.38 ± 0.31d | 4.49 ±0.18e | 4.87 ± 0.30ABC | |
| PediococcusacidilacticiDSM 20238 | |||||||
| Control | 0 | 9.63 ± 0.12a | 8.30 ± 0.12b | 7.36 ± 0.03c | 6.41 ± 0.18d | 4.38 ±0.19e | 5.24 ± 0.30A |
| Green Tea | 0.05 | 9.71 ± 0.06a | 8.48 ± 0.06b | 8.09 ± 0.11c | 7.64 ±0.12d | 6.13 ± 0.08e | 3.58 ± 0.13D |
| 0.1 | 9.47 ± 0.25a | 8.52 ± 0.10b | 7.43 ± 0.17c | 7.48 ± 0.14c | 5.73 ± 0.10d | 3.73 ± 0.35CD | |
| Moringa | 0.05 | 9.82 ± 0.13a | 8.73± 0.21b | 8.01± 0.26c | 7.36 ± 0.24d | 6.24 ± 0.21e | 3.22 ± 0.33E |
| 0.1 | 9.81 ± 0.08a | 8.84± 0.11b | 7.78 ± 0.16c | 7.28 ± 0.23d | 6.02 ± 0.21e | 3.79 ± 0.26CD | |
| Fennel | 0.05 | 9.51 ± 0.04a | 8.58 ±0.15b | 7.65 ± 0.07c | 6.57 ± 0.34d | 4.86 ± 0.08e | 4.65 ± 0.13BC |
| 0.1 | 9.28 ± 0.25a | 8.34±0.32b | 7.36 ± 0.13c | 6.43 ± 0.11d | 4.81 ± 0.09e | 4.46 ± 0.28C | |
| Marmaria | 0.05 | 9.33 ± 0.30a | 8.0 ± 0.14b | 7.81±0.18b | 6.03 ± 0.07c | 4.41 ± 0.20d | 4.92 ± 0.30AB |
| 0.1 | 9.57 ± 0.12a | 8.10±0.11b | 7.70 ±0.20c | 5.77 ± 0.14d | 4.74 ± 0.08e | 4.83 ± 0.07ABC | |
Means in the same row or column followed by the same lower case or capital letters respectively are not significantly different (P<0.05). Each data point is the average of three replications.
In the present investigation, during refrigerated storage, the addition of 0.05% moringa extract to calcium alginate as the encapsulating material had a positive effect on probiotic cells. To date, it has been reported to improve probiotic stability by encapsulation with moringa extract. Referring to previous studies in this area, Coz-Bolaסos et al.28 and Wang et al.29 revealed that Moringa oleifera has a high content of antioxidant compounds. Hence, this extract can therefore create an anaerobic environment that promotes probiotic survival due to its oxygen – scouring properties30. This finding was confirmed by Shah et al.31, who found that fruit juices containing antioxidant compounds showed superior probiotic bacteria stability during 6 weeks of storage compared with the control sample, which was consistent with the positive effect of moringa extract on the survival and stability of probiotic strains. In summary, L. plantarum DSM 20205 and P. acidilactici DSM 20238 encapsulated with 0.05% moringa extract shown the highest survivability throughout storage at 4°C. Consequently, this amount of extract was elected for evaluating the effects of refrigerated storage on novel encapsulated probiotic cells in fruit juices and drinkable yogurt compared to the negative control treatment and treatments with green tea extract as the positive control.
