ISSN: 0973-7510
E-ISSN: 2581-690X
In recent days, antibiotic producing fluorescent pseudomonads (FPs) has been used as a bioorganic tool for the control of sheath blight disease of rice. Combined application of antagonistic microorganism showed that significant bio control activity and enhances plant growth by induced systemic resistance (ISR). The present study, we carryout morphological, physiological and biochemical analysis and then identified, the selected isolate VSMKU2 is Pseudomonas sp. Maximum level of phenylalanine ammonia lyase (PAL) was quantified in the treatment of Pseudomonas sp VSMKU2 + R. solani on the 7th day (97.50 nmol trans-cinnamic acid/min/g). Similarly, the cell free culture filtrate of VSMKU2 challenged with R. solani demonstrated lower level of PAL activity on 7th day (91.76 nmol trans-cinnamic acid/min/g) compared to control. Peroxidase (PO) and polyphenoloxidase (PPO) gave higher activity in Pseudomonas sp VSMKU2 challenged with R. solani on 7th day (0.94 and 0.95 unit/min/g of proteinrespectively) but 14th and 21st day after challenged inoculation of R. solani had been reduced (0.92, 0.75 and 0.82, 0.65 unit/min/g of protein) compared to control. The total phenol content activity was significantly increased with Pseudomonas sp VSMKU2 (148.27 µg catechol/mg/g of protein) and cell free culture filtrate of VSMKU2 (137 µg catechol/mg/g of protein) treated in rice seedlings on 7th day after challenged inoculation of R. solani compared to control. The results obtained in the current study imply to Pseudomonas sp VSMKU2 was able to rise defence response, thereby contribute resistance to sheath blight disease.
Pseudomonas sp VSMKU2, R. solani, Rice seedlings, defence related enzymes.
Sheath blight of rice (ShB) is a critical diseases caused by Rhizoctonia solani. Rice yield was condensed up to 69% in tropical Asian countries like India and China1. For management of ShB of rice using chemical fungicides cause severe threat to the environment and public health. Earlier days, chemical fungicides are used for control of soil borne fungal pathogens, but these chemical fungicides persist in the agriculture ecosystem and cause toxicity to beneficial microbes and develop resistance to the plant pathogens2. Hence, we need to find out alternative approach for control of plant disease through bio control methods without causing any environmental problems and health hazards3. In recent days, biological control is one of the best choices and extensively documented as both safe and consistent clarification for sustainable agriculture. Bio control method is ecofriendly approach to minimize the risk of possible resistance under selection pressure4. Recent findings reported many microorganism considered as potential biocontrol agents such as Pseudomonas aeruginosa MML2212, Burkholderia, Ceratobasidium, Bacillus pumilus MTCC7615 and Streptomyces aurantiogriseus VSMGT10143,5-8. Among different beneficial microbial population, fluorescent pseudomonads have drawn much attention worldwide, since, it has plant growth promotion efficiency and major biocontrol potential for fungal pathogens. Moreover it does not cause any environmental problems and health hazards9. Fluorescent pseudomonads (FPs) are reported to be a major associated bacteria10. FPs demonstrates has the ability to produce IAA, ACC deaminase, siderophore, hydrogen cyanide and lytic enzymes. Previous report showed that FPs strains facilitate to raise seed germination, plant growth and yield 11.
Pseudomonas spp are activates systemically in the plant system through induced systemic resistance (ISR). Recent report demonstrated that, plant growth promoting rhizobacteria (PGPR) activating defence genes encoding chitinase, POX, PPO and PAL in plants12. P. fluorescens is providing plant growth promotion against plant diseases such as sheath blight, sheath rot, blast of rice, bacterial blight of cotton, ground nut, Pythium disease of tomato and hot pepper13-16. The ISR induced by Pseudomonas sp was established in bean, carnation, rice, cucumber and raddish13,17-20.
Previous reports showed, the seed treatment with soil application of P. fluorescens DABBV4 enhanced seed germination and vigour index. Further, wilt disease was considerably reduced by P. fluorescens treated seeds challenge with R. solanacearum21. The objectives of the current study deal with the identification of selected isolate VSMKU2. To carry out green house experiment for sheath blight of rice with treatment of VSMKU2 and their cell free culture filtrate against R. solani. After 1-3 weeks of R. solani inoculation, we examine PAL, PO, PPO activity and total phenol content.
Antagonistic and Pathogenic culture collection and maintenance
The culture collection and maintenance of selected antagonistic isolate VSMKU2 and pathogen R. solani were obtained from our lab culture collection in the Department of Microbial Technology, School of Biological Sciences, Madurai Kamaraj University, which was isolated from rice rhizosphere. Sheath blight disease causing R. solani was used in this study. The selected antagonistic isolate VSMKU2 and R. solani were kept at 4°C in King’s B and Potato Dextrose Agar (PDA) for regular research work. For long time storage, the isolate VSMKU2 was stored in 40% glycerol at – 80°C.
Morphology and Biochemical analysis
The selected isolate VSMKU2 was identi ed by colony morphology, cells shape, including gram staining and pigmentation. The pyocyanin pigment was observed on King’s B medium. Isolate VSMKU2 was observed as rod shape under light microscope by staining with Grams reaction. Biochemical analysis was performed by Bergey’s Manual of Determinative Bacteriology22.
Green house experiment
The greenhouse experiments were performed in earthenware pots with rice seedling in complete randomized block design (CRBD) with triplicate. Wet nursery was organized in earthenware pots filled with sterilized field soil and rice seeds of IR-50 were sown as per the treatments. After 25 days, rice seedlings were transplanted to bigger pots for various treatments.
