ISSN: 0973-7510
E-ISSN: 2581-690X
Members of the family Enterobacteriaceae are the major cause of nosocomial infections, including approximately 70% of gastrointestinal infections, 60% to 70% of urinary tract infections (UTIs), and septicemias. Since 1990, members of Enterobacteriaceae have emerged as predominant pathogens causing UTIs. In this study, we performed phenotypic testing of the virulence factors in uropathogens. A total of 118 uropathogens belonging to the family Enterobacteriaceae were isolated from suspected UTI cases in patients aged > 18 years. Uropathogens were tested for virulence factors, such as hemolysin, phospholipase, gelatinase, and lipase production, as well as for mannose resistance, mannose-sensitive hemagglutination (MRHA, MSHA), serum resistance, and cell surface hydrophobicity. Among 118 uropathogenic Enterobacteriaceae isolates, E. coli was the most prevalent (62,52.5%), followed by Klebsiella (28,23.7%), Citrobacter (23,19.5%), and, Proteus species (5, 4.2%). Serum resistance was the most common virulence factor and was seen in 54(87%) of E. coli, 24 (85.7%) of Klebsiella species, 22 (95.7%) of Citrobacter species, and 5(100%) of Proteus species isolates. Furthermore, 10 (16.1%) isolates of E. coli showed mannose resistance hemagglutination and 7(11.3%) showed mannose-sensitive hemagglutination. Two (8.7%) isolates of Citrobacter species showed mannose resistance and mannose-sensitive hemagglutination. Hemolysis was observed in 33(53.2%) isolates of E. coli, 11(39.3%) of Klebsiella species, 15(65.2%) Citrobacter species, and 1(20%) Proteus isolate. Gelatinase production was observed in 14(22.6%) of E. coli, 6(21.4%) of Klebsiella species, 7(30.4%) of Citrobacter species, and 5(100%) of Proteus isolates. All E. coli, Klebsiella species, and Citrobacter species isolates tested negative for phospholipase. This study showed that the Enterobacteriaceae family plays a role in UTIs by evading the host immune response through the production of various virulence factors.
Urinary Tract Infection, Enterobacteriaceae, Virulence Factors, Hemagglutination, Cell Surface Hydrophobicity, Gelatinase, Phospholipase
Urinary Tract Infections (UTIs) form one of the most common bacterial infections in both community and hospital settings, and there has been an increase in morbidity and mortality due to UTIs in the recent past worldwide.1,2
Approximately 10% of humans develop UTIs in their lives at some time. The prevalence of UTIs is age-and sex-dependent. During the first year of life, UTIs occur around 2% of males and females; however, in the latter half, the UTI infection increases in females.3
Prolonged hospital stay due to medical or surgical ailments and catheterization of the urinary bladder are risk factors for both sexes in older age.
Among microbial agents that cause UTIs, bacteria are more common than viral or fungal agents. Gram-negative organisms constitute 90% of UTI cases, and the remaining 10% are Gram-positive organisms amongst the bacterial etiology. About 65%–90% of the most frequently isolated uropathogens remain Escherichia coli (E. coli).4,5
The most common uropathogens in the community and healthcare facilities belong to the Enterobacteriaceae family. Many of these bacteria are significant human pathogens that cause various infections.6 Some of the members which belong to this family are E. coli, Klebsiella species, Citrobacter species, Proteus species, Enterobacter species, etc., are found in the normal gut flora of mammals and can be isolated from the environment.
Certain virulence factors are expressed by uropathogens to combat the host’s immune system or to colonize the organ, which is either a part of the microorganism or secreted by them.