Survival of herbal extract-encapsulated probiotics and control capsules cells in simulated digestive fluids
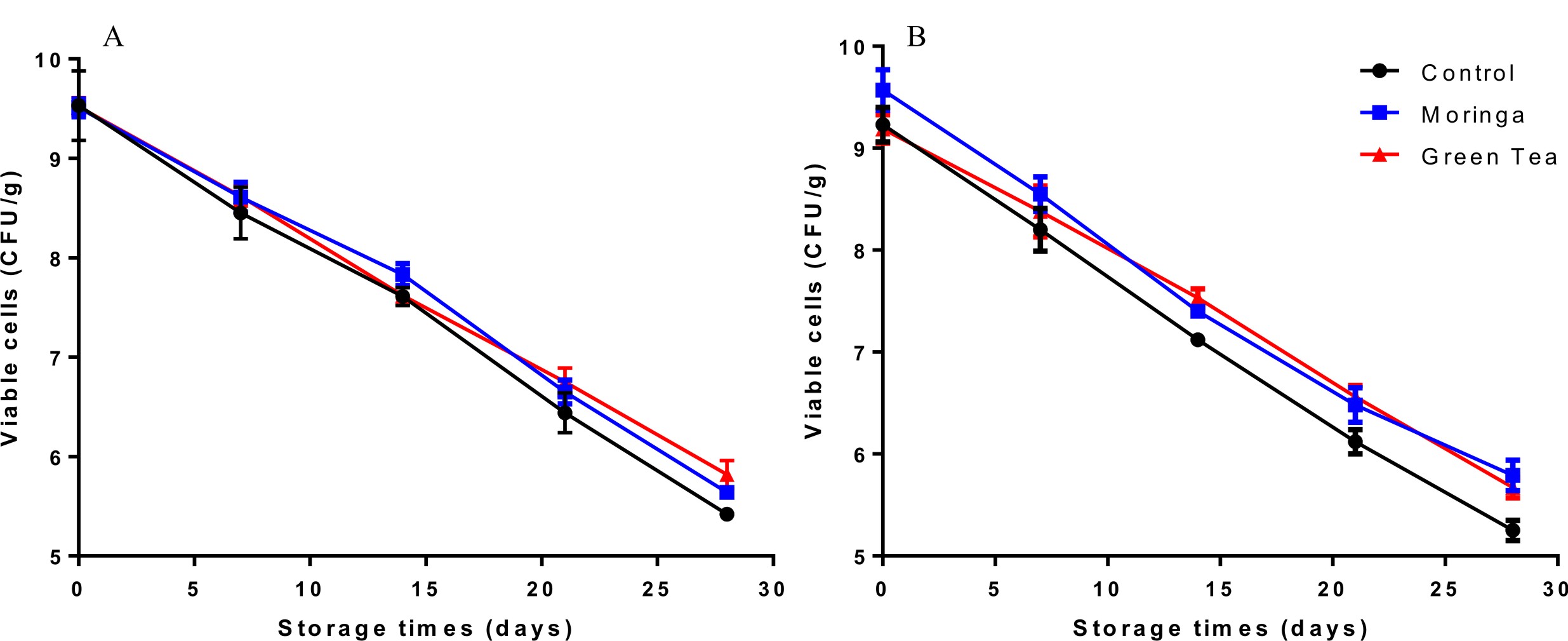
Probiotics can survive under acidic stomach conditions and throughout the intestines in suitable numbers between 106 and 108 cfu/g. However, their viability must be conformed early in any study18,32,33. The survival or viability of the control capsules cells (CC) contains calcium alginate only and novel capsules (NC) contains herbal extracts of L. plantarum DSM 20205 and P. acidilactici DSM 20238 in simulated digestive fluids (SSF, SGF, and SIF) are displayed in Figs. 1 & 2.