Pseudomonas sp VSMKU2 treatment and challenge inoculation with R. solani in green house
Pseudomonas sp VSMKU2 was used for defence reaction against R. solani. The treatments included (1) Healthy control (IR-50 seeds treated with sterile distilled water) (2) Inoculation of 25g of R. solani hull/rice seedlings (3) Pseudomonas sp VSMKU2 treated in seeds + soil application (25 mL bacterial cells, 7 x 108 Cfu/ml) with R. solani. (4) A 25 ml of cell free culture filtrate of Pseudomonas sp VSMKU2 and R. solani. The treatments were duplicated for three times. In 21 days’ time course of study, every one week interval; the samples were taken from all the treatments for defence related stress enzymes assay such as PAL, PO, PPO and total phenol.
Preparation of rice leaf extracts
Defence related enzyme assay was performed using rice leaf extracts. Rice leaves were collected on 7th, 14th and 21st days after challenged inoculation of R. solani and were stored at -80°C until extract was prepared. From all the treatments, rice leaves were collected every three rice seedlings about 10cm.
Phenylalanine ammonia lyase (PAL)
Phenylalanine ammonia lyase estimation was performed according to Dickerson et al. (1984)23. Briefly, One gram of rice leaves were taken and homogenized then followed by the above said method.
Peroxidase (PO)
Peroxidise activity was carryout at 30°C by the method of Hammerschmidt et al 24. Briefly one gram of rice leaves were homogenized using 2ml of 0.1 M phosphate buffer (pH 7.0) at 4 °C and then followed the above said method.
Polyphenol Oxidase (PPO)
Polyphenol oxidase activity was examined by the method of Mayer et al. (1965)25. One gram of rice leaf tissues were homogenized using two ml of 0.1 M sodium phosphate buffer (pH 6.5) and centrifuged at 16,000 g for 15 min at 4 °C and then followed by the above said method.
Total phenol content
Total phenol content was quantified according to Mayer et al, (1966)26. One gram of fresh leaf tissues were homogenized with 10 ml of 80% methanol and then followed the above mentioned method.
Statistical analysis
The pot experiments were carryout in a randomized design. The enzyme activity was presented as means ± standard deviations (S.D.) All treatments were repeated in triplicates with three plantlets per pots.
Identification of VSMKU2 and Characterization
PGPR have the ability to improve plant growth promotion and indirectly control fungal pathogens like R. solani, Pythium aphanidermatum, Colletotrichum orbiculare, Fusarium oxysporum3, 27-29. In the same way the current study, discover the potential antagonistic rhizobacterium enhance rice defence related stress enzymes in the inoculation and noninoculation of pathogen R. solani. Based on the plant growth promotion and bio control potential against fungal pathogen R. solani by the isolate VSMKU2 (data not shown) was selected for defence related stress enzymes in rice plants and its role for the management of sheath blight of rice.
Morphology
Isolate VSMKU2 exhibited good growth on King’s B agar medium with fluorescent colonies (Fig.1). It secretes a variety of pigments, including blue-green (pyocyanin) in different growth media. Light microscopic visualization revealed that the VSMKU2 is a rod-shaped bacterial cell. The light microscope images of VSMKU2 exhibited a detailed structure showing a rod shape.
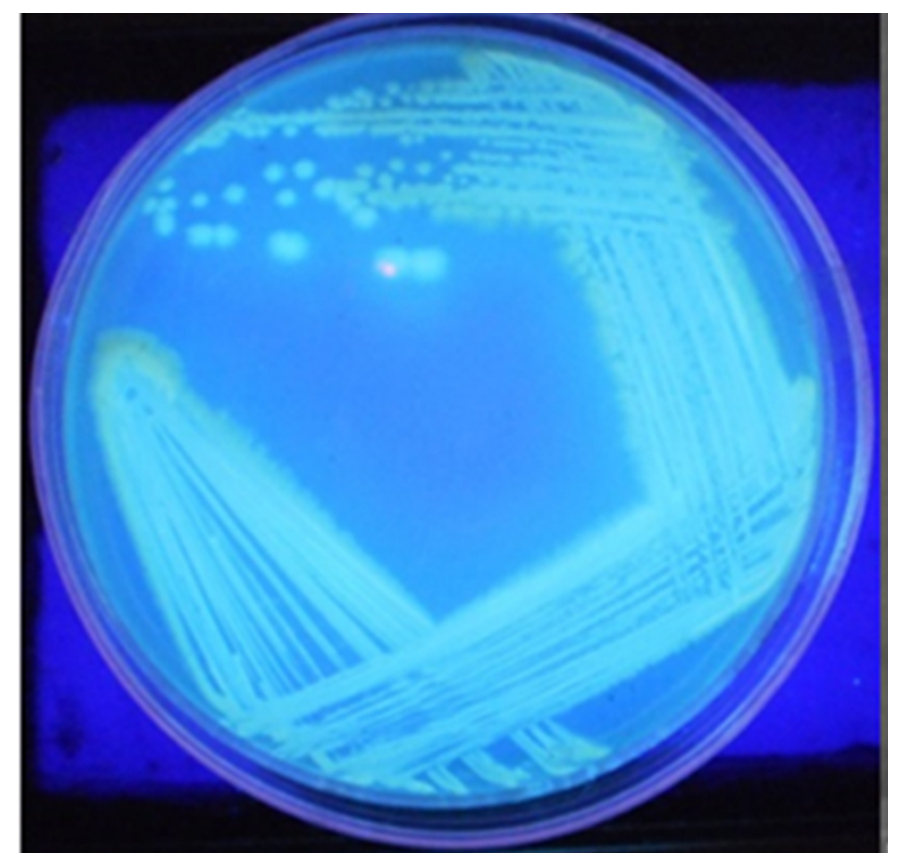 Fig. 1. Morphology of Pseudomonas sp. VSMKU2. Culture plate showing fluorescent pigment production under UV-transilluminator at 365 nm.