These can be divided into two categories7:
The following virulence factors were described using uropathogenic E. coli as a prototype. [Table 1]8
- Microorganism cell wall surface-associated factors
- Secreted proteins
Table (1):
Virulence factors: Surface proteins8
| • Fimbriae | |
| • Type 1(Mannose sensitive)· | – Adherence, invasion, biofilm |
| • P fimbriae | – Adherence, cytokine secretion |
| • Capsule | – Antiphagocytic, anti-complement & Serum resistance |
| • Lipopolysaccharide | – Endotoxin, cytokines, serum resistance |
| • Outer membrane proteins | – Receptor, transport |
| Exported factors | |
| • Toxins· | |
| • Hemolysin (α)· | – Cytotoxicity, hemolysin |
| • Cytotoxic necrotizing factor 1 (CNF1)· | -Entry into the cell, interferes with phagocytosis. |
| • Secreted autotransporter toxin (Sat) | – Cytotoxicity |
| • Iron acquisition system· | – Siderophore |
| • Enterobactin· | |
| • Salmochelin· | |
| • Aerobactin· | |
| Yersiniabactin | |
The first step in pathogenicity is the adhesion of the pathogens through adhesins such as fimbriae or pili, which help them adhere to the host cell and play a role in evading the host’s immune response. Type P fimbriae play a key role in pyelonephritis, and type 1 fimbriae (mannose-sensitive adhesins) promote the development of cystitis 9,10. Capsular polysaccharide (K antigen)blocks the alternate complement pathway and impairs the classical complement pathway, especially by K1, to protect the bacteria from serum killing; the degree of serum resistance is proportional to the amount of capsular material present, and smooth strains are more serum resistant than rough strains.11,12 Hemolysin, a cytolytic protein secreted by pathogenic strains, lyses erythrocytes, leading to the release of iron and other nutrients that are beneficial to bacteria. They are pro-inflammatory and contribute to inflammation, tissue injury, and impairment of chemotaxis and host defenses.13
This study aimed to identify the phenotypic characteristics of uropathogen virulence factors of the Enterobacteriaceae family in adults aged> 18 years.
Aims and objectives
- Phenotypic detection of virulence factors of the uropathogenic Enterobacteriaceae.
Type of Study: a prospective study
Place: Tertiary care hospital
Inclusion criteria
- Non-repetitive urine samples were collected from patients aged > 18 years with clinical presentation of suspected UTI in the microbiology laboratory.
- Samples with significant bacteriuria.
Exclusion criteria
- Patients on antibiotics, diuretics, and those with chronic kidney disease.
- Polymicrobial growth in culture.
In this study, all significant uropathogens of the family Enterobacteriaceae were initially identified by conventional biochemical reactions (Indole, Triple sugar iron, mannitol motility media, urease production by Christensen’s urease agar, and citrate utilization by Simmons Citrate Agar) and later screened for virulence factors, namely hemolysin, lipase, gelatinase, lecithinase (phospholipase) production, hemagglutination inhibition assay, serum resistance, and cell surface hydrophobicity.
Production of Hemolysin
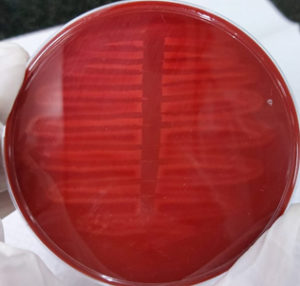
Zone of hemolysis was observed on 5% sheep blood agar after overnight incubation at 37°C as described by Mittal S et al.14 [Figure 1]
Property of Serum Resistance
The viable count of the inoculum was compared after 3 h of incubation with serum to their original count, as described by Mittal S et al. 14 [Figure 2]
- A mixture was prepared with a bacterial suspension made in Hank’s balanced salt solution from an overnight culture on blood agar and an equal volume of serum and incubated at 37°C for 3 h.
- Following this, 10 µL of this mixture was streaked on a blood agar medium and incubated overnight at 37°C.
- The uropathogen was considered sensitive if the viable count fell to <1% of the original count and serum resistant if > 90% of organisms survived.
Cell Surface Hydrophobicity (CSH)
Determined by salt aggregation test (SAT) as described by Mittal S et al.14
- A bacterial suspension was prepared in phosphate-buffered saline (PBS) at pH 6.8 matching the turbidity of McFarland tubes 6 and 7.
- Ammonium sulfate with molar concentration ranging from 0.3125 M to 5M was prepared.
- In a VDRL slide, 40 µL of PBS was added to the 1st well, and to the rest of the wells, the 40 µL bacterial suspension along with a different molar concentration of ammonium sulfate was added.
- The slide was rotated for 1 min and observed for visible clumping of bacteria at the highest molar concentration of ammonium sulfate.
- Auto aggregate: isolates showing clumping in PBS alone.
- Hydrophobic: SAT < or equal to 1.25 M.
Hemagglutination
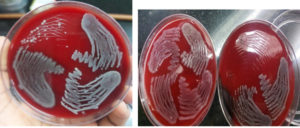
Agglutination of RBC by bacteria was performed using the slide method as described by R Hassan et al. 15 [Figure 3]
- For full fimbriation, the isolates were incubated in nutrient broth at 37°C for 48 h.