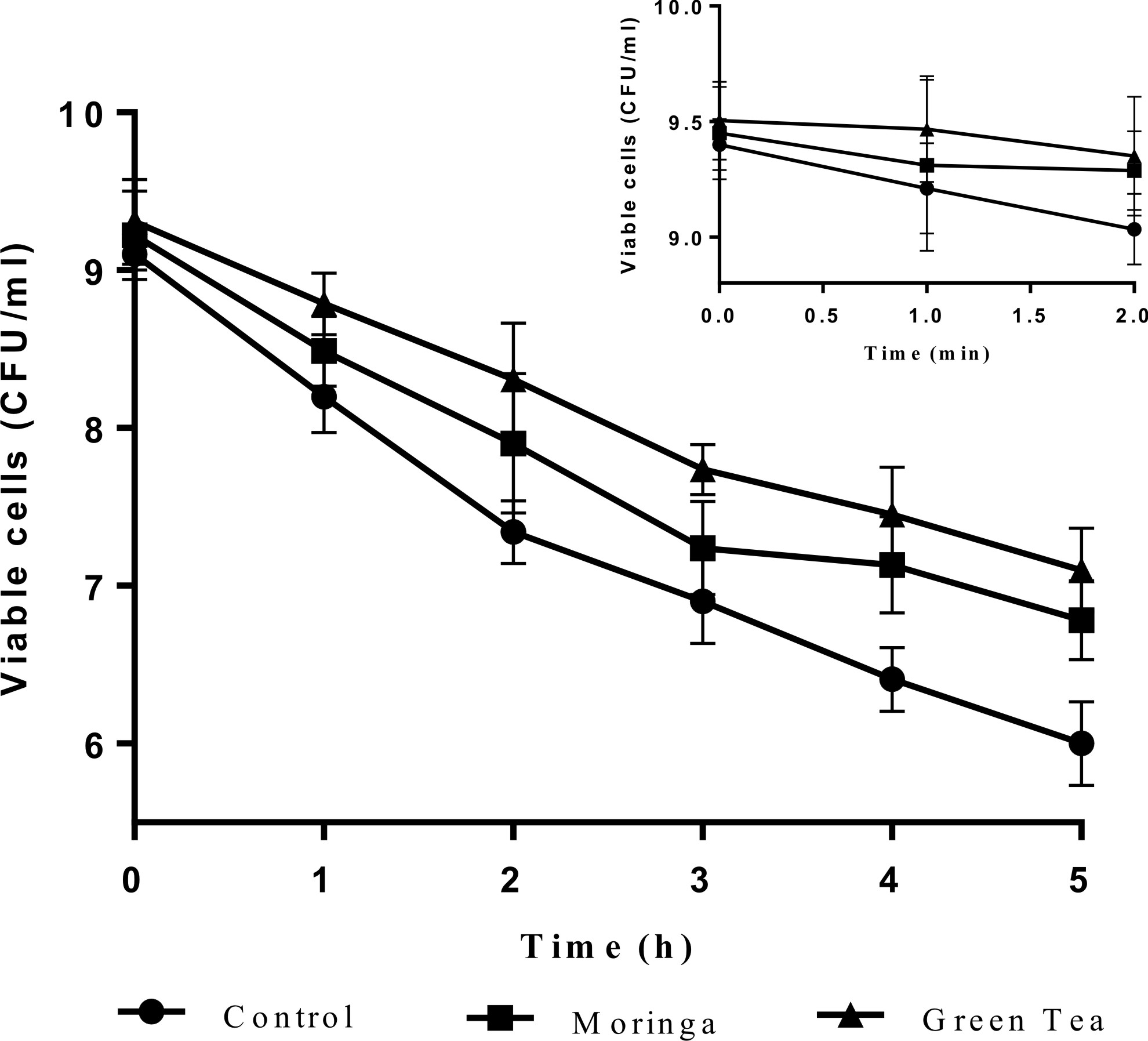
Fig. 1. Survival of control encapsulatedprobioticcells (CC) and novel encapsulatedprobioticcells (NC) with herbalextractsL. plantarum DSM 20205 upon sequential exposure to simulated salivary fluid (SSF) for 2 min, simulatedgastric fluid (SGF) for 2 h and simulated intestinal fluid (SIF) for 3 h. The insetrepresents the survival of cells when exposed in SSF for 2 min.
