ISSN: 0973-7510
E-ISSN: 2581-690X
The COVID-19 outbreak was a serious challenge for countries around the globe. With the objective of mitigating the spread of the virus, both national and international health organizations swiftly enacted quarantine measures across numerous cities around the globe. This presented a unique chance to evaluate the consequences of human actions on the quality of the air. This study aimed to investigate airborne microbial levels in different outdoor locations in Al-Madinah City, Kingdom of Saudi Arabia (KSA), during the COVID-19 pandemic by comparing lockdown against non-lockdown conditions. Twelve outdoor locations were investigated in terms of microbial total counts using the open plate method during and after the COVID-19 lockdown. Environmental factors that could affect the airborne microbial load, including humidity, temperature and wind speed, were recorded during the assessment. The means of the total colony-forming units (CFU) for each cubic meter (m3) of air were calculated. Lockdown restrictions caused significant decreases in the biological contaminants in all locations compared with the numbers after the pandemic. Gram-positive bacteria represented most of the samples, with fewer fungal strains detected. The outdoor average total bacterial counts ranged between 0.00±0.00-8337.50±248.98 CFU/m3, compared with 2903.75±407.60-19722.50±475.03 CFU/m3 after the pandemic. The mean concentrations of total fungi were lower than those of bacteria and ranged between 0.00±0.00-143.75±131.75 CFU/m3 during the COVID-19 lockdown and were elevated after the lockdown to reach 28.75±49.80-776.25±298.78 CFU/m3. Based on the available data, there are no studies comparing outdoor microbial counts during and after the COVID-19 pandemic. Therefore, this research offers additional perspectives on the air quality experienced amidst the COVID-19 pandemic and the subsequent implementation of lockdown measures and could serve as a valuable resource for monitoring and implementing measures to control air pollution.
COVID-19, Lockdown Measures, Outdoor Air, Air Quality, Airborne Bacteria, Airborne Fungi, Anthropogenic Activities
Air quality, both indoors and outdoors, is the most important factor that impacts our everyday lives. Every day, we breathe approximately 10 cubic meters of air.1 Air can be full of biocontaminants, which allows for their spread and dispersal. Biocontaminants are microscopic, suspended particles in the air that are made up of or derived from living organisms. Other examples of these substances are spores, pollen particles, endotoxins, or fragments of animal shedding.2 They can be released into the environment as singular cells, in clumps of multiple organisms, or as cells or pieces of cells attached to other particles in the air. Inhaling these biocontaminants can lead to infectious, allergic, and toxic effects. The past few decades have seen heightened global interest in biocontaminant exposure. This is largely due to the understanding that exposure to these contaminants is linked to many serious health issues, ranging from contagious diseases to malignancies. Such conditions can lead to considerable public health burdens.3 Every year, outdoor air pollution claims the lives of almost 3.3 million individuals.4 There is a significant impact of air pollution on life expectancy worldwide that is estimated to be more than twice as large as the combined effects of water, soil, and occupational pollution.5
Airborne microbes constitute a significant proportion of particulate matter present in the atmosphere,6 which can be hazardous to human health, as well as the ecological balance of wildlife and plant species. Additionally, they can be transported with wind, thus affecting the entire ecosystem.7,8 Adverse health effects have been observed due to spread of airborne microorganisms.9-11 The findings of epidemiological studies indicate that exposure to high concentrations of microbes in the air can induce respiratory disorders,12 allergic reactions and infectious diseases,10,13 hypersensitivity,14,15 fatigue, headache and sinusitis, asthma attacks11,12 along with alveolitis.16 Immunocompromised individuals, young children, pregnant women, the elderly, and patients suffering respiratory and cardiovascular diseases, are more susceptible to air pollutants.11,14
Previous studies investigated airborne microorganisms in outdoors and indoor environments in urban and rural regions.17-22 There are a variety of bacterial communities found in urban air microbiomes; however, Proteobacteria, Actinobacteria and Firmicutes tend to be the most abundant.18,20,21 Some of the fungal spores detected in many studies are associated with allergies, such as Ascomycota, Basidiomycota, and Zygomycota.22,23 Nevertheless, the levels of microorganisms in air are strongly correlated with various environmental factors, including temperature, humidity, air movement, light intensity, and types of human activities.24-27
Air quality in terms of microbes can be analysed to ensure safety and protection from potential risks.28 It allows the detection of the existence of biological agents, recognizes crucial situations, and ensures that the safety measures taken are effective. Moreover, air sampling serves educational, scientific, and quality control objectives.29
In December 2019, the world was in a panic caused by a new infection officially recognized as coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO). It was initially detected in Wuhan, Hubei, China, and subsequently extend all over the world.30 Our laboratory has published several previous works concerning COVID-19.31-33 The effects of COVID-19 can vary widely, from individuals who show no symptoms at all to those with severe cases that might necessitate the use of a ventilator or even result in fatalities. The worldwide count of confirmed COVID-19 cases and fatalities has exceeded 12 million and 550,000, respectively, as indicated by a global database. All countries were striving to contain the virus. To execute the “flattening the curve” plan, the world faced an unprecedented lockdown with a range of measures from individual to global levels. Closing global boundaries, educational facilities, and nonessential businesses, as well as restricting the movement of people in some areas, were among the steps taken to implement lockdown.34 In many cities, air quality, in terms of non-biological pollutants, improved during the lockdown period. The strict restriction placed on billions of people globally had at least one beneficial consequence. Satellite images and measurements of contaminants conducted from ground-based locations in several cities clearly demonstrate this study.34-37 This study aimed to investigate airborne microbial levels, mainly bacteria and fungi, in twelve different outdoor locations in Al-Madinah City, KSA, during the COVID-19 outbreak by comparing lockdown conditions against non-lockdown conditions.