- On a slide, a drop of the bacterial suspension and a drop of 3% RBC suspension were added and incubated for 5 min at room temperature.
- If agglutination was seen, the bacterial suspension was further tested with the addition of 2% w/v D-mannose. If clumping was seen, they were considered mannose-resistant hemagglutination (MRHA).
Detection of Lipase
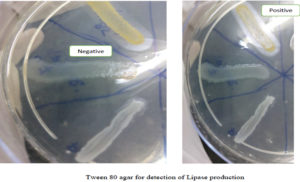
Production of lipase by the isolates was determined by spot inoculating on Tween 80 medium and incubating for a week at 37°C and observing for a zone of opaque halo around the spot as performed by R Hassan et al.15 [Figure 4]
Detection of phospholipase (Lecithinase)
Production of Lecithinase by the isolates was determined by spot inoculating on Egg yolk agar medium and incubating them at 37÷C for 24–48 as described by Pramodhini S et al. 16 and observed for the appearance of opaque (precipitation) halo around the colony. [Figure 5]
Detection of Gelatinase
This was performed as described by Pramodhini S et al 16 by the stab method wherein a heavy inoculum of the isolate was stabbed 3–4 times in the nutrient gelatin and incubated at 37°C for 48 h following which the tubes were kept in the refrigerator for 30 min and looked for free flow of the medium indicating the liquefaction of gelatin by the production of gelatinase. [Figure 6]
A total of 118 isolates belonging to the Enterobacteriaceae family were tested for virulence factors.
Among 118 isolates, 72 (45.6%) were isolated from inpatients and 46 (29.1%) from outpatients.
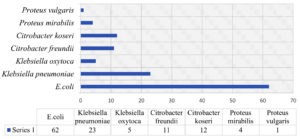
Out of 118 isolates from the family Enterobacteriaceae, E. coli was the most prevalent (62, 52.5%), followed by Klebsiella pneumoniae (23,19.5%), Klebsiella oxytoca (5,4.2%), Citrobacter freundii (11,9.3%), Citrobacter koseri (12,10.2%), Proteus mirabilis (4,3.4%) and, Proteus vulgaris (1, 0.8%). [Figure 7]
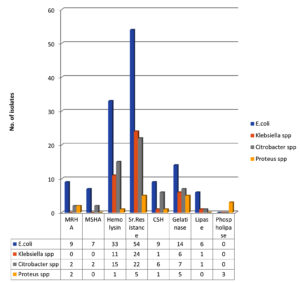
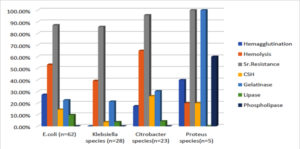
Phenotypic detection of virulence factors revealed that 10(16.1%) of E. coli, 2(8.7%) of Citrobacter species, and 2(40%) of Proteus isolates showed MRHA. [Figure 8 and 9; Table 2]
Seven (11.3%) of E. coli and 2(8.7%) of Klebsiella species isolates showed Mannose-sensitive hemagglutination (MSHA).
Hemolysis was noted in 33(53.2%) isolates of E. coli, 11(39.3%) of Klebsiella species, 15(65.2%) of Citrobacter species, and 1(20%) of Proteus isolates. [Figure 8 and 9; Table2]
54(87.1%) of E. coli, 24(85.7%) of Klebsiella species, 22(95.7%) of Citrobacter species, and 5(100%) of Proteus isolates showed the property of serum resistance. [Figure 8 and 9; Table 2]
Seven (14.5%) isolates of E. coli, 1(3.6%) of Klebsiella species, 6(26.1%) of Citrobacter species, and 1(20%) of Proteus isolates showed cell surface hydrophobicity. [Figure 8 and 9; Table 2] Gelatinase production was noted in 14(22.6%) isolates of E. coli, 6(21.4%) of Klebsiella species, 7(30.4%) of Citrobacter species, and 5(100%) Proteusisolates. [Figure 8 and 9; Table 2]
Lipase production was observed in 6 (9.7%) isolates of E. coli, 1(3.6%) of Klebsiella species, and 1(4.3%) of Citrobacter isolates. [Figure 8 and 9; Table 2]
Phospholipase production was not observed in E. coli, Klebsiella species, or Citrobacter isolates, but was observed in 3(60%) Proteus isolates. [Figure 8 and 9; Table 2]
Table (2):
Detection of virulence factors among the uropathogens of the Enterobacteriaceae family.