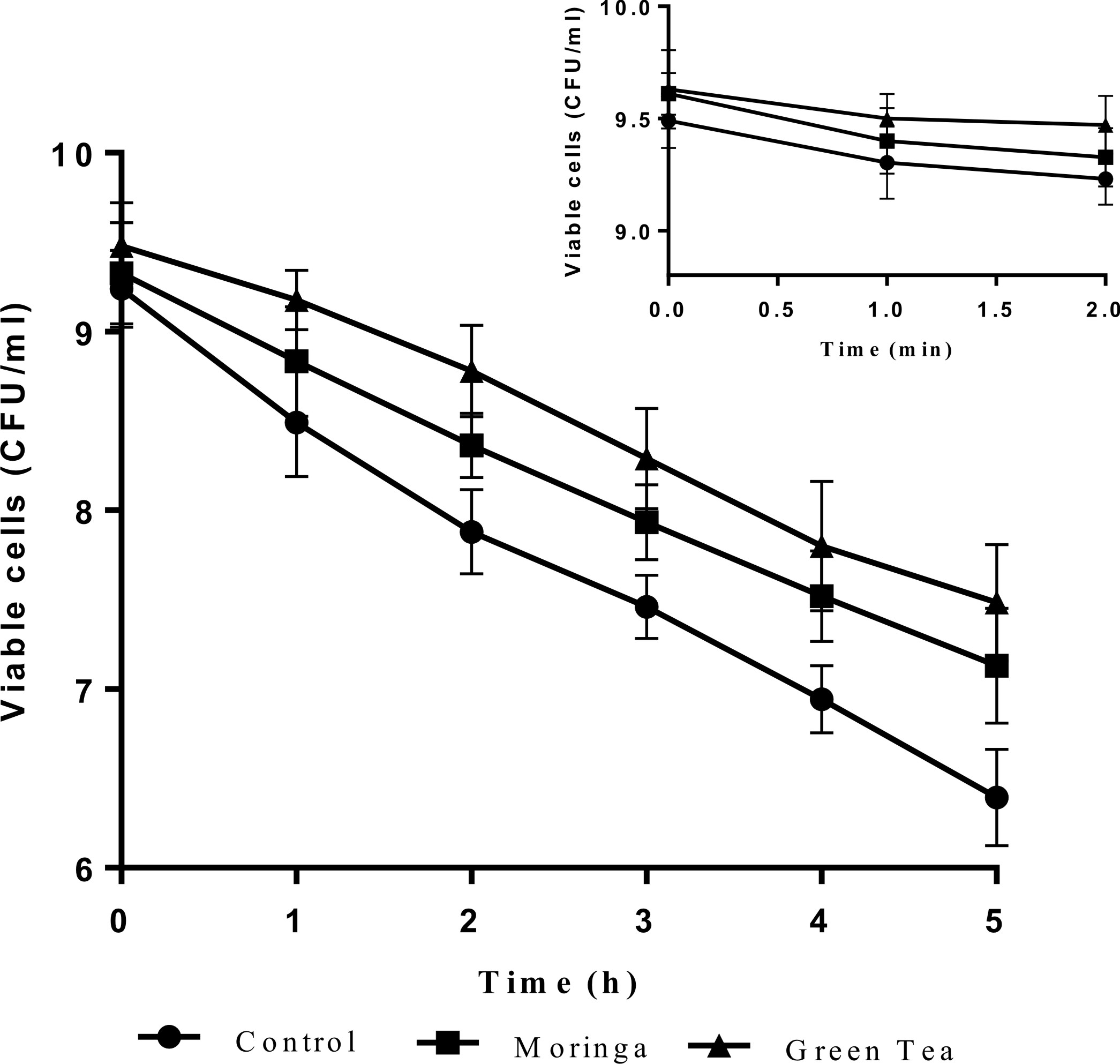
Fig. 2. Survival of control encapsulatedprobioticcells (CC) and novel encapsulatedprobioticcells (NC) with herbalextractsP. acidilacticiDSM 20238upon sequential exposure to simulated salivary fluid (SSF) for 2 min, simulatedgastric fluid (SGF) for 2 h and simulated intestinal fluid (SIF) for 3 h. The insetrepresents the survival of cells when exposed in SSF for 2 min.