Fig. 1. Morphology of Pseudomonas sp. VSMKU2. Culture plate showing fluorescent pigment production under UV-transilluminator at 365 nm.Biochemical characteristics
Biochemical tests exposed that the isolate VSMKU2 is a Gram negative organism. It demonstrates positive reactions such as oxidase, catalase, citrate utilization, indole production, nitrate reduction and triple sugar iron (acid butt, alkaline slant, H2S production) tests. It exhibited negative reactions to MR and VP tests (Table 1). The isolate VSMKU2 effectively fermented for various carbon sources like glucose, fructose, sucrose and mannitol. However, it did not ferment arabinose, lactose, maltose and xylose (Table 1). The hydrolytic enzyme assay on different substrate amended medium was exposed, the VSMKU2 isolate secrete only amylase, cellulase, gelatinase and protease, however not produced pectinase and chitinase (Table 1) compared to control. Our present findings coherence with previous results 30-32.
Table (1):
Physiochemical and biochemical characteristics of Pseudomonas sp VSMKU2
Test |
Results |
|---|---|
Biochemical test
Gram’s staining reaction |
– |
Motility |
Motile |
Colony |
small, circular and yellow in colour |
Pigment production |
+ |
Optimum temperature for growth |
37ºC |
Optimum pH for growth |
7 |
Salt tolerance for growth |
0.1-1M |
Indole test |
+ |
Methyl red test |
– |
Voges-Proskauer |
– |
Citrate utilization |
+ |
Gelatin liquefaction |
+ |
Nitrate reduction |
+ |
TSI |
acid butt, alkaline slant, H2S production |
Catalase |
+ |
Oxidase |
+ |
Carbohydrate utilization test |
|
Glucose
Fructose |
+
+ |
Sucrose
Mannitol Lactose Maltose Xylose Arabinose Lytic enzyme Production Amylase Cellulase Gelatinase Protease Chitinase Pectinase |
+
+ – – – –
+ + + + – – |
Note: – absence, + presence.
Induction of defence related stress enzymes
Isolate VSMKU2 belongs to genus Pseudomonas sp were involved for the control of fungal and bacterial plant pathogens through antagonism, competition and by developing straight communications with host plants through Induced systemic resistance (ISR). In this study, we discuss the following defence related stress enzymes such as PAL, PO, PPO and total phenol.
Phenylalanine ammonia lyase (PAL)
The seed treatment and soil application of Pseudomonas sp VSMKU2 significantly induced maximum level of PAL activity (97.50 nmol trans-cinnamic acid/min/g) on 7th day inoculation of R. solani, whereas 14th and 21st day after inoculation of R. solani, the PAL activity reduced compared to control (Fig. 2). But, the cell free treatment of Pseudomonas sp VSMKU2 about 20 to 30% reduction of PAL activity was observed for all three consequent days of evaluation compared to pathogen and untreated control. Similar result was reported by Reshma et al. (2018)33 , showed that seed treatment and root dipping of Pseudomonas sp maximum in PAL activity on 7th day of challenge inoculation of R. solani in rice plants. Numerous fluorescent pseudomonads were showed to induce ISR33. Similarly, ISR condensed infection and enhanced plant growth promotion was reported in several crops34. Since, PAL is the primary