Organism |
Hemagglutination |
Hemolysin |
Serum Resistance |
CSH |
Gelatinase |
Lipase |
Phospholipase |
|---|---|---|---|---|---|---|---|
E. coli (n=62) |
MRHA – 10(16.1%) MSHA – 7 (11.3%) |
33 (53.2%) |
54(87.1%) |
9 (14.5%) |
14(22.6%) |
6 (9.7%) |
0 |
Klebsiella spp (n=28) |
0 |
11(39.3%) |
24(85.7%) |
1(3.6%) |
6(21.4%) |
1(3.6%) |
0 |
Citrobacter spp (n=23) |
MRHA – 2 (8.7%) MSHA – 2 (8.7%) |
15(65.2%) |
22(95.7%) |
6(26.1%) |
7(30.4%) |
1(4.3%) |
0 |
Proteus spp. (n=5) |
MRHA – 2 (40%) |
1 (20%) |
5 (100%) |
1 (20%) |
5 (100%) |
0 |
3 (60%) |
Diseases caused by an organism and their severity depend on virulence factors associated with the microorganism. In this study, a few virulence factors were phenotypically detected in the uropathogens.
In the present study, E. coli was the predominant organism isolated which was comparable to other studies by Bharati et al.17 (E. coli – 36.84%; Klebsiella species – 7.66%; Citrobacter species – 2.55% & Proteus species – 2.07%), Nazmeen A, et al.18 (E. coli – 40%; Klebsiella species – 17%; Citrobacter species – 13% & Proteus species – 10%), and Rafalskiy V19 (E. coli – 49.1%; Klebsiella species – 9.5%; Citrobacter species – 0 &Proteus species – 2.9%).
Escherichia coli (n=62)
- Hemolysis was observed in 53.2% of the isolates. Studies conducted by Mittal et al.,14 Raksha et al.,20 Tabasi M et al., 21 and Shah C et al.22 reported hemolysis in 47.4%, 41.36%, 34%, and 32.3% of the isolates, respectively, which are comparable to the results obtained in this study.
- Studies by Raksha et al.,20 and Vagarali et al.23 reported MRHA in 30.9% and 25% of isolates, respectively. In this study, it was observed that 16.1% of isolates demonstrated MRHA which was comparable to the results obtained by Vagarali et al.23 However, MSHA was observed only in 11.3% of isolates.
- A study by Hasan R et al.15 noted MSHA in 28% of isolates. A study conducted by Tabasi Met al21 observed that 62.8% of isolates showed MSHA and 37.2% of isolates showed MRHA. Shah C et al.22 observed that 5.7% of isolates showed MSHA and 52.3% showed MRHA.
- Serum resistance in the present study was noted to be 87.1%, which was comparable to that reported by Vaish et al.24 (95.38%).
- Cell surface hydrophobicity was observed in 14.5% of isolates, similar to a study done by Raksha et al.20 where it was noted to be 26.36%.
- In the present study, 9.7% of the isolates exhibited lipase activity; however, in a study conducted by Bhattacharyya et al.25 lipase activity was noted in 100% of the isolates.
In the present study, serum resistance was noted in a majority of isolates (87.1%), followed by hemolysis (53.2%), which could play a role in disease pathogenesis by evading the immune response and causing inflammation, thus predisposing to severe disease.
Klebsiella species (n=28)
There are limited studies on the phenotypic detection of virulence factors in uropathogenic Klebsiella.
- In the present study, 39.3% of Klebsiella isolates showed hemolysis. Studies conducted by Hassan R et al.,15 and Chaudhary N et al.26 reported it to be 7% and 43.75%, respectively.
- Serum resistance was noted in 85.7% of isolates, which was higher than that reported by El-Naggar et al.27 (33%).
- Only one isolate was noted to have cell surface hydrophobicity.
- In this study, the production of gelatinase and lipase were observed in 21.4% and 3.6% of isolates, respectively, which are lower in comparison to those reported by Ramakrishna K et al. 28: 40.9% and 58.2%, respectively. However, the study conducted by Ramakrishna K et al.28 included other clinical specimens also.
Citrobacter species (n=23)
This is an emerging pathogen in hospitals; however, only a limited number of studies are available on Citrobacter virulence factors.
- A study conducted by Nayar R et al.29 noted that 86.6% of isolates were serum resistant and 17.1% of strains were positive for the cell surface hydrophobicity test. Similar findings were noted in the present study, where 95.7% of isolates showed serum resistance, and 26.1% of isolates showed cell surface hydrophobicity.