These s trains showed a steadily loss of viability in simulated digestive fluids, but their sensitivity to SSF, SGF and SIF differed considerably. Dramaticaly reductions in the number of probiotic cells after exposure to simulated mouth fluid (SSF) were observed for both cells control capsules (CC) and novel capsules (NC) L. plantarum DSM 20205 and P. acidilactici DSM 20238 cells (Fig. 1 and 2), and a significant loss in (CC) viability corresponds to ~ 0.5 log cfu/ml. During the second step, exposure to SGF, which is a two-hour process and includes normal ingested foods also present in the stomach34, there was a remarkable decrease in both (CC) and (NC) numbers compared to the initial state after the first hour of incubation, but the cell numbers increased in the second hour. Novel capsules (NC) showed significantly better survivability (p < 0.05) than was seen with (CC), and the NC counts were 1.3 and 1 log cfu/ml for moringa extract and green tea extract-encapsulated cells), respectively, compared to 1.76 log cfu/ml for (CC). The last step, which is exposure to SIF, was the most important part and the target of this work as this step determines if these beneficial bacteria can reach the intestine in reasonable live numbers to be able to exert their effects. Our novel capsules (NC) successfully improved the survival relative to ordinary (CC). The same results were obtained by Mandal et al. (2006) in their study on free Lactobacillus casei NCDC-29 cells revealed to different concentrations of bile salts; they noticed a decrease in the cell counts from 9.34 to 5.60 log cfu ml-1. These results show that the presence of plant extracts will protect bacterial cells throughout the simulated gastric-intestinal system. This effect may be due to their strong antioxidant activity, which has been confirmed by many previous studies27. As mention above, these results strongly support the improvement of the survival of probiotic bacteria in the human digestive system by encapsulating probiotic bacteria with plant extracts.
Survival of probiotics encapsulated with plant extracts in fruit juices and drinkable yogurt during storage at 4°C
The survivals of L. plantarum DSM 20205 and P. acidilactici DSM 20238 encapsulated with 0.05% moringa extract and green tea extract during storage at 4°C for 30 days were evaluated after aseptically transferring the encapsulated substance into various fruit juices, like kiwi, prickly pear and carrotjuices. The results in Table 3 show that the number of cells of all encapsulated cultures in each fruit juice decreased continuously (P<0.05) with increasing storage time. Moreover, all cultures survived better in prickly pear juice than in kiwi and carrotjuices.
Table (3):
Survivability of probiotics encapsulated with and without plant extracts in fruit juices during refrigerated storage.
| Fruit juices | Herbal extracts | Number of survival cells (CFU/ml fruit juices) | Cell loss (log CFUs) | ||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |||
| Lactobacillus plantarumDSM 20205 | |||||||
| Kiwi juice | Control | 9.51 ±0.30a | 8.44± 0.14b | 7.47 ±0.18c | 6.23 ±0.06d | 4.78 ±0.17e | 4.73±0.42AB |
| Moringa | 9.54 ± 0.08a | 8.58±0.17b | 7.53 ±0.06c | 6.43 ±0.15d | 5.21 ±0.15e | 4.32±0.21BCDE | |
| Green Tea | 9.24 ± 0.11a | 8.44±0.17b | 7.84±0.06c | 6.44±0.17d | 5.10± 0.09e | 4.13±0.17DEF | |
| Prickly pear juice | Control | 9.55 ±0.19a | 8.44 ±0.06b | 7.47 ±0.05c | 6.51 ±0.04d | 5.26 ± 0.12e | 4.29±0.10CDE |
| Moringa | 9.40 ±0.43a | 8.67 ±0.07b | 7.62 ±0.09c | 6.57 ±0.17d | 5.47±0.25e | 3.93±0.17 DEFG | |
| Green Tea | 9.26 ± 0.17a | 8.57 ± 0.21b | 7.41 ± 0.08c | 6.45 ± 0.12d | 5.36 ± 0.09e | 3.89±0.10 DEFG | |