enzyme in phenylpropanoid metabolism and phenolics and phytoalexins which reduced the development of pathogen35. The present study showed increased PAL activity due to Pseudomonas sp VSMKU2 action, which has the capacity to prevent the establishment of R. solani in rice roots and leaves. PAL has been involved a significant task in phenylpropanoid pathway, since lignin is a major product. Lignin accumulation is a provoke defence mechanism and strengthening against infection development. Superior PAL activity by Pseudomonas spp was reported in tomato 36, pearl millet37, cucumber12, tomato16, 20 and mulberry38.
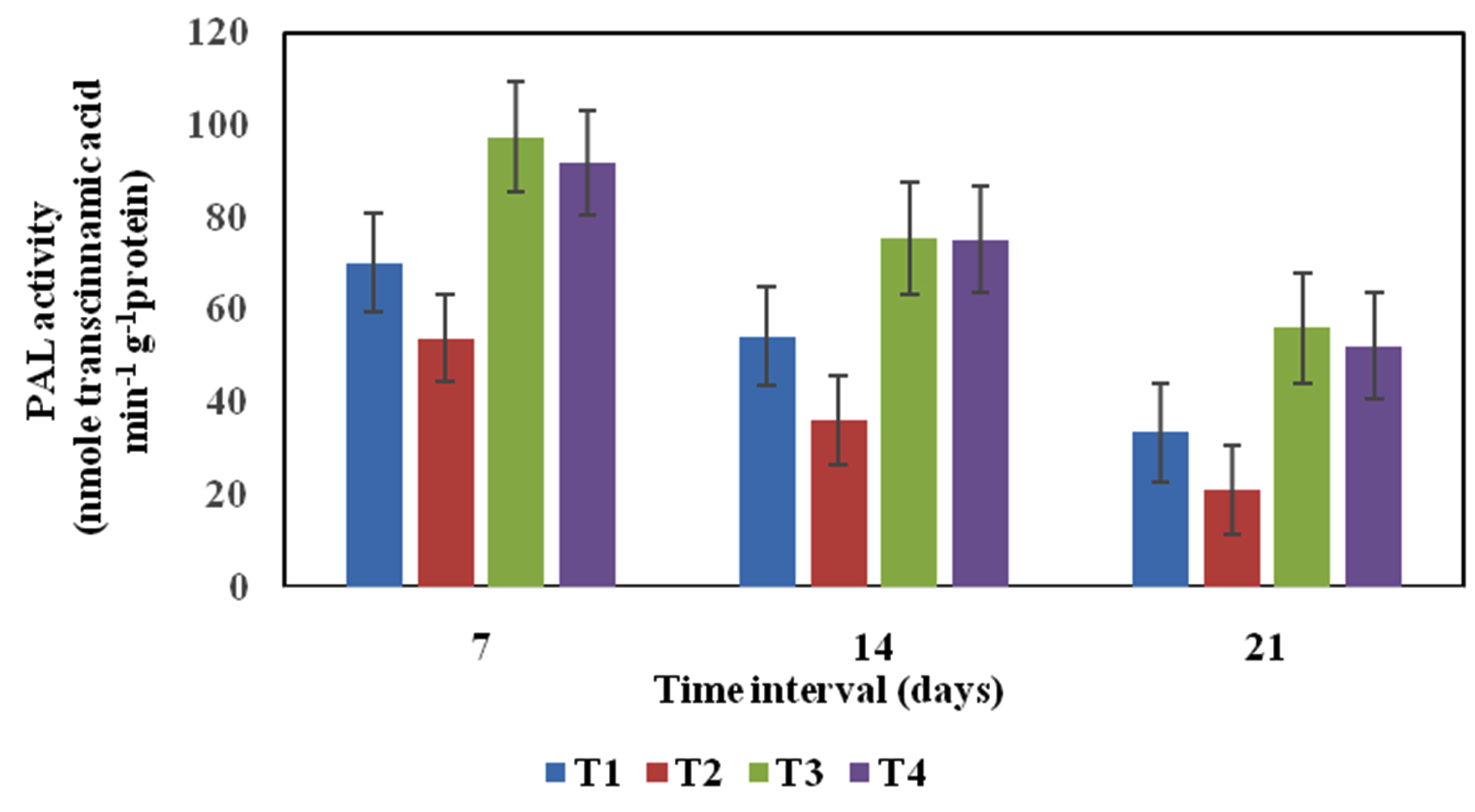 Fig. 2. Phenylalanine ammonia lyase (PAL) activity profile of rice leaves variety IR-50 in different treatments. T1- Healthy control (rice variety IR-50 treated with sterile distilled water); T2- Disease control (rice seed inoculated with R. solani); T3- P. aeruginosa VSMKU2 culture + R. solani; T4- P. aeruginosa VSMKU2 culture filtrate + R. solani at 7th, 14th and 21st day. The data represent the mean values based on three replicates in each treatment, Vertical bar indicate standard error.
Fig. 2. Phenylalanine ammonia lyase (PAL) activity profile of rice leaves variety IR-50 in different treatments. T1- Healthy control (rice variety IR-50 treated with sterile distilled water); T2- Disease control (rice seed inoculated with R. solani); T3- P. aeruginosa VSMKU2 culture + R. solani; T4- P. aeruginosa VSMKU2 culture filtrate + R. solani at 7th, 14th and 21st day. The data represent the mean values based on three replicates in each treatment, Vertical bar indicate standard error.Peroxidase (PO)
Peroxidase is the principal enzyme during biosynthesis of lignin. Due to production of PO, it gives strengthening to plant tissues and avoids pathogen entry in to the plants. PO could afford fortification from oxidative stress, through which lipid peroxidation ensuing in damage to the macromolecules, thereby inhibiting photosynthesis and other enzyme activities. In our study, PO activity has been increased on 7th and 14th days of challenged inoculation of R. solani with Pseudomonas sp VSMKU2 as seed and soil treatment in rice seedlings compared to control. Whereas, in the cell free culture filtrate treatment showed significant activity of PO on 14th days after challenge inoculation of R. solani in comparison to other two (7th and 21st) consequent days (Fig. 3). In concurrence with our result, Podile and Lakshmi (1998) 39 reported that PO activity was increased in pea plants treated by Bacillus subtilis against Fusarium udum after 7 day of inoculation. On the other hand, PO level has been improved after immunization of pathogen and accomplish its greatest at 9th hours after Ralstonia solancearum inoculation in tomato plants. Similarly, PAL and PO lower activity was observed in tomato seedlings treated with R. solancearum but superior activity was noticed in plants treated with Pseudomonas fluorescens and control seedlings40. The enhancement of PO activity obtains by P. fluorescens in various seedlings such as cucumber12, rice13, tomato16 and mulberry38.
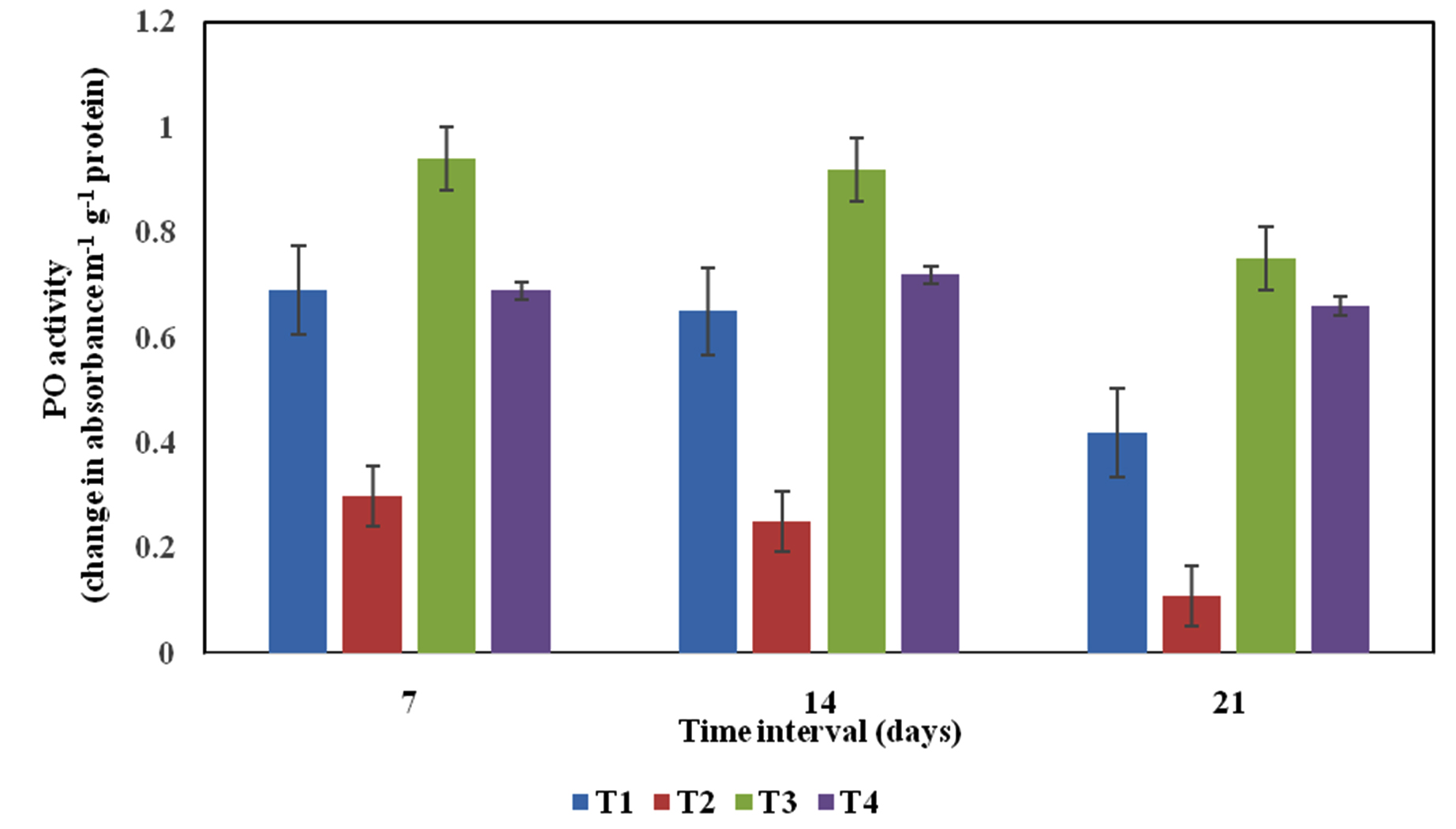 Fig. 3. Phenol oxidase (PO) activity profile of rice leaves variety IR-50 in different treatments. T1- Healthy control (rice variety IR-50 treated with sterile distilled water); T2- Disease control (rice seed inoculated with R. solani); T3- P. aeruginosa VSMKU2 culture + R. solani; T4- P. aeruginosa VSMKU2 culture filtrate + R. solani at 7th, 14th and 21st day. The data represent the mean values based on three replicates in each treatment, Vertical bar indicate standard error.
Fig. 3. Phenol oxidase (PO) activity profile of rice leaves variety IR-50 in different treatments. T1- Healthy control (rice variety IR-50 treated with sterile distilled water); T2- Disease control (rice seed inoculated with R. solani); T3- P. aeruginosa VSMKU2 culture + R. solani; T4- P. aeruginosa VSMKU2 culture filtrate + R. solani at 7th, 14th and 21st day. The data represent the mean values based on three replicates in each treatment, Vertical bar indicate standard error.Polyphenol oxidase (PPO)
High level of PPO activity was recorded in seed treatment, soil application and culture filtrate of Pseudomonas sp VSMKU2 on 7th day of R. solani inoculation in rice seedlings compared to treated and untreated control (Fig. 4). Similar to other enzyme activity PPO level reduced on 14th and 21st day after pathogen inoculation. PPO catalyses, the oxidation of phenolic compounds to more toxic quinones are a key role in plant disease resistance. In tomato seedlings treated with P. fluorescens, the ISR induced PPO activities were observed. PPO activity was maximum in plant pre-treated with P. fluorescens and inoculation with R. solanacearum. In contrary, R. solanacaerum treated plants were showed PPO activity was less (Chen et al)12. Also they reported that many rhizobacteria and P. aphanidermatum induce PPO activity in cucumber plants. PPO level was increased in P. fluorescens treated banana plants41, tomato20 and mulberry38.