Proteus species (n=5)
The isolation rate of Proteus in the present study was low compared to that of other organisms; hence, a higher percentage was observed for a few virulence factors compared to others.
- In the present study, 20% of Proteus isolates showed hemolytic properties. Studies by Filipiak A et al.30 & Jebur MH et al.31 noted hemolysin production in 16% and 100% of isolates, respectively.
In the present study, 3 of the patients had urosepsis (2 E. coli and 1 Citrobacter species) and had hemolysin, serum resistance, and gelatinase properties. The other virulence factors observed in these cases were cell surface hydrophobicity and mannose-sensitive hemagglutination.
In cases of pyelonephritis, renal calculi, and post-infectious glomerulonephritis, the most common virulence factors observed in this study were hemolysin and serum resistance, followed by cell surface hydrophobicity and gelatinase.
In the present study, the most common uropathogens isolated were E. coli and Klebsiella pneumoniae. Among the virulence factors, serum resistance followed by hemolysin production was the most common, followed by hemagglutination and gelatinase production. Serum resistance and hemolysin properties set the pace for the pathogenesis of renal disease by facilitating the secretion of interleukins, evading the host immune response, and promoting ascending infection. Other virulence factors, such as lipase and phospholipase (lecithinase), digest the extracellular matrix, thereby facilitating the entry of the organism into the host.
Future scope
Effective development of a vaccine against these virulence factors may decrease the number and severity of UTIs that are still in the trial phase.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
GSB conceptualized the study and reviwed the manuscript. BRS performed data acquisition and wrote the manuscript. BRS and GSB performed literature search, data analysis, designed the study and edited the manuscript.Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study has been approved by the Institutional Ethics Committee, Ramaiah Medical College, India, with reference number EC/PG-16/2018
- Mohapatra S, Panigrahy R, Tak V, et al. Prevalence and resistance pattern of uropathogens from community settings of different regions: an experience from India. Access Microbiol. 2022;4(2):000321.
Crossref - Tandogdu Z, Wagenlehner FM. Global epidemiology of urinary tract infections. Curr Opin Infect Dis. 2016;29(1):73-79.
Crossref - Patricia M. Tille. Bailey & Scott’s Diagnostic Microbiology, 14th ed. 987-996.
- Seifu WD, Gebissa AD. Prevalence and antibiotic susceptibility of Uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infect Dis. 2018;18(1):30.
Crossref - Christy VR, Athinarayanan G, Mariselvam R, Dhasarathan P, Singh RAJA. Epidemiology of urinary tract infection in south India. Biomed Res Clin Prac. 2019;4.
Crossref - Octavia S, Lan R. The Family Enterobacteriaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F. (eds) The Prokaryotes. Springer, Berlin, Heidelberg. 2014.
Crossref - Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4(1):80-128.
Crossref - Stamm WE. Theodore E. Woodward Award: host-pathogen interactions in community-acquired urinary tract infections. Trans Am Clin Climatol Assoc. 2006;117:75-84.
- Roberts JA, Marklund BI, Ilver D, et al., The Gal (alpha 1-4) Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci USA. 1994;91(25):11889- 11893.
Crossref - Ofek I, Mirelman D, Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977;265:623-625.
Crossref - Orskov F, Orskov I, Jann B, Jann K. Immunoelectrophoretic patterns of extracts from all Escherichia coli 0 and K antigen test strains. Correlation with pathogenicity. Acta Pathol Microbiol Scand. 1971;79:142-152.
Crossref - Leying H, Suerbaum S, Kroll HP, Stahl D, Opferkuch W. The capsular polysaccharide is a major determinant of serum resistance in K-1-positive blood culture isolates of Escherichia coli. Infect Immun. 1990; 58:222-227.
Crossref - Bhakdi S, Mackman N, Nicaud JM, Holland IB. Escherichia coli hemolysin may damage target cell membrane by generating transmembrane pores. Infect Immun. 1986;52(1):63-69.
Crossref - Mittal S, Sharma M, Chaudhary U. Study of virulence factors of uropathogenic Escherichia coli and its antibiotic susceptibility pattern. Indian J Pathol Microbiol. 2014;57(1):61-64.
Crossref - Hassan R, El-Naggar W, El-Sawy E, El-Mahdy A. Characterization of some virulence factors associated with Enterobacteriaceae isolated from urinary tract infections in Mansoura Hospitals. N Egypt J Med Microbiol. 2011;20(2):9-17.