| Carrotsjuice | Control | 9.58 ± 0.17a | 8.13 ± 0.15b | 7.10 ± 0.10c | 6.13 ± 0.06d | 4.97 ± 0.15e | 4.61±0.17ABC |
| Moringa | 9.50 ± 0.19a | 8.43 ± 0.10b | 7.41 ± 0.10c | 6.39 ± 0.09d | 5.19 ± 0.16e | 4.30±0.31BCDE | |
| Green Tea | 9.34 ±0.07a | 8.28 ± 0.14b | 7.51 ± 0.10c | 6.54 ± 0.07d | 5.32 ± 0.24e | 4.02±0.16DEFG | |
| Pediococcus acidilactici DSM 20238 | |||||||
| Kiwi juice | Control | 9.69±0.13a | 8.16±0.15b | 7.23±0.15c | 6.43±0.12d | 4.81±0.19e | 4.87±0.29A |
| Moringa | 9.51±0.08a | 7.92±0.10b | 7.32±0.03c | 6.59±0.09d | 5.27±0.19e | 4.24±0.20CDEF | |
| Green Tea | 9.22±0.07a | 8.07±0.11b | 7.58±0.06c | 6.72±0.11d | 5.12±0.08e | 4.10±0.16DEF | |
| Prickly pear juice | Control | 9.46±0.20a | 8.12±0.08b | 7.08±0.17c | 6.50±0.19d | 5.33±0.12e | 4.13±0.14DEF |
| Moringa | 9.50±0.24a | 8.44±0.19b | 7.31±0.11c | 6.75±0.11d | 5.66±0.39e | 3.83±0.40FG | |
| Green Tea | 9.51±0.27a | 8.47±0.22b | 7.53±0.08c | 6.83±0.11d | 5.86±0.15e | 3.64±0.26G | |
| Carrotsjuice | Control | 9.55±0.25a | 7.80±0.17b | 6.94±0.07c | 6.11±0.07d | 5.19±0.14e | 4.36±0.13BCD |
| Moringa | 9.40±0.17a | 8.10±0.10b | 7.21±0.11c | 6.35±0.10d | 5.40±0.16e | 4.00±0.26 DEFG | |
| Green Tea | 9.28±0.25a | 8.23±0.11b | 7.35±0.12c | 6.51±0.07d | 5.3±0.10e | 3.95±0.16 DEFG | |
Means in the same row or column followed by the same lower case or capital letters respectively are not significantly different (P<0.05). Each data point is the average of three replications.
Nualkaekul et al.35 showed that Lactobacillus plantarum encapsulated with chitosan-coated alginate beads could survive in pomegranate juice throughout storage at 4°C. Chaikham36 mentioned the effect of alginate encapsulation with Thai herbal extracts, including extracts of yanang, pennywort and cashew flower, on the survival of Lactobacillus casei 01, Lactobacillus acidophilus LA5 and Bifidobacterium lactis Bb-12 suspended in melon, longan, maoberry and mulberry juices during storage at 4°C. Similar results were reported by Ding and Shah33 in apple and orange juices containing encapsulated Bifidobacterium longum, B. lactis and Lactobacillus plantarum which could survive six weeks of storage at 4°C, whilst the free cells lost their viability during five weeks.
Our results show that encapsulation with 0.05% moringa extract or green tea extract significantly increased the stability of L. plantarum DSM 20205 and P. acidilactici DSM 20238 compared to control in fruit juices during storage (Table 3). Similar to fruit juices, during storage, the surviving populations of probiotics with plant extracts suspended in drinkable yogurt and without them tended to decrease (Fig. 3). This investigation established that probiotics entrapped with 0.05% moringa extract or green tea extract survived better than probiotics encapsulated without plant extracts. The survival of L. plantarum DSM 20205 seemed to be higher than that of P. acidilactici DSM 20238 after 30 days of storage (Fig. 4). Our findings were consistent with the findings of Krasaekoopt and Watcharapoka15 who reported the survivability of microencapsulated probiotics in a simulated digestive system, fruit juice and drinkable yogurt. Brinques and Ayub37 studied the effects of immobilization techniques on the survival of lactobacilli in yogurt during refrigerated storage. The addition of green tea extracts has been positive impact on survival of B. animalis spp. lactis LAFTI-B94, L. acidophilus LAFTI-L10 and L. paracasei LAFTI-L26 during incubation for 72h at 37°C 27. They found that the addition of green tea extract could lead to a favourable an anaerobic environment for probiotic bacteria due to the oxygen-scavenging and antioxidant characteristics.