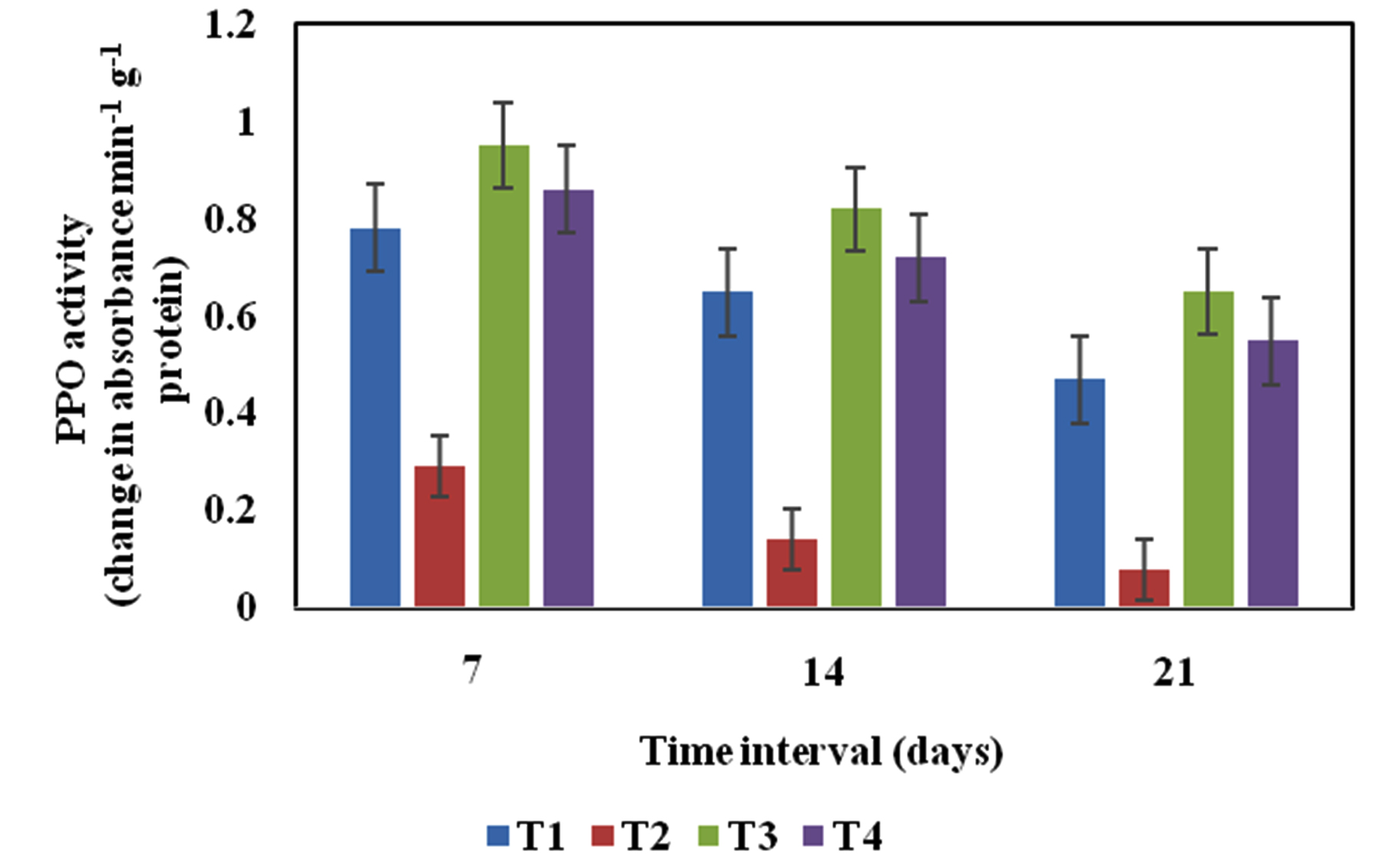 Fig. 4. Polyphenol oxidase (PPO) activity profile of rice leaves variety IR-50 in different treatments. T1- Healthy control (rice variety IR-50 treated with sterile distilled water); T2- Disease control (rice seed inoculated with R. solani); T3- P. aeruginosa VSMKU2 culture + R. solani; T4- P. aeruginosa VSMKU2 culture filtrate + R. solani at 7th, 14th and 21st day. The data represent the mean values based on three replicates in each treatment, Vertical bar indicate standard error.
Fig. 4. Polyphenol oxidase (PPO) activity profile of rice leaves variety IR-50 in different treatments. T1- Healthy control (rice variety IR-50 treated with sterile distilled water); T2- Disease control (rice seed inoculated with R. solani); T3- P. aeruginosa VSMKU2 culture + R. solani; T4- P. aeruginosa VSMKU2 culture filtrate + R. solani at 7th, 14th and 21st day. The data represent the mean values based on three replicates in each treatment, Vertical bar indicate standard error.Total Phenol content
Phenol content increased in seed treatment and soil application and cell free culture filtrate of Pseudomonas sp VSMKU2 on 7th day after the pathogen R. solani inoculation in rice seedlings compared to treated and untreated control (Fig. 5). Whereas, 14th and 21st day up on the pathogen inoculation, the activity of total phenol content almost same in both seed and soil application and culture filtrate of Pseudomonas sp VSMKU2 compared to control. Our result in coherence with the reports of Anita and Samiyappan (2012)42, the induced defence mechanism exposed more amount of phenol present in bacterized rice roots treated with Meloidogyne graminicola. However, the accretion of phenol was reported after seven days of inoculation, where as maximum level of phenol was observed in bacterized seedlings on 14th day after nematode inoculation. Phenolic compounds are known to be a key role for plant defence mechanism against various pathogens. P. fluorescens liberate lytic enzymes to build up of phenolic compounds15 and secretion of indole acetic acids are involved in the phenol metabolism in plants43.
 Fig. 5. Profiling of total phenol content in rice leaves variety IR-50 in different treatments. T1- Healthy control (rice variety IR-50 treated with sterile distilled water); T2- Disease control (rice seed inoculated with R. solani); T3- P. aeruginosa VSMKU2 culture + R. solani; T4- P. aeruginosa VSMKU2 culture filtrate + R. solani at 7th, 14th and 21st day. The data represent the mean values based on three replicates in each treatment, Vertical bar indicate standard error.
Fig. 5. Profiling of total phenol content in rice leaves variety IR-50 in different treatments. T1- Healthy control (rice variety IR-50 treated with sterile distilled water); T2- Disease control (rice seed inoculated with R. solani); T3- P. aeruginosa VSMKU2 culture + R. solani; T4- P. aeruginosa VSMKU2 culture filtrate + R. solani at 7th, 14th and 21st day. The data represent the mean values based on three replicates in each treatment, Vertical bar indicate standard error.The induction of ISR by beneficial rhizobacteurium can put forth for protective mechanism against soil borne pathogens. Hence, our present findings concluded that Pseudomonas sp VSMKU2 could be used as a bioinoculant for the management of sheath blight of rice.
Acknowledgements
The authors VS and KN are thankful to UGC, New Delhi, India for financial support. VS thankful to DBT- IPLS and DST- PURSE program for financial support and instrument facilities. VS and KN thankful to Chairperson, School of Biological Sciences for providing laboratory facilities.
Conflict of Interest
The authors declares that there is no conflict of interest.
Author’s Contribution
KN, contributed the data and drafted the manuscript. VS interpretation supervised and reviewed the manuscript. NB helped for preparation of figures, interpretation and draft improvisation. KN, VS and NB read and approved the manuscript.
Funding
University Grants Commission, New Delhi, India (39-214/2010 (SR) dated: 27.12.2010).
Data Availability
All datasets generated or analyzed during this study are included in the manuscript.
Ethics Statement
Not applicable.
- Rush M.C., Lee F. Rice sheath blight: a major rice disease. Plant. Dis., 1983; 67(7): 829-832.
Crossref - Rainey P.B., Moxon E.R. When being hyper keeps you fit. Science, 2000; 288: 1186-1187.
Crossref - Shanmugaiah V., Mathivanan N., Varghese B. Purification, crystal structure and antimicrobial activity of phenazine 1 carboxamide produced by a growth promoting biocontrol bacterium, Pseudomonas aeruginosa MML2212. J. Appl Microbiol., 2010; 108(2):703-11.
Crossref - Singh R., Singh B.P., Singh A., Singh U.P, Kureel R.S. Management of sheath blight in rice through application of Validamycin, Trichoderma harzianum and Pseudomonas fluorescence. J. Appl. Nat. Sci., 2010; 2(1): 121-125.
Crossref - Cuong N.D. Nicolaisen M.H., Sorensen J., Olsson S. Hyphae-colonizing Burkholderia sp.—a new source of biological control agents against sheath blight disease (Rhizoctonia solani AG1-IA) in rice. Microb. Ecol., 2011; 62(2): 425-434.
Crossref - Mosquera-Espinosa A.T., Bayman P., Prado G.A., Gףmez-Carabalם A., Otero J.T., The double life of Ceratobasidium: orchid mycorrhizal fungi and their potential for biocontrol of Rhizoctonia solani sheath blight of rice. Mycologia, 2013; 105(1): 141-150.
Crossref - Harikrishnan H., Shanmugaiah V., Balasubramanian N., Sharma M.P., Kotchoni S.O. Antagonistic potential of native strain Streptomyces aurantiogriseus VSMGT1014 against sheath blight of rice disease. World J. Microbiol Biotech., 2014; 30(12): 3149-3161.
Crossref - Padaria J.C., Tarafdar A., Raipuria R., Lone S.A., Gahlot P., Shakil N.A., Kumar, J. Identification of phenazine 1 carboxylic acid gene (phc CD) from Bacillus pumilus MTCC7615 and its role in antagonism against Rhizoctonia solani. J. Basic Microbiol., 2016; 56(9): 999-1008.
Crossref - Shanmugaiah V., Nithya K., Harikrishnan H., Jayaprakashvel M., Balasubramanian N. 2015. Biocontrol mechanism of siderophore against bacterial plant pathogens. Suisolateable Approaches to Controlling Plant Pathogenic Bacteria, pp 167-190. CRC Press, Taylor and Francis group, New York.
- Berg G., Hallmann J. 2006. Control of plant pathogenic fungi with bacterial endophytes. In Microbial root endophytes, pp 53-69. Springer, Berlin, Heidelberg.
Crossref - Anjaiah V., Cornelis P., Koedam, N. Effect of genotype and root colonization in biological control of fusarium wilts in pigeonpea and chickpea by Pseudomonas aeruginosa PNA1. Can. J. Microbiol.,2003; 49(2): 85-91.
Crossref - Chen C., Belanger R.R., Benhamou N., Paulitz, T.C. Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol. Mol. Plant Pathol., 2000; 56(1): 13-23.
Crossref - Nandakumar R., Babu S., Viswanathan R., Raguchander T., Samiyappan R. Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol Biochem., 2001; 33(4-5): 603-612.
Crossref - Commare R.R., Nandakumar R., Kandan A., Suresh S., Bharathi M., Raguchander, T., Samiyappan R. Pseudomonas fluorescens based bio-formulation for the management of sheath blight disease and leaffolder insect in rice. Crop Prot., 2002; 21(8): 671-677.
Crossref - Meena B., Radhajeyalakshmi R., Marimuthu T., Vidhyasekaran P., Doraiswamy S., Velazhahan R. Induction of pathogenesis-related proteins, phenolics and phenylalanine ammonia-lyase in groundnut by Pseudomonas fluorescens. J. Plant Dis. Protect., 2000; 107(5): 514-527.
- Ramamoorthy V., Raguchander T., Samiyappan R. Enhancing resistance of tomato and hot pepper to Pythium diseases by seed treatment with fluorescent pseudomonads. Eur. J. Plant Pathol., 2002; 108: 429–441.
Crossref - Alstrom S. Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterization with rhizosphere pseudomonads. J Gen Appl Microbiol., 1991; 37(6): 495-501.
Crossref - Wei G., Kloepper J.W., Tuzun, S. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology, 1991; 81(11): 1508-1512.
Crossref - Hoffland E., Hakulinen J., Van Pelt J.A. Comparison of systemic resistance induced by avirulent and nonpathogenic Pseudomonas species. Phytopathology, 1996; 86(7): 757-762.
Crossref - Anand T., Chandrasekaran A., Kuttalam S., Raguchander T., Prakasam V., Samiyappan, R. Association of some plant defense enzyme activities with systemic resistance to early leaf blight and leaf spot induced in tomato plants by azoxystrobin and Pseudomonas fluorescens. J. Plant Interact.,2007; 2(4): 233-244.
Crossref - Vanitha S.C., Niranjana S.R., Mortensen C.N., Umesha S. Bacterial wilt of tomato in Karnataka and its management by Pseudomonas fluorescens. Biocontrol, 2009; 54(5): 685-695.
Crossref - Holt J.G., Krieg N.R., Sneath P.H., Staley J.T., Williams, S.T., 1994. Bergey’s manual of determinative bacteriology. 9th. Ed., The Williams and Wilkins Company. In Library of Congress, Baltimore, MA.
- Dickerson D.P., Pascholati S.F., Hagerman A.E., Butler L.G., Nicholson R.L. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol.,1984; 25(2): 111-123.
Crossref - Hammerschmidt R., Nuckles E.M., Kuז, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol., 1982; 20(1): 73-82.
Crossref - Mayer A.M., Harel E., Shaul, R.B. Assay of catechol oxidase—a critical comparison of methods. Phytochemistry, 1966; 5(4): 783-789.
Crossref - Kavitha K., Nakkeeran S., Chandrasekar G., Rhizobacterial-mediated induction of defense enzymes to enhance the resistance of turmeric (Curcuma longa L) to Pythium aphanidermatum causing rhizome rot. Arch. Phytopathology Plant Protect., 2012; 45:199-219.
Crossref - Sang M.K., Kim E.N., Han G.D., Kwack M.S., Jeun Y.C., Kim, K.D. Priming-mediated systemic resistance in cucumber induced by Pseudomonas azotoformans GC-B19 and Paenibacillus elgii MM-B22 against Colletotrichum orbiculare. Phytopathology, 2014; 104(8): 834-842.
Crossref - Bubici G., Kaushal M., Prigigallo M.I., Gףmez-Lama Cabanבs C., Mercado-Blanco J. Biological control agents against Fusarium wilt of banana. Front. Microbiol., 2019; 10: 616.
Crossref - Charulatha R., Harikrishnan H., Manoharan P.T., Shanmugaiah V, 2013. Characterization of Groundnut Rhizosphere Pseudomonas sp. VSMKU 2013 for control of phytopathogens. In: Velu, R.K. (Ed) Microbiological Research in Agroecosystem Management, pp 121-127. Springer India, New Delhi.
Crossref - Shanmugaiah V., Mathivanan N., Balasubramanian N., Manoharan P.T. Optimization of cultural conditions for production of chitinase by Bacillus laterosporous MML2270 isolated from rice rhizosphere soil. Afr. J. Biotech., 2008; 7(15): 2562-2568.
- Kalimuthu R., Suresh P., Varatharaju G., Balasubramanian N., Rajasekaran K.M., Shanmugaiah V. Isolation and characterization of Indole Acetic Acid (IAA) producing tomato rhizobacterium Pseudomonas sp VSMKU4050 and its potential for Plant Growth Promotion. Int. J. Curr. Microbiol. App. Sci., 2019; 8(6): 443-455.
Crossref - Reshma P., Naik M.K., Aiyaz M., Niranjana S.R., Chennappa G., Shaikh S.S., Sayyed R.Z. Induced systemic resistance by 2, 4-diacetylphloroglucinol positive fluorescent Pseudomonas strains against rice sheath blight. Indian J. Exp.Biol., 2018; 56: 207-212.
- Audenaert K., Pattery T., Cornelis P., Hצfte M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: role of salicylic acid, pyochelin, and pyocyanin. Mol Plant Microbe Interact., 2002; 15(11): 1147-1156.
Crossref - Mariutto M., Duby F., Adam A., Bureau C., Fauconnier M.L., Ongena M., Thonart P., Dommes J. The elicitation of a systemic resistance by Pseudomonas putida BTP1 in tomato involves the stimulation of two lipoxygenase isoforms. BMC Plant Biol., 2011; 11(1): 1-15.
Crossref - Kandan A., Radjacommare R., Ramiah M., Ramananthan A., Samiyappan R. Plant growth promoting rhizobacteria induce systemic resistance in cowpea (Vigna unguicalata) against tomato spotted wilt virus by activating defense related enzymes and compound. Proceedings of the 6th International workshop on Plant growth promoting rhizobacteria, 2003; 480-486.
- Niranjan Raj S., Sarosh B. R., Shetty N. P., Shetty H. S., Reddy M. S. Plausible biochemical and molecular mechanisms involved in plant growth promoting rhizobacteria mediated resistance induction against pearl millet downy mildew disease. Proceedings of the 6th International workshop on Plant growth promoting rhizobacteria, 2003; 520-529.
- Ganeshamoorthi P., Anand T., Prakasam V., Bharani M., Ragupathi N., Samiyappan R. Plant growth promoting rhizobacterial (PGPR) bioconsortia mediates induction of defense-related proteins against infection of root rot pathogen in mulberry plants. J. Plant Interact., 2008; 3(4): 233-244.
Crossref - Podile A.R., Laxmi V.D.V. Seed Bacterization with Bacillus subtilis AF 1 Increases Phenylalanine Ammonia lyase and Reduces the Incidence of Fusarial Wilt in Pigeonpea. J. Phytopathol., 1998; 146(5 6): 255-259.
Crossref - Vanitha S.C., Umesha, S. Pseudomonas fluorescens mediated systemic resistance in tomato is driven through an elevated synthesis of defense enzymes. Biologia plantarum, 2011; 55(2): 317-322.
Crossref - Harish S. Molecular biology and diagnosis of Banana bunchy top virus and its management through induced systemic resistance. Ph.D. Thesis. Tamil Nadu agricultural University, Combatore 2005.
- Anita B., Samiyappan R., Induction of systemic resistance in rice by Pseudomonas fluorescens against rice root knot nematode Meloidogyne graminicola. Journal of Biopesticides., 2012; 5: 53-59.
- Shabaev V. P., Olyunina L. N., Smolin Y. Y. Functional activity of maize roots after inoculation with growth promoting rhizosphere bacteria, Pseudomonas. India Biological Bulletin of Russian Academic Science, 1999; 26: 30- 35.
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