- Pramodhini S, Umadevi S, Seetha KS. Detection of virulence determinants and its association with drug resistance in clinical isolates of Pseudomonas aeruginosa. Int J Res Med Sci. 2016;4(9):3917-3923.
Crossref - Bharti AK, Farooq U, Singh S, Kaur N, Ahmed R, Singh K. Incidence of Enterococcal Urinary Tract Infection and it’s Sensitivity Pattern among Patients Attending Teerthanker Mahaveer Medical College and Research Centre, Moradabad, India. Int J Sci Study. 2016;12(3):115-119.
Crosssref - Nazmeen A, Maiti S. Prevalence, Types and Antibiotic Sensitivity pattern in Urinary Tract Infection (UTI) In Midnapore Town, India. Journal of Clinical and molecular Pathology. 2018:2(1:16). https://www.imedpub.com/articles/prevalence-types-and-antibiotic-sensitivity-pattern-in-urinary-tract-infectionuti-in-midnapore-town-india.php?aid=24299
- Rafalskiy V, Pushkar D, Yakovlev S, et al., Distribution and antibiotic resistance profile of key Gram-negative bacteria that cause community-onset urinary tract infections in the Russian Federation: resource multicentre surveillance 2017 study. J Glob Antimicrob Resist. 2020(21);188-194.
Crossref - Raksha R, Srinivasa H, Macaden RS. Occurrence and characterisation of uropathogenic Escherichia coli in urinary tract infections. Indian J Med Microbiol. 2003;21(2):102-107.
Crossref - Tabasi M, Karam MRA, Habibi M, Yekaninejad MS, Bouzari S. Phenotypic Assays to Determine Virulence Factors of Uropathogenic Escherichia coli (UPEC) Isolates and their Correlation with Antibiotic Resistance Pattern. Osong Public Health Res Perspect. 2015;6(4):261-268.
Crossref - Shah C, Baral R, Bartaula B, Shrestha LB. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 2019;19(1):204.
Crossref - Vagarali MA, Karadesai SG, Patil CS, Metgud SC, Mutnal MB. Haemagglutination and siderophore production as the urovirulence markers of uropathogenic Escherichia coli. Indian J Med Microbiol. 2008;26(1):68-70.
Crossref - Vaish R, Pradeep M, Setty C, et al.Evaluation of Virulence Factors and Antibiotic Sensitivity Pattern of Escherichia Coli Isolated from Extraintestinal Infections. Cureus. 2016;8(5):e604:1-13.
- Bhattacharyya S, Kumari S, Sarfraz A, et al. Comparative efficacy of Levofloxacin and Prulifloxacin against Uropathogenic Escherichia coli and Klebsiella spp. from a tertiary care hospital and their correlation with expression of lipase and lecithinase. Eastern J Med Sci. 2016;1(1):31-33.
Crossref - Chaudhary N, Murthy S. Urinary tract infection: etiology and antimicrobial resistance with reference to adhesive organelles. Journal of Drug Delivery and Therapeutics. 2013;3(4):93-98.
Crossref - El-Naggar W, Hassan R, El-Sawy E, et al. Comparative Study of Phenotypic and Genotypic Expression of some Virulence Factors in Escherichia Coli and Klebsiella Pneumoniae Isolated from Urinary Tract Infections in Mansoura Hospitals. Egypt J Med Microbiol. 2011;20(3):67-68.
- Ramakrishna K, Johnsi PJ, Seetha KS. Clinical isolates of Klebsiella pneumoniae and its virulence factors from a tertiary care hospital. Indian J Microbiol Res. 2019;6(2):109-112.
Crossref - Nayar R, Shukla I, Ali SM. Evaluation of the virulence markers in the clinical isolates of Citrobacter species: the first report from India. J Clin Diagn Res. 2013;7(6):1031-1034.
Crossref - Filipiak A, Chrapek M, Literacka E, et al. Pathogenic Factors Correlate with Antimicrobial Resistance Among Clinical Proteus mirabilis Strains. Front Microbiol. 2020.
Crossref - Jebur M H, Bunyan I, Trad J K. Isolation of Proteus mirabilis and Proteus vulgaris from Different Clinical Sources and Study of some Virulence Factors. Journal of Babylon University/Pure and Applied Sciences. 2013;21(1):43-48.
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.