This study evaluated the effect of a novel encapsulation technique using calcium alginate and plant extracts on the stability of probiotic bacteria, including L. plantarum DSM 20205 and P. acidilactici DSM 20238,in fruit juices and drinkable yogurt during storage at 4°C for 28days. After 4 weeks of the storage period, the survivability of cells encapsulated with 0.05% moringa extract was significantly higher than those of probiotics encapsulated with fennel and sage extracts. Upon refrigerated storage, the extracts of both green tea and moringa improved the constancy of probiotic capsules in fruit juices and drinkable yogurt compared to the control capsules. Overall, the novel capsules improved the survival of L. plantarum DSM 20205 and P. acidilactici DSM 20238 in prickly pear juice and drinkable yogurt throughout storage. The novel capsules were sequentially subjected to simulated digestive fluids (SSF, SGF, and SIF) In vitro, and the results showed that the extracts enhanced the survival and intestinal adhering capacity and supported to keep a higher balance of probiotics in the human digestive system.
None
The author declares that there are no conflict of interest.
- Najgebauer-Lejko, D. Effect of green tea supplementation on the microbiological, anti-oxidant, and sensory properties of probiotic milks. Dairy Sci. Technol., 2014; 94: 327–339.
- FAO/WHO. Food and Agriculture Organization of the United Nations; World Health Organization. Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, 2001; (Cףrdoba, 34 pp.).
- Tripathi, M.K. and S.K. Giri. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods., 2014; 9: 225–241.
- Vasiljevic, T. and N.P. Shah. Probiotics from Metchnikoff to bioactives. Int. Dairy J., 2008; 18: 714–728.
- Zhu, Y., T.M., Luo, C. Jobin, and H.A. Young. Gut microbiota and probiotics in colon tumori-genesis. Cancer let., 2011; 309: 119–127.
- Palomar, M.M., C.M. Galdeano, and G. Perdigףn. Influence of a probiotic lactobacillus strain on the intestinal ecosystem in a stress model mouse. Brain BehavImmun., 2014; 35: 77–85.
- Stanton, C., G. Gardiner, H. Meehan, K. Collins, G. Fitzgerald and P.B. Lynch. Market potential for probiotics. Am. J. Clin. Nutr., 2001; 73: 476–483.
- Sanders, M.E. and M.L. Marco. Food formats for effective delivery of probiotics. Ann. Rev. Food Sci. Technol., 2010; 1: 65–85.
- Siro, I., E. Kבpolna, , B. Kapolna, and A. Lugasi. Functional food. Product development, marketing and consumer acceptance – A review. Appetite., 2008; 51: 456–467.
- Shah, N.P. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci., 2000; 83: 894–907.
- Antunes, A.E.C., E.T.G. Marasca, I. Moreno, F.M. Dourado, L.G. Rodrigues, and A.L.S. Lerayer. Desenvolvimento de buttermilk probiotico. Ciךncia e Tecnologia de Alimentos., 2007; 27: 83–90.
- Chaikham, P., A. Apichartsrangkoon, T. George, and W. Jirarattanarangsri. Efficacy of polymer coating of probiotic beads suspended in pressurized and pasteurized longan juices on the exposure to simulated gastrointestinal environment. Int. J. Food Sci. Nutr., 2013; 64: 862–869.
- Krasaekoopt, W., B. Bhandari, and H. Deeth. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J., 2003; 13: 3–13.
- Sultana, K., G. Godward, N. Reynolds, R. Arumugaswamy, P. Peiris, and K. Kailasapathy. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int J Food Microbiol., 2000; 62: 47–55.
- Krasaekoopt, W. and S. Watcharapoka. Effect of addition of inulin and ga- lactooligo saccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT–Food Sci Technol., 2014; 57: 761–766.
- Sathyabama, S., M. Ranjithkumar, P. Brunthadevi, R. Vijayabharathi, and V. Brindhapriyadharisini. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT– Food Sci Technol., 2014; 57: 419–425.
- Chavarri, M., I. Maraסףn, R. Ares, F.C. Ibanez, F. Marzo, and M. C. Villaran. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int J Food Microbiol., 2010; 142: 185–189.
- Nualkaekul, S., M. T. Cook, V. V. Khutoryanskiy, and D. Charalampopoulos. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res Int., 2013; 53: 304-311.
- Maisuthisakul, P., M. Suttajit, and R. Pongsawatmanit. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem., 2007; 100: 1409–1418.
- Siriwatanametanon, N., B.L. Fiebich, T. Efferth, J.M. Prieto, and M. Heinrich. Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. J Ethnopharmacol., 2010; 130: 196–207.
- Abdel-Razek, A. G., A. Noah Badr, and M. G. Shehata. Characterization of olive oil byproducts: Antioxidant activity, its ability to reduce aflatoxigenic fungi hazard and its aflatoxins. Ann Res Rev Bio., 2017; 14: 1-14.
- Badr, A. N.; M. G. Shehata, and A. G. Abdel-Razek. Antioxidant Activities and Potential Impacts to Reduce Aflatoxins Utilizing Jojoba and Jatropha Oils and Extracts, Int J Pharm. DOI: 10.3923/ijp., 2017.
- Shehata, M.G. A.N. Badr, A.G. Abdel-Razek, M. M. Hassanein, and H. A. Amra. Oilbioactive Films as an Antifungal Application to Save Post-harvest Food Crops, Ann. Res. Rev. Bio., 2017; 16: 1-16.
- Shehata, M.G.; S.A. El Sohaimy, A.M. El-Sahn and M.M. Youssef. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agri. Sci., 2016; 61: 65–75.
- Minekus, M., M. Alminger, P. Alvito, S. Ballance, T. Bohn, and C. Bourlieu. A standardised static in vitro digestion method suitable for food an international consensus. Food & Funct., 2014; 5: 1113–1124.
- Marhamatizadeh, M.H., E. Ehsandoost, and P. Gholami. The influence of green tea (Camellia sinensis L.) extract on characteristic of probiotic bacteria in milk and yoghurt during fermentation and refrigerated storage. IJFAS, 2013; 2: 599–606.
- Lopez de Lacey, A. M., E. Pיrez-Santםn, M. E. Lףpez-Caballero, and P. Montero. Survival and metabolic activity of probiotic bacteria in green tea. LWT–Food Sci. Technol., 2014; 55: 314–322.
- Coz-Bolaסos, X., R. Campos-Vega, R. Reynoso-Camacho, M. Ramos-Gףmez, G. Flavia Loarca-Piסa, and S.H. Guzmבn-Maldonado. Moringa infusion (Moringa oleifera) rich in phenolic compounds and high antioxidant capacity attenuate nitric oxide pro-inflammatory mediator in vitro. Ind. Crops Prod., 2018; 118: 95-101.
- Wang, Y., Y. Gao, H. Ding, S. Liu, X. Han, J. Gui, and D. Liu. Subcritical ethanol extraction of flavonoids from Moringa oleifera leaf and evaluation of antioxidant activity. Food Chem., 2017; 218: 152–158.
- Huang, D. J, B.X. Ou, and R.L. Prior. The chemistry behind antioxidant capacity assays. J Agric Food Chem., 2005; 53: 1841–1856.
- Shah, N.P., W.K. Ding, M.J. Fallourd, and G. Leyer. Improving the Stability of Probiotic Bacteria in Model Fruit Juices Using Vitamins and Antioxidants. J. Food Sci., 2010; 75: 278-282.
- Cook, M. T., G. Tzortzis, D. Charalampopoulos, and V. V. Khutoryanskiy. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release., 2012; 162: 56-67.
- Ding, W. K. and N. P. Shah. An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. J. Food Sci., 2008; 74: 53-61.
- Mandal, S., A.K. Puniya, and K. Singh. Effect of alginate concentrations on survival of microen-capsulated L. casei NCDC-298. Int Dairy J., 2006; 16: 1190–1195.
- Nualkaekul, S., D. Lentona, M.T. Cook, V.V. Khutoryanskiy, and D. Charalampopoulos. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Cat. Rev., 2012; 90: 1281- 1287.
- Chaikham, P.. Stability of probiotics encapsulated with Thai herbal extracts in fruit juices and yoghurt during refrigerated storage. Food Biosci., 2015; 12: 61–66.
- Brinques, G.B. and M.A.Z. Ayub. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J Food Eng.,2011; 103: 123–128.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.