To date, there is no definite agreement on when air sampling should be done, what technique would be applied, and how to analyse the results in order to apply control and safety measures that are effective. The techniques employed for microbial air sampling can be categorized as either passive or active.28,38 The active technique provides a way to measure the concentration of cultivable microorganisms in the atmosphere. It uses various tools to take a known amount of air that is then blown onto a nutrient medium. The final result is quantified in terms of the quantity of colony forming units per cubic meter. On the other hand, Passive methods involve exposing settle plates to air for a predetermined time period to measure the rate of sedimentation of microorganisms on surfaces. Findings are given as CFU/plate/time. In general, the choice of the technique is determined by the objectives of the study, and no particular method is preferred over another.39
Sampling sites
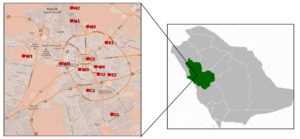
The present study was done at twelve locations in Madinah, KSA. The framework of this study is shown in Figure 1.40 The investigated districts include C1 and C2 located in the centre; N1 and N3 in the northern part; S1 and S2 in the southern part; E1 and E2 in the eastern part; and W1 and W4 in the western part of Madinah City.
Figure 1. Geographical description and marked sampling locations in Madinah, KSA. C, centre; N, north; S, south; E, east; W, west
Study period and meteorological conditions
The sampling studies were conducted in June 2020 and July 2023. All the samples were collected in the afternoon between approximately 16:00–18:00 o’clock. The meteorological factors (wind speed, temperature, and relative humidity) were recorded during sampling.
Collection of samples
Bacterial and fungal samples were collected using the open plate method. Petri dish plates of 9.0 cm diameter containing media (nutrient agar and MacConkey agar were used to obtain bacterial total counts and Sabouraud Dextrose agar for fungal culturing) were exposed for 5 min at 150 cm height, which represents the breathing zone of an individual. A minimum of 10 m was kept between walls and other obstructions. The plates were transported to the laboratory in sealed microbiological plastic bags. The incubation process on bacterial samples was done at a temperature of 37°C for 24-48 hours. Fungal samples were incubated for 5 days at 28°C.41,42 To obtain data variation, all air analyses were performed in 3 parallel repetitions at each sampling location. Air samples were analysed at the microbiology laboratory, College of Science, Taibah University.
Enumeration of bacteria and fungi
After incubation, the calculated number of colonies was then converted into CFU/m3 considering the Omelyansky formula.43,44
N =5 a × 104(bt)-1 (1)
where
N = CFU/m3, a = colonies number per Petri dish, b = plate surface (cm2), and t = the time duration for opening of the plate (min), according to estimates that on an area of 100 cm2, in 5 minutes, as many microorganisms as there are deposited in 10 m3 of air.
Statistical analysis
Microsoft Excel 2010 (Microsoft Corporation, New York, USA) was used for statistical analysis. One-way analysis of variance (ANOVA) with multiple comparison tests (Tukey’s) (Minitab®19 statistical software) was applied to verify the likelihood of statistically significant differences among the concentrations of bacteria and fungi at diverse locations. The values are presented as mean±standard deviation (SD). A p-value less than 0.05 indicates that the difference is statistically significant.
In this study, a total of 216 outdoor air samples from twelve locations in Madinah City were included during and after the COVID-19 pandemic. The wind speed during sampling varied between 14 and 23 km h−1. The air temperature ranged from 44°C to 45°C (summer season), and the relative humidity was approximately 5%. The numbers of bacteria and fungi at these sampling points had significant differences based on the location. Data, arithmetic means and standard deviations of bacteria and fungi recorded at each sampling location are presented in Tables 1-3. There was a significant decline (p<0.05) in the biological contaminants during the COVID-19 lockdown in all sampling areas compared with their numbers after the pandemic.
Table (1):
Descriptive statistics of bacteria at different sampling locations during the COVID-19 pandemic
| No. | Location | Bacteria CFU/m3 | |||
|---|---|---|---|---|---|
| Nutrient agar | MacConkey agar | ||||
| 24 hours | 48 hours | 24 hours | 48 hours | ||
| 1 | C1 | 4772.5±388.9228 | 4830.00±524.64 | 28.75±49.80 | 57.50±99.59 |
| 2 | C2 | 6957.5±348.58 | 7848.75±480.22 | 0.00±0.00 | 57.50±49.80 |
| 3 | N1 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| 4 | N2 | 5491.25±277.25 | 5606.25±228.20 | 57.50±99.59 | 57.50±99.59 |
| 5 | N3 | 0.00±0.00 | 0.00±0.00 | 316.25±131.75 | 977.50±217.06 |
| 6 | S1 | 0.00±0.00 | 0.00±0.00 | 345.00±345.00 | 1236.25±302.90 |
| 7 | S2 | 0.00±0.00 | 0.00±0.00 | 230.00±263.50 | 977.50±131.75 |
| 8 | E1 | