ISSN: 0973-7510
E-ISSN: 2581-690X
Recent studies have explored Stevia rebaudiana Bertoni leaf extracts for their antibacterial potential and phytochemical content. However, the impact of extraction methods and solvents on aquaculture bacteria remains understudied. This research aimed to evaluate the antibacterial, radical scavenging, and phytochemical properties of S. rebaudiana extracts against Aeromonas species. Dried S. rebaudiana leaves were extracted using methanol (Mt) and ethanol (Et) through Soxhlet and maceration methods (SMt, SEt, MMt and MEt respectively). Soxhlet extraction yielded higher amounts (36.29% for Mt, 23.87% for Et) compared to maceration. Phytochemical analysis identified phenolics, flavonoids, alkaloids, saponin, tannin, and steroids in all extracts. Notably, MEt had elevated phenolic and flavonoid content, while SEt contained more tannins. MEt exhibited the strongest antioxidant activity (IC50 = 67.95µg/mL), aligning with its high phenolic and flavonoid levels. In antibacterial assays against Aeromonas strains, ethanol extract showed the largest zone of inhibition (ZOI) of 16.67mm for A. salmonicida, followed by methanol extract (15mm) at 250 mg/mL, using maceration and Soxhlet methods, respectively. However, none of the extracts displayed activity against A. hydrophila. This suggests that cold maceration is a cost-effective method that preserves heat-sensitive secondary metabolites within a shorter extraction time. In conclusion, this study highlights the significance of extraction techniques and solvents in obtaining potent antibacterial and antioxidant extracts from S. rebaudiana leaves. The findings emphasize the potential of these extracts in aquaculture practices and open avenues for further research in utilizing natural compounds for sustainable aquaculture strategies.
Stevia rebaudiana, Aeromonas, Antioxidant, Anti-inflammatory, Secondary Metabolites, Maceration
Medicinal plants are an unavoidable component of human well-being. They produce a vast range of secondary metabolites, of which around 50,000 have been examined and their structures determined.1 The plant’s secondary metabolites have been used for ages to treat ailments, build immunity against a variety of bacterial and viral diseases and improve digestion.2-5 The efficacy of these plant extracts, however, is dependent on the phytochemical composition, plant matrix, and operator expertise.6 The extraction procedure is a vital stage in the process of identifying secondary metabolites from plant parts. The research has already shown that the biological activity of extracts obtained by different procedures might differ greatly, emphasizing the need to select the appropriate extraction technique.7 Both conventional and unconventional extraction methods can be used to isolate active compounds from plants. The unconventional techniques include supercritical fluid extraction, pressurized liquid extraction, microwave-assisted extraction, ultrasonic-assisted extraction etc. Most of the above-mentioned extraction methods require high temperatures. Despite the fact that higher temperatures encourage the release of more secondary metabolites, a possibility exists for the degradation of thermally unstable compounds.8 However, most industries and laboratories are still using conventional methods like Soxhlet and cold maceration due to low cost, lower solvent consumption, low energy consumption and simple procedure.9
Researchers frequently analyse several extraction methods in terms of efficiency, yield, and cost-effectiveness to select the best methodology for their specific needs. Soxhlet extraction has been used for many years, although it is highly labour-intensive and requires significantly more solvents than the maceration approach.10 The fundamental advantage of cold maceration, on the other hand, is that it helps maintain the volatile and thermolabile chemicals found in the original plant material.11 Plant secondary metabolites are important in a variety of sectors, including aquaculture.12 Because of the possible consequences for animal and human health, as well as the environment, the use of antimicrobials in aquaculture is a source of worry and continuous discussion. One of the major concerns associated with the use of antimicrobials in aquaculture is the potential development of antimicrobial resistance (AMR) bacteria that leads to treatment failures.13 These AMR bacteria can spread through the environment, posing risks to other aquatic organisms and potentially impacting human health if transferred through the food chain. Disease prevention and mitigation are essential in aquaculture to ensure the health and welfare of the farmed species and the sustainability of the industry. It is proven that plant-based products can stimulate the fish immune system in a specific or nonspecific way.14-17 There are numerous ways that medicinal plants have been used, such as a crude substance, an extract, or an active component. For fish farmers in particular, using crude plants has the advantage of requiring little effort to apply; however, there was occasionally a lesser impact than when the extract was utilised.18
Stevia rebaudiana is a herbaceous short-day plant of the Asteraceae family. It is the only species that belongs to genera Stevia, having sweetening, stevioside compound, found distributed in leaf, stem and flower and was considered as a sugar alternative in the early 1970s by Japanese people.19,20 The major glycosides present in the plant are stevioside, rebaudioside A, rebaudioside C and dulcoside A with an order of sweetness of 210, 242, 30 and 30 times more than sucrose and the chemical composition varies according to cultivar and geographical area.21,22 Stevia has gained popularity as a natural alternative to artificial sweeteners due to its therapeutic values like antioxidant, anticancerous, anti-inflammatory, antihyperglycemic and antimicrobial properties.23-25 Recent research confirmed that the addition of stevioside could enhance liver antioxidant activity, immune functions and growth performance in juvenile mirror carp.26 It has a promising natural remedy to avail disease management in the aquaculture field, as it is facing issues due to the use of antibiotics.
A scientific comparative study determining the superiority of one extraction method over another for extraction would be extremely beneficial to researchers and enterprises interested in Stevia processing and applications. Such a study would shed light on the efficiency, yield, and quality of extracts obtained using various procedures. Thus, the primary goal of the study was to carry out preliminary phytochemical screening, investigate antioxidant activity, and assess the in vitro antibacterial activity of S. rebaudiana extracts produced by two different extraction methods, Soxhlet and maceration, against selected aquaculture pathogens.
Plant material
Fresh and mature Stevia rebaudiana leaves were obtained from ‘Stevia World Agrotech’ in Bangalore, India, and identified by professionals from the Central Ayurveda Research Institute in Uttarahalli, Bangalore, India (Authentication No. SMPU/CARI/BNG/2021-22/2207). After collection, the leaves were cleaned using distilled water and dried for four days in the shade at room temperature before being milled into powder. For the current investigation, leaf powders were always made fresh.
Preparation of leaf extracts
For the present study, two different extraction methods, Maceration (cold extraction) and Soxhlet (hot extraction) were adopted using ethanol and methanol as solvents separately, to ensure the suitable method and solvents to assess. Thus, four extracts were prepared, ethanolic extract of Soxhlet (SEt) & Maceration (MEt) and methanolic extract of Soxhlet (SMt) & Maceration (MMt) and were used to screen against selected aquaculture pathogens.
Extraction by maceration
Approximately 20g of powdered dry leaf (as described above) was soaked in 100 mL methanol and ethanol separately in an enclosed conical flask for 72 hours. Then it was filtered through Whatman filter paper 1. Then the solvent was evaporated by a rotary vacuum evaporator at 60°C.27
Extraction by soxhlet
About 20 grams of the powdered leaf was placed in a thimble holder and about 200mL of the respective extraction solvent (methanol and ethanol) was filled in a round bottom flask. A rotary vacuum evaporator was used to evaporate and concentrate the solvent at 60°C.28 The leftover concentrated residue from both extraction methods was placed in Petri dishes and let the solvent completely evaporate. The crude extracts were then stored at 4°C.
Screening of phytochemicals (qualitative)
All four extractions (SEt, MEt, SMt & MMt) methods were tested for the presence of bioactive compounds such as phenolics, flavonoids, alkaloids, saponin, steroids, anthraquinone, tannin, carbohydrates and protein using the standard protocol of Harborne (1998) with minor modification.29
Phytochemical analysis (Quantitative)
Determination of total phenolic content
The total phenolic content of the four extracts (SEt, MEt, SMt, and MMt) was evaluated using the Folin-Ciocalteu colourimetric method with gallic acid as a standard phenolic component.30 The quantification was conducted using a calibration curve (y = 0.0012x + 0.0013, R2 = 0.997) using various concentrations of gallic acid solutions such as 0, 100, 200, 300, 400 and 500 µg/mL and 1 mg/mL concentrations of all extracts were prepared. To 40 µl of each extract, 400 µl of Folin–Ciocalteu reagent (FCR) (10%)and 360 µl of Na2CO3(7%) were added for a total volume of 800 µl. The blue-coloured reaction mixture was thoroughly agitated and incubated for 30 minutes. The FCR reagent oxidises phenols in sample solutions, resulting in a dark blue colour, that is quantified using a UV-visible spectrophotometer at 760 nm against a reagent blank. The samples were prepared in triplicate for each analysis. The total phenolic content of the extracts was expressed in milligrams of gallic acid equivalents (GAE) per gram of dry weight of the sample (mg/g).
Determination of total flavonoid content
The total flavonoid content of all four extracts was measured using an aluminium chloride colourimetric assay with quercetin as a reference flavonoid component.31 The quantification was conducted using a calibration curve (y = 0.0081x + 0.0124, R2 = 0.998) using various concentrations of quercetin (0, 100, 200, 300, 400 and 500µg/mL) and 1 mg/mL concentrations of all extracts was prepared. Briefly, to 100 µl of each extract, 100 µl AlCl3 (10%) and 100 µl of Na2CO3(1M) were added. The reaction mixture was thoroughly agitated and incubated for 45 minutes. Then, the absorbance was measured using a UV-visible spectrophotometer at 760 nm against blank. All the experiments were carried out in triplicates. The amount of flavonoids in the extracts was expressed as milligrams of quercetin equivalents (QE) per gram of sample dry weight (mg/g).
Determination of tannin content
Total tannin contents in ethanol and methanol extract of S. rebaudiana leaf prepared through the Soxhlet and maceration method were determined by colourimetric assay using tannic acid as a standard tannin compound.32 The quantification was carried out by means of the calibration curve (y = 0.0017x + 0.0022, R2 = 0.998) using various concentrations of tannic acid(0, 100, 200, 300, 400 and 500µg/mL) and 1 mg/mL concentration of all extracts were prepared. To about 1000 µl of each extract, 1000 µl Folin–Ciocalteu reagent (FCR) (10%) and 1000 µl of Na2CO3(35%) were added. The reaction mixture was thoroughly agitated and incubated for 30 minutes. Then, the absorbance was measured using a UV-visible spectrophotometer at 700 nm against blank. All the experiments were carried out in triplicates. The amount of tannin in the extracts was expressed as milligrams (mg) of tannic acid equivalents (TAE) per gram of sample dry weight (mg/g).
The GC-MS analysis
Gas chromatography-mass spectrometry (GC-MS) analysis was performed on different extracts of S. rebaudiana leaves using GC-MSQP2010 SE SHIMADZU model. Spectroscopic detection by GC–MS involved a pressure of 76.2KPa Helium gas (99.995% purity) was employed as the carrier gas, with a flow rate of 4.3 mL/min. A very small amount (1 µL) of a diluted extract (prepared at 1% concentration) was introduced into the GC-MS system using the splitless injection technique (280°C). The compounds present in the sample were identified by comparing the acquired mass spectra to a mass spectral library provided by the National Institute of Standards and Technology (NIST).
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay
The free radicle scavenging activity of all four extracts of S. rebaudiana was assessed in terms of hydrogen donating ability using DPPH radicle. Ascorbic acid was used as a standard reference compound (All chemicals were analytical grade and procured from VASA Scientific, Bangalore). The assay was carried out according to the method of Sanna et al.33 The radical scavenging activity was determined using the calibration curve (y = 2.6938x + 0.196, R2 = 0.998) using various concentrations of ascorbic acid (0, 2,4, 6, 8, 10 and 12µg/mL). All four samples were prepared in methanol at different concentrations (0, 20, 40, 60, 80, 100, 120 µg/mL). To about 1000 µl of each extract, 3 mL of 0.1mM DPPH solution was added. The tubes containing the reaction mixture were thoroughly agitated and incubated for 30 minutes in the dark. The absorbance at 520 nm was measured using a UV-VIS spectrophotometer against blank. All of the experiments have been performed in triplicate. The percentage inhibition activity at different concentrations was determined and half maximal inhibitory concentration (IC50) of extracts was calculated from the graph and compared with the standard.
Antibacterial assay
Two bacterial pathogens, Aeromonas hydrophila (MCC2052 1) and Aeromonas salmonicida (MCC2318 1) were selected for the present study as these are common fish pathogens. These isolates were obtained from the National Centre for Microbial Resource, Pune, India. The reference strains were cultured on nutrient agar slants, subcultured regularly and stored at -20°C. Nutrient agar (HiMedia) was used as media for subculture and antibacterial assay. The antimicrobial screening was performed in a laminar hood to avoid contamination by the test organisms. Neomycin (µg/disc) standard disc and 50µL of DMSO were used as the reference (positive and negative control, respectively). The agar well diffusion method was used to assess antibacterial activity.34 Inoculums of test organisms (106 CFU/mL) were spread on nutrient agar plates. Wells (6 mm diameter) were punched on the agar plates with sterile cork borer and filled with 50µl of various concentrations (50,100, 150, 200 and 250 mg/mL) of each extract. The plate was maintained at room temperature for 1 hour to allow for diffusion before being incubated at 30°C for 24 hours. Antimicrobial activity was determined by measuring the zone of inhibition against the test organisms. The growth was compared with both the positive and negative control. All experiments were conducted in triplicate.
Statistical analysis
The data were expressed as mean and standard deviation. The computational analysis was done by IBM SPSS software. The statistical significance was evaluated by One-way analysis of variance (ANOVA) followed by a post hoc test such as Duncan’s test (P<0.05 was considered statistically significant).
Percentage yield of extraction
The kind of extraction techniques/solvents used and their extraction ability had a direct impact on the extraction yield.35 The extraction efficiency is linked to the solvent volume, extraction time, as well as the impact on the environment and people. Therefore, selecting an effective and environmentally friendly extraction technique or solvent for the separation of phytochemicals from plant materials is crucial.36 The percentage yield of extraction is given in Table 1. The findings showed that the percentage extractive yield was significantly influenced by the solvents and technique followed. The results proved that the soxhlet extraction was the most effective method resulting in an extraction yield of 36.29% and 23.87% for methanol and ethanol, respectively, compared to maceration. According to the results, soxhlet seems to be the suitable method of extraction of S. rebaudiana leaves. However, it should be noted that the high temperature used in the soxhlet method reduces the viscosity and surface tension of solvents, thereby allowing the solvents to penetrate deeply into the plant material and also permitting the co-extraction of the fibres. These add to the dry extract weight. The exposure of plant material to several rinses of warm solvent may also be the cause of the maximum extraction.37 According to the results, methanol solvent showed better yield when compared to ethanol in both methods. Methanol is more polar than ethanol, hence favours more extraction yield. Truong et al.38 also showed similar results to the present study. Severinia buxifoliua extraction yield was maximum when methanol was used as the extraction solvent.38 This might be a result of the high concentrations of polar phytochemicals found in plant material, which are soluble in highly polar solvents like methanol and ethanol.
Table (1):
Yield of extraction of S. rebaudiana
Method of extraction |
Yield (%) |
|---|---|
Methanol maceration (MMt) |
8.43 ± 0.58 |
Ethanol maceration (MEt) |
7.13 ± 0.09 |
Methanol Soxhlet (SMt) |
36.29 ± 0.38 |
Ethanol Soxhlet (SEt) |
23.87 ± 0.72 |
Phytochemical screening (qualitative)
The phytochemical composition analysis (qualitative) of S.rebaudiana leaf extracts showed the presence of phenolic compounds, saponins, flavonoids, steroids, carbohydrates, alkaloids, proteins and tannins as summarized in Table 2. While anthraquinones were absent in all extracts. The phenolic and flavonoids have proven to have antibacterial and antioxidant activity.39 So that their presence in all extracts could be responsible for the respective biological activities. Even though the different extraction methods can influence the potency of extracts.
Table (2):
Phytochemical screening of S.rebaudiana leaf extracts
| Phytochemical component | Extraction type | Test followed | |||
|---|---|---|---|---|---|
| MEt | MMt | SEt | SMt | ||
| Phenolic compounds | + | + | + | + | Ferric chloride test |
| Alkaloid | + | + | + | + | Draganodorff’s Test |
| Saponin | + | + | + | + | Froth test |
| Flavonoids | + | + | + | + | Lead acetate test |
| Tannin | + | + | + | + | Ferric chloride test |
| Steroid | + | + | + | + | Salkowski test |
| Anthraquinone | – | – | – | – | Borntrager’s test |
| Carbohydrate | + | + | + | + | Molisch’s test |
| Protein | + | + | + | + | Million’s test |
Phytochemical analysis (quantitative)
Phenolic compounds are known to possess antioxidant, antimicrobial and anti-inflammatory properties.40-42 According to the present study, all extracts had a high phenolic content that ranged from 45.25 to 61.69 mg GAE/g dry extract (Table 3). Plant extracts abundant in phenolic compounds or individual isolates can serve as natural additives for food preservation. Their incorporation can extend the longevity of edibles by suppressing the proliferation of spoilage microorganisms and slowing oxidative processes.43 Thus, it can be an indication that Stevia is being used in the food industry. The findings showed that MEt had the highest phenolic concentration of 61.69±1.52 mg GAE/g followed by MMt, 54.42±1.5 mg GAE/g, SMt, 52.80±1.25 mg GAE/g and SEt, 45.25±1.49 mg GAE/g; having a notable difference between them (p < 0.05) (Table 3). The total phenolic concentration could serve as a basis for a screening of antioxidant activity since their hydroxyl groups facilitate their ability to scavenge free radicals.44 Similar results obtained from the studies conducted by Shukla et al.45 have shown that the ethanolic leaf extract of S. rebaudiana possesses a phenolic content of 61.50 mg GAE/g. The findings suggest that phenolic compounds from Stevia can be readily extracted using ethanol and can be used to serve as a basis for a screening of antioxidant activity since their hydroxyl groups facilitate their ability to scavenge free radicals.
Table (3):
Quantification of phytochemicals from different extracts of S. rebaudiana
| Phytochemical Parameters (mg/g) | |||||
|---|---|---|---|---|---|
| Extracts | Total phenolic content | Total flavonoid content | Tannin content | Carbohydrate | Protein |
| MEt | 61.69±1.53a | 22.62±0.28a | 10.90±0.81a | 107.07±0.43b | 204.99±1.67a |
| MMt | 54.42±1.50b | 14.55±0.07c | 6.58 ±0.52b | 111.07±0.86a | 193.33±1.67b |
| SEt | 45.25±1.49c | 12.45±0.17d | 5.41±0.53bc | 110.61± 0.93a | 175.55±1.67c |
| SMt | 52.80±1.25b | 18.71±0.11b | 5.02±0.30c | 100.08 ± 0.30c | 171.48±1.92d |
Mean values in the same column with different alphabets were significantly different (p<0.05)
Flavonoids are one of the most studied groups of plant phenol with notable medicinal action. In the present study, all extracts had a high flavonoid content that ranged from 13.12 to 22.52 mg QE/g of dry extract (Table 3). The findings showed that MEt had the highest flavonoid concentration of 22.62±0.28 mg GAE/g followed by SMt (18.71±0.11 mg QE /g), MMt (14.55±0.07 mg QE /g) and SEt (12.45±0.17mg QE/g); with a notable difference between them (p < 0.05). The findings from this current investigation are in accordance with Zaidan et al.,46 who showed that the leaf ethanol extract of S. rebaudiana has the highest flavonoid content of TFC of 10.91 mg QE/g.46 Some flavonoids, such as flavones and flavonols, are also employed in cosmetic formulation because of their anti-ageing and anti-inflammatory characteristics.47,48 Quercetin, protocatechuic acid and ferulic acid is the most abundant flavonoid in Stevia extracts.49 Quercetin is found to have biological activities like apoptosis induction, inhibiting angiogenesis and modifying cell cycles.50 This is evidence of the pharmacological importance of Stevia.
In the present investigation, all extracts had a high tannin content that ranged from 5.48 to 11.25 mg GAE/g of dry extract (Table 3). The findings showed that MEt had the highest tannin concentration of 10.90±0.81 mg GAE/g followed by MMt (6.58±0.52 mg GAE/g), SEt (5.41±0.53 mg GAE/g) and SMt (5.02±0.30mg GAE/g); with considerable difference between them (p < 0.05). The highest yield of tannin in MEt could be due to the release of additional hydrogen bonds between tannins and protein as a result of prolonged exposure of the sample to solvent by maceration.51 Similar findings reported by Farahmandfar et al.52 in Arum maculatum leaf extract, showed the highest tannin (5.33mg/g) obtained by the maceration method using ethanol: water(1:1) as solvent. Due to the fact that tannin is a polyphenol component, polar solvents can easily extract it. The findings of Naima et al.53 witnessed that ethanol is the best solvent to extract condensed tannins from Acacia mollissima barks with an extraction yield of 18.50±0.06 Cya/g.
The results of the quantitative analysis showed that maceration is an effective method for extracting secondary metabolites like phenolics, flavonoids and tannin. The soaking of leaf powder for an extended period allowed the above-mentioned polar compounds to dissolve in alcohol. However, in Soxhlet, the continuous refluxing of solvents over the samples may lead to the degradation of some heat-sensitive secondary metabolites and lower their composition in it.54 As per the current study, ethanol was able to extract phytochemicals in terms of quantity. The possible reason is that it is more polar than methanol, making them for strong hydrogen bonds and dipole-dipole interaction with polar compounds in plants. Also, the fact that ethanol is safer for extraction without causing a higher risk to human health.55 The findings indicated that the maceration method is the simple and low-cost way and ethanol is the best solvent to extract secondary metabolites such as phenolics, flavonoids and tannin from S. rebaudiana leaf.
GC-MS analysis of S. rebaudiana
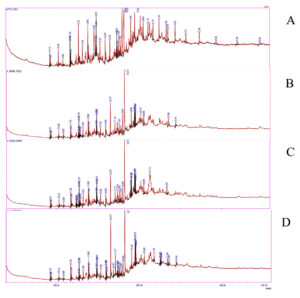
GC-MS analysis of four different extracts of S. rebaudiana showed that the chemical constituents belong to different classes like alkane, alkene, fatty alcohol, fatty acids and carboxylic acid. The chromatogram is depicted in Figure 1. A total of 34 similar compounds were identified from all four extracts, which are listed in Table 4. The abundant compound from all extracts was n-hexadecanoic acid. The antibacterial and antioxidant of n-Hexadecanoic acid have been reported in many research works.56 Neophytadiene, a diterpenoid was also identified from all extracts with various quantities. The highest amount was obtained from MEt with a relative percentage composition of 12%, followed by SMt (8.94%). Previous research has proven the antibacterial and anti-inflammatory activities of neophytadiene. Al-Rajhi et al.57 have found that high neophytadiene content in Mentha pulegium directly correlated with the increased antibacterial activity against Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa etc. There are few reports on the occurrence of phthalates in plants. GC-MS results also revealed the existence of dibutyl phthalate in all four extracts of S. rebaudiana. It is reported that plant-derived dibutyl phthalate is having many biological properties such as antibacterial activities.58 Dibutyl phthalate isolated from Begonia malabarica showed significant antibacterial activity against E. coli, Staphylococcus epidermis, Streptococcus pneumoniae, Micrococcus luteus, Vibrio cholerae and Shigella flexneri.59 The majority of these compounds have been discovered to have significant biological activity, like antibacterial, antioxidant, and anti-inflammatory action against specific diseases or pathogens.
Table (4):
Compounds identified in the leaf extracts of S. rebaudiana and their significant biological activities
| Class | Compounds | Rt (min) | Reported biological activities | Reference |
|---|---|---|---|---|
| Alkene | 1-Dodecene | 9.31 | ||
| 2-Dodecene | 9.31 | |||
| 1-tetradecene | 9.31 | |||
| 1-Heptadecene | 13.90 | |||
| 1-Pentadecen | 13.90 | |||
| 1-Tridecene | 13.90 | |||
| 2-Phytene | 16.57 | Antimicrobial, antioxidant and cytotoxic | Larayetan et al.60; Hasan et al.61 | |
| 1-Octadecene | 16.00 | |||
| Fatty alcohol | 1-Dodecanol | 9.31 | Antibacterial | Zhang et al.62 |
| 1-Decanol, 2-hexyl | 20.60 | |||
| Alkane | Tridecane | 9.41 | ||
| Undecane | 9.41 | |||
| Hexadecane | 9.41 | |||
| Dodecane, 4,6-dimethyl | 10.34 | |||
| Dodecane, 3,7-dimethyl | 10.34 | |||
| Tetradecane | 10.34 | Antimicrobial | Nasr et al.63 | |
| Undecane, 3,8-dimethyl | 10.34 | |||
| Undecane, 3,7-dimethyl | 10.91 | |||
| Octadecane | 12.78 | |||
| Heptadecane | 12.78 | |||
| 1-Octadecyne | 16.57 | |||
| Nonadecane | 15.38 | Antioxidant | Kazemi M.64 | |
| Tetratetracontane | 21.17 | |||
| Tetracosane | 21.17 | |||
| Octocosane | 21.17 | |||
| Docosane | 21.17 | |||
| Heneicosane | 15.38 | Antioxidant Antimicrobial | Vanitha et al.65; Mohammadi et al.66 | |
| Aromatic carboxylic acid | Benzoic acid | 14.64 | Antimicrobial Antioxidant | Park et al.67 Velika, Kron68 |
| Ester | Dibutyl phthalate | 18.04 | Antibacterial | Bi et al.69 |
| Carboxylic acid | Phthalic acid | 18.04 | ||
| Fatty acids | Pentadecaflurooctanoic acid | 20.60 | ||
| Eicosanoic acid | 18.26 | |||
| Docosanoic acid | 18.26 | |||
| n-hexadecanoic acid | 18.26 | Antibacterial Antioxidant, nematicide | Abubakar, Majinda70 | |
| Terpenoids | Neophytadiene | 16.57 | Antibacterial, anti-inflammatory | Bhardwaj et al.71 |
DPPH radicle scavenging assay
The phytochemical analysis of the current study has revealed the existence of phenolics and flavonoids in all four extracts, which is directly correlated with antioxidant activity. Hence the analysis of radicle scavenging activity is a mandatory tool to determine the antioxidant activity of four extracts of S. rebaudiana. The antioxidant activity of natural products can be analysed in terms of electron donation ability by using 2,2-diphenyl-1- picrylhydrazyl (DPPH) radicle scavenging assay. The present study revealed that all extracts showed concentration-dependent inhibition of free radicles. As reported by previous studies the antioxidant potential of extracts was found to be lower than ascorbic acid.72 The percentage inhibition was significantly different among all concentrations for all four extracts (p < 0.05). Among all extracts, MEt showed the highest antioxidant activity in terms of inhibition of free radicle with IC50 of 67.95µg/mL. This is followed by SEt (75.39µg/mL), MMt (94.31µg/mL) and SMt (95.40µg/mL) (Table 5). The greatest capacity of superoxide radicle scavenging of MEt can be correlated with high phenolics and flavonoid content. In the study conducted by Ahmad et al.73, the methanolic extract of S. rebaudiana leaf (obtained by maceration method) showed percentage inhibition of 77.68% for the DPPH scavenging assay, which was in close agreement with the results of the current investigation. A similar trend of DPPH scavenging was observed for ethanol extracts of Buhinia purpurea leaf obtained by maceration and soxhlet method.74 Their research found that macerated extract outperformed soxhlet in terms of scavenging activity with percentage inhibition of 88.6±3.0 and 71.4±0.9 % at 40µg/mL, respectively. According to the findings of the current study, ethanolic leaf extract of S. rebaudiana obtained by the maceration method can be employed as a readily available source of natural antioxidants with positive health effects. In view of the fact that maceration permits extraction at room temperature and subsequent solvent removal at decreased pressure, ensuring the recovery of more antioxidant compounds of interest (phenolics) without the risk of denaturation or other changes brought on by high temperatures as in hot extraction techniques like soxhlet.75
Table (5):
Antioxidant activity of different extracts of S. rebaudiana
| Concentration (μg/mL) | MEt | MMt | SEt | SMt |
|---|---|---|---|---|
| 0 | 0.00±0.00g | 0.00±0.00g | 0.00±0.00g | 0.00±0.00g |
| 20 | 14.83±0.00f | 10.86±0.06f | 10.94±0.00f | 4.62±0.18f |
| 40 | 29.21±0.00e | 20.04±0.06e | 22.04±0.06e | 18.81±0.16e |
| 60 | 45.92±0.06d | 33.60±0.10d | 32.18±0.06d | 27.92±0.14d |
| 80 | 60.56±0.00c | 43.30±0.23c | 44.99±0.06c | 40.66±0.11c |
| 100 | 71.68±0.00b | 54.27±0.00b | 62.90±0.00b | 51.88±0.09b |
| 120 | 87.34±0.00a | 61.54±0.06a | 69.72±0.06a | 66.41±0.09a |
| IC50 | 67.95 | 94.31 | 75.39 | 95.40 |
| IC50 standard (Ascorbic acid) | 18.49 | |||
Mean values in the same column with different alphabets were significantly different (p<0.05).
Antibacterial assay
The bactericidal activity of S.rebaudiana leaf extracts against A. hydrophila and A. salmonicida is depicted in Table 6. The outcome revealed that all extracts showed concentration-dependent antibacterial activity against A. salmonicida (Figure 2). However, none of the extracts demonstrated a significant zone of inhibition when used in different concentrations against A. hydrophila. The possible reason for not showing antibacterial activity may be due to the formation of biofilms by this particular species. Previous studies have shown that the formation of biofilms, which promote the spread of antibiotic-resistant genes among bacteria in biofilms than in planktonic cells, is a key element in the onset of chronic infections and drug resistance in A. hydrophila. The study conducted by Bakhtiari et al.76 witnessed that 19 strains of A. hydrophila were resistant to 75% of studied antibiotics.76 Similar findings were reported by Jayaraman et al.77 with chloroform and aqueous extract of S.rebaudiana leaf.77 Only a few studies reported the antibacterial activity of S. rebaudiana against fish bacterial pathogens such as Aeromonas spp.
Table (6):
Antibacterial activity of various extracts of S. rebaudiana against A. salmonicida
Concentration (mg/mL) |
SEt (mm) |
SMt (mm) |
MEt (mm) |
EMt (mm) |
|---|---|---|---|---|
50 |
7.33±0.58d |
8.33±0.58d |
8.33±0.58d |
0.00±0.00c |
100 |
10.00±1.00c |
11.00±0.00c |
12.00±1.00c |
0.00±0.00c |
150 |
12.00±1.00b |
12.67±0.58b |
13.67±0.58b |
10.00±1.00b |
200 |
13.33±0.58ab |
13.67±1.15ab |
15.33±0.58a |
10.33±0.58b |
250 |
14.67±0.58a |
15.00±1.00a |
16.67±1.15a |
11.67±0.58a |
Mean values in the same column with different alphabets were significantly different (p<0.05).
Figure 2. Antibacterial activity of S. rebaudiana leaf extracts against A. salmonicida (Zone of Inhibition)
Maximum antibacterial activity (DZI) against A. salmonicida was demonstrated by MEt (16.67±1.15 mm) and SMt (15.00±1.00 mm) at 250 mg/mL. SEt showed DZI of 14.67±0.58 mm and MMt showed 11.67±0.58 mm. Statistically, there was a significant difference in the inhibitory activity among SEt and SMt at 50, 100 and 150 mg/mL against A. salmonicida (p < 0.05). Previous studies have reported the antibacterial activity of ethanol, methanol and chloroform extracts of S. rebaudiana are effective against Pseudomonas aeruginosa, Escherichia coli and S. aureus.78 The solubility, concentration and composition of secondary metabolites might be responsible for the antibacterial activity of different extracts against A. salmonicida. At the same time, there was no significant difference between 200 and 250 mg/mL concentration of all extracts against A. salmonicida. The variation in the activity among different extracts might be caused by their chemical makeup.79 The bioactive compounds such as phenolics, flavonoids, tannins, alkaloids and saponins can hinder the growth and metabolism of microorganisms in a negative way. Phenolics and flavonoids can inhibit bacterial peptidogly can synthesis and modify membrane permeability.80 Alkaloids are proven to be intercalated with nuclear DNA resulting in cell death.81 Tannins are able to inactivate cell envelop transport protein and adhesins.82 The presence of volatile phytochemicals detected by GC-MS analysis is proven to have antibacterial, antioxidant and anti-inflammatory activities. The findings of the current work have proven that S. rebaudiana could serve as a source of a potential antimicrobial agent against fish pathogens such as A. salmonicida as the usage of natural plant extracts is so widespread.83 The overall data of this study were consistent with previous results.
Plant extracts possess combined antioxidant and antibacterial attributes, rendering them valuable across medical, cosmetic, and food sectors. They offer natural alternatives to synthetic counterparts, reducing dependence on chemical additives. Our study highlights maceration as the optimal method for efficiently extracting phenolics, flavonoids, and tannins from S. rebaudiana leaves. Ethanol extracts from maceration, rich in phytochemicals, effectively inhibit fish pathogens, like A. salmonicida. Plant-derived compounds, such as alkaloids, terpenoids, tannins, and flavonoids, underpin the antibacterial potential. These disrupt bacterial structure, enzymes, and metabolism, curtailing growth. Phenolic compounds, by quenching free radicals and reducing agents, limit radical chain reactions. While plant extracts find utility in medicine, cosmetics, and food, their antibacterial efficacy varies based on species, extraction methods, and bacterial strains. Our ongoing research underscores the ease and cost-effectiveness of cold maceration in preserving heat-sensitive metabolites during S. rebaudiana extraction. Further investigation is vital to decipher mechanisms and optimize plant extracts for antibacterial use. In conclusion, S. rebaudiana extracts emerge as a viable resource for pioneering aquaculture antibacterial treatments.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Life Sciences, CHRIST (Deemed to be University), Bangalore, for extending necessary facilities and support throughout the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AR and KV designed the experiments, analyzed the data and wrote the manuscript. AR performed the experiment and collected the data. KV performed supervision and revised the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
This study was supported by the University Grants Commission under the scheme of Junior Research Fellowship (965/(CSIRNETJUNE2019).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Pichersky E, Gang DR. Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends Plant Sci. 2000;5(10):439-445.
Crossref - Selvi S, Polat R, Cakilcioglu U, Celep F, Dirmenci T, Ertug ZF. An ethnobotanical review on medicinal plants of the Lamiaceae family in Turkey, Turkey. Turk J Bot. 2022;46(4):283-332.
Crossref - Dong S, Yang X, Zhao L, Zhang F, Hou Z, Xue P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind Crops Prod. 2020;149:112350.
Crossref - Qadri H, Shah AH, Ahmad SM, Alshehri B, Almilaibary A, Mir MA. Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi J Biol Sci. 2022;29(9):103376.
Crossref - Cakylcyoglu U, Turkoglu I. Plants used for hemorrhoid treatment in Elazig central district. Acta Hortic. 2007;826:89-96.
Crossref - Azmir J, Zaidul ISM, Rahman MM, et al. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117(4):426-436.
Crossref - Hayouni EA, Abedrabba M, Bouix M, Hamdi M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007;105(3):1126-1134.
Crossref - Osorio-Tobon JF. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J Food Sci Technol. 2020;57:4299-4315.
Crossref - Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: A comprehensive review. Chin Med. 2018;13:1-26.
Crossref - Bras T, Neves LA, Crespo JG, Duarte MF. Effect of extraction methodologies and solvent selection upon cynaropicrin extraction from Cynara cardunculus leaves. Sep Purif Technol. 2020;236:116283.
Crossref - Oreopoulou A, Tsimogiannis D, Oreopoulou, V. Extraction of polyphenols from aromatic and medicinal plants: an overview of the methods and the effect of extraction parameters. Polyphenols in Plants, 7nd Ed. Academic Press, Cambridge, USA. 2019:243-259.
Crossref - Rangel-Lopez L, Rivero-Perez N, Valladares-Carranza B, et al. Antibacterial Potential of Caesalpinia coriaria (Jacq) Willd Fruit against Aeromonas spp. of Aquaculture Importance. Animals. 2022;12(4):511.
Crossref - Abdallah EM, Adil AH, Al-Mijalli SH. GC-MS and Antibacterial Potential of Methanolic Extract Hyphaene Thebaica L. Fruit Pulp against Antibiotics-resistant Pathogens. J Pure Appl Microbiol. 2021;15(3):1655-1664.
Crossref - Rashmeei M, Shekarabi SPH, Mehrgan MS, Paknejad H. Stimulatory effect of dietary chasteberry (Vitex agnus-castus) extract on immunity, some immune-related gene expression, and resistance against Aeromonas hydrophila infection in goldfish (Carassius auratus). Fish Shellfish Immunol. 2020;107:129-136.
Crossref - Motlagh HA, Safari O, Selahvarzi Y, Baghalian A, Kia E. Non-specific immunity promotion in response to garlic extract supplemented diets in female Guppy (Poecilia reticulata). Fish Shellfish Immunol. 2020;97:96-99.
Crossref - Adeniyi OV, Olaifa FE, Emikpe BO. Effects of dietary tamarind pulp extract on growth performance, nutrient digestibility, intestinal morphology, and resistance to Aeromonas hydrophila infection in Nile tilapia (Oreochromisniloticus L.). J Appl Aquacul. 2022;34(1):43-63.
Crossref - Mohanasundari L, Devi GB, Musthafa MS, Madhavi M. Effects of Illicium verum Hook. f. (Chinese herb) enriched diet on growth performance, immune response and disease resistance in Catla catla [Hamilton] fingerlings against Aeromonas hydrophila. Fish Shellfish Immunol. 2022;127:455-462.
Crossref - Rashidian G, Shahin K, Elshopakey GE, et al. The dietary effects of nutmeg (Myristica fragrans) extract on growth, hematological parameters, immunity, antioxidant status, and disease resistance of common carp (Cyprinus carpio) against Aeromonas hydrophila. J Mar Sci Eng. 2022;10(3):325.
Crossref - Chughtai MFJ, Pasha I, Zahoor T, et al. Nutritional and therapeutic perspectives of Stevia rebaudiana as emerging sweetener; a way forward for sweetener industry. CyTA J Food. 2020;18(1):164-177.
Crossref - Putnik P, Bezuk I, Barba FJ, et al. Sugar reduction: Stevia rebaudiana Bertoni as a natural sweetener. Agri-food industry strategies for healthy diets and sustainability, Academic Press. Cambridge, USA, 2020:123-152.
Crossref - Ameer K, Bae SW, Jo Y, Lee H-G, Ameer A, Kwon J-H. Optimization of microwave-assisted extraction of total extract, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves, using response surface methodology (RSM) and artificial neural network (ANN) modelling. Food Chem. 2017;229:198-207.
Crossref - Formigoni M, Milani PG, da Silva Avincola, et al. Pretreatment with ethanol as an alternative to improve steviol glycosides extraction and purification from a new variety of Stevia. Food Chem. 2018;241:452-459.
Crossref - Iatridis N, Kougioumtzi A, Vlataki K, Papadaki S, Magklara A. Anti-cancer properties of Stevia rebaudiana; more than a sweetener. Molecules. 2022;27(4):1362.
Crossref - Covarrubias-Cardenas, Ana G, Martinez-Castillo, et al. Antioxidant capacity and UPLC-PDA ESI-MS phenolic profile of Stevia rebaudiana dry powder extracts obtained by ultrasound assisted extraction. Agronomy, 2018;8(9):170.
Crossref - Ruiz-Ruiz JC, Moguel-Ordonez YB, Matus-Basto AJ, et al. Antidiabetic and antioxidant activity of Stevia rebaudiana extracts (Var. Morita) and their incorporation into a potential functional bread. J Food Sci Technol. 2015;52:7894-7903.
Crossref - Wang J, Li K, Wang L, Xu Q. Effects of different levels of stevioside on growth performance, digestive enzyme activity, antioxidant capacity and gene expression of juvenile mirror carp (Cyprinus carpio). Aquaculture. 2021;543:737019.
Crossref - Bouabid K, Lamchouri F, Toufik H, Faouzi MEA. Phytochemical investigation, in vitro and in vivo antioxidant properties of aqueous and organic extracts of toxic plant: Atractylis gummifera L. J Ethnopharmacol. 2020;253:112640.
Crossref - Redfern J, Kinninmonth M, Burdass D, et al. Using soxhlet ethanol extraction to produce and test plant material (essential oils) for their antimicrobial properties. J Microbiol Biol Educ. 2014;15(1):45-46.
Crossref - Harborne AJ. Phytochemical methods a guide to modern techniques of plant analysis, springer science & business media, 3rd Ed. New York. 1998.
- Jafri L, Saleem S, Ullah N, Mirza B. In vitro assessment of antioxidant potential and determination of polyphenolic compounds of Hedera nepalensis K. Koch. Arab J Chem. 2017;10(2):s3699-s3706.
Crossref - Hossain MA, Shah MD, Gnanaraj C, Iqbal M. In vitro total phenolics, flavonoids contents and antioxidant activity of essential oil, various organic extracts from the leaves of tropical medicinal plant Tetrastigma from Sabah. Asian Pac J Trop Med. 2011;4(9):717-721.
Crossref - Kaushik R, Narayanan P, Vasudevan V, Muthukumaran G. Nutrient composition of cultivated stevia leaves and the influence of polyphenols and plant pigments on sensory and antioxidant properties of leaf extracts. J Food Sci Technol. 2010;47(1):27-33.
Crossref - Sanna D, Delogu G, Mulas M, Schirra M, Fadda A. Determination of free radical scavenging activity of plant extracts through DPPH assay: An EPR and UV-Vis study. Food Anal Methods. 2012;5(4):759-766.
Crossref - Kumari K, Sharma S, Kaundal K. Production, purification and efficacy of bacteriocin isolated from natural lactic acid fermentation of wild Himalayan fig fruit. J Pure Appl Microbiol. 2018;12(2):879-885.
Crossref - Sharma A, Cannoo DS. A comparative study of effects of extraction solvents/techniques on percentage yield, polyhenolic composition, and antioxidant potential of various extracts obtained from stems of Nepeta leucophylla: RP HPLC DAD assessment of its polyhenolic constituents. J Food Biochem. 2017;41(2):e12337.
Crossref - Johnson WM, Kido Soule MC, Ujawinski EB. Extraction efficiency and quantification of dissolved metabolites in targeted marine metabolomics. Limnol Oceanogr Methods. 2017;15(4):417-428.
Crossref - Devi V, Khanam S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J Clean Prod. 2019;207:645-657.
Crossref - Truong DH, Nguyen DH, Ta NTA, Bui AV, Do TH, Nguyen HC. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifoliua. J Food Qual. 2019;8178294.
Crossref - Hemalatha R, Nivetha P, Mohanapriya C, Sharmila G, Muthukumaran C, Gopinath M. Phytochemical Composition, GC-MS Analysis, In Vitro Antioxidant and Antibacterial Potential of Clove Flower Bud (Eugenia Caryophyllus) Methanolic Extract. J Food Sci Technol. 2016;53(2):1189-1198.
Crossref - Tohma H, Gulcin I, Bursal E, Goren AC, Alwasel S, Kiksal E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas Charact. 2017;11(2):556-566.
Crossref - Roby MH, Abdelaliem YF, Esmail AHM, Mohdaly AA, Hassanien MFR. Evaluation of Egyptian honeys and their floral origins: Phenolic compounds, antioxidant activities, and antimicrobial characteristics. Environ Sci Pollut Res. 2020;27(2):20748-20756.
Crossref - Harbeoui H, Hichami A, Wannes WA, Lemput J, tunsi MS, Khan NA. Anti-inflammatory effect of grape (Vitis vinifera L.) seed extract through the downregulation of NF-kB and MAPK pathways in LPS-induced RAW264. 7 macrophages. S Afr J Bot. 2019;125:1-8.
Crossref - Stagos D. Antioxidant activity of polyphenolic plant extracts. Antioxidants. 2020;9(1):19.
Crossref - Pahlavan F, Lamanna A, Park KB, Kabir SF, Kim JS, Fini EH. Phenol-rich bio-oils as free-radical scavengers to hinder oxidative aging in asphalt binder. Resour Conserv Recycl. 2022;187:106601.
Crossref - Shukla S, Mehta A, Bajpai VK, Shukla S. In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food ChemToxicol. 2009;47(9):2338-2343.
Crossref - Zaidan UH, Zen NIM, Amran NA, Shamsi S, Gani SSA. Biochemical evaluation of phenolic compounds and steviol glycoside from Stevia rebaudiana extracts associated with in vitro antidiabetic potential. Agric Biotechnol. 2019;18:101049.
Crossref - Szewczyk K, Pietrzak W, Klimek K, et al. Flavonoid and phenolic acids content and in vitro study of the potential anti-aging properties of Eutrema japonicum (Miq.) Koidz cultivated in Wasabi Farm Poland. Int J Mol Sci. 2021;22(12):6219.
Crossref - Silva B, Biluca FC, Gonzaga LV, et al. In vitro anti-inflammatory properties of honey flavonoids: A review. Food Res Int. 2021;141:110086.
Crossref - Tang SM, Deng XT, Zhou J, Li Q-P, Ge X-X, Miao L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother. 2020;12:109604.
Crossref - Gao Y, Zietsman AJ, Vivier MA, Moore JP. Deconstructing wine grape cell walls with enzymes during winemaking: New insights from glycan microarray technology. Molecules. 2019;24(1):165.
Crossref - Gil-Martin E, Forbes-Hernandez T, Romero A, Cianciosi D, Giampieri F, Battino M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022;378:131918.
Crossref - Farahmandfar R, EsmaeilzadehKenari R, Asnaashari M, Shahramour D, Bakhshandeh T. Bioactive compounds, antioxidant and antimicrobial activities of Arum maculatum leaves extracts as affected by various solvents and extraction methods. Food Sci Nutr. 2019;7(2):465-475.
Crossref - Naima R, Oumam M, Hannache H, et al. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind Crop Prod. 2015;70:245-252.
Crossref - Noore S, Rastogi NK, O’Donnell C, Tiwari B. Novel bioactive extraction and nano-encapsulation. Encyclopedia. 2021;1(3):632-664.
Crossref - Birkova A, Hubkova B, Cizmarova B, Bolerazska B. Current view on the mechanisms of alcohol-mediated toxicity. Int J Mol Sci. 2021;22(18):9686.
Crossref - Ganesan T, Subban M, Britto D, Bharathi S, Seedevi P. Structural characterization of n-hexadecanoic acid from the leaves of Ipomoea eriocarpa and its antioxidant and antibacterial activities. Biomass Convers Biorefin. 2022:1-12.
Crossref - Al-Rajhi AM, Qanash H, Almuhayawi MS, et al. Molecular interaction studies and phytochemical characterization of Mentha pulegium L. constituents with multiple biological utilities as antioxidant, antimicrobial, anticancer and anti-hemolyticagents. Molecules. 2022;27(15):4824.
Crossref - Shobi T, Viswanathan M. Antibacterial activity of di-butyl phthalate isolated from Begonia malabarica. J Appl Biotechnol Bioeng. 2018;5(2):101-104.
Crossref - Buyun L, Tkachenko H, Kurhaluk, Goralczyk A, Tomin V, Osadowski Z. Screening for antimicrobial activity of nine ethanolic extracts obtained from leaves of Begonia plant: A possible alternative in the treatment of infections caused by Citrobacter freundii. Agro Biodiv Improv Nutr Health Life Q. 2019;(3):312-322. https://agrobiodiversity.uniag.sk/scientificpapers/article/view/279
- Larayetan RA, Ayeni G, Yahaya A, et al. Chemical composition of Gossypiumher baceumlinn and its antioxidant, antibacterial, cytotoxic and antimalarial activities. Clinical Complementary Medicine and Pharmacology, 2021;1(1):100008.
Crossref - Hasan M, Hwija I, Mossa Y. Essential oils from Plumbago europaea L. aerial parts (leaves, flowers): GC-MS analyses and literature biological properties. Nat Prod Res. 2023:1-10.
Crossref - Zhang H, Feng F, Fu X, Du Y, Zhang L, Zheng X. Antimicrobial effect of food-grade GML microemulsions against Staphylococcus aureus, Eur Food Res Technol. 2007;226(1):281-286.
Crossref - Nasr ZS, El-shershaby H, Sallam KM, Abed N, Abd- El ghany I, Sidkey N. Evaluation of Antimicrobial Potential of Tetradecane Extracted from Pediococcus acidilactici DSM: 20284-CM Isolated from Curd Milk. Egypt J Chem. 2022;65(3):705-713.
Crossref - Kazemi M. Phenolic profile, antioxidant capacity and anti-inflammatory activity of Anethum graveolens L. essential oil. Nat Prod Res. 2015;29(6):551-553.
Crossref - Vanitha V, Vijayakumar S, Nilavukkarasi M, Punitha VN, Vidhya E, Praseetha PK. Heneicosane-A novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind Crops Prod. 2020;154:112748.
Crossref - Mohammadi R, Moradi M, Tajik H, Molaei R. Potential application of postbiotics metabolites from bioprotective culture to fabricate bacterial nanocellulose based antimicrobial packaging material. Int J Biol Macromol 2022;220:528-536.
Crossref - Park ES, Moon WS, Song MJ, Kim MN, Chung KH, Yoon JS. Antimicrobial activity of phenol and benzoic acid derivatives, Int Biodeterior Biodegrad. 2001;47(4):209-214.
Crossref - Velika B, Kron I. Antioxidant properties of benzoic acid derivatives against superoxide radical. Free Radic. Antioxid. 2012;2(4):62-67.
Crossref - Bi Y, Liu G, Yu Q, et al. Anti-Vibrio dibutyl phthalate from marine-derived Streptomyces sp. S073. Res Vet Sci. 2021;140:198-202.
Crossref - Abubakar MN, Majinda RR. GC-MS analysis and preliminary antimicrobial activity of Albiziaadianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines. 2016;3(1):3.
Crossref - Bhardwaj M, Sali VK, Mani S, Vasanthi HR. Neophytadiene from Turbinariaornata suppresses LPS-induced inflammatory response in RAW 264.7 macrophages and Sprague Dawley rats. Inflammation. 2020;43(3):937-950.
Crossref - Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012;12:1-12.
Crossref - Ahmad N, Fazal H, Abbasi BH, et al. Efficient free radical scavenging activity of Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous leaves through DPPH (2, 2-diphenyl-1-picrylhydrazyl). Int.l J. Phytomed, 2010;2(3):231-239. https://ijp.arjournals.org/index.php/ijp/article/view/42. Accessed August 25, 2023.
- Annegowda HV, Mordi MN, Ramanathan S, Hamdan MR, Mansor SM. Effect of extraction techniques on phenolic content, antioxidant and antimicrobial activity of Bauhinia purpurea: HPTLC determination of antioxidants. Food Anal Methods. 2012;5:226-233.
Crossref - Vieitez I, Maceiras L, Jachmanian I, Albores S. Antioxidant and antibacterial activity of different extracts from herbs obtained by maceration or supercritical technology. J Supercrit Fluids. 2018;133(Part 1):58-64.
Crossref - Bakhtiari NM, Tulabi Z, Alishahi M. Biofilm-producing ability and antibiotic resistance pattern of pathogenic strains of Aeromonas hydrophila. Jundishapur J Microbiol. 2019;12(12):e97640.
Crossref - Jayaraman S, Manoharan MS, Illanchezian S. In-vitro antimicrobial and antitumor activities of Stevia rebaudiana (Asteraceae) leaf extracts, Trop J Pharm Res. 2008;7(4):1143-1149.
Crossref - Lemus-Mondaca R, Vega-Galvez A, Roja P, et al. Antioxidant, antimicrobial and anti-inflammatory potential of Stevia rebaudiana leaves: effect of different drying methods. J Appl Res Med Aroma Plants. 2018;11:37-46.
Crossref - Ababutain IM. Antimicrobial Activity and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Saudi Arabian Ocimum basilicum Leaves Extracts. J Pure Appl Microbiol. 2019;13(2):823-833.
Crossref - Rizwana H, Al Otibi F, Al-Malki N. Chemical composition, FTIR studies and antibacterial activity of Passiflora edulis f. edulis (Fruit). J Pure Appl Microbiol. 2019;13(4):2489-2498.
Crossref - Gupta PD, Birdi TJ. Development of botanicals to combat antibiotic resistance. J Ayurveda Integr Med. 2017;8(4):266-275.
Crossref - Utaipan T, Athipornchai A, Suksamrarn A, et al. Carbazole alkaloids from Murraya koenigii trigger apoptosis and autophagic flux inhibition in human oral squamous cell carcinoma cells. J Nat Med. 2017;71(1):158-169.
Crossref - Yalcin, S, Akan H, Cakilcioglu U. Medicinal Plants Sold at Herbal Markets in Suruc District (Sanliurfa-Turkey). Int J Nat Life Sci. 2021;5:40-51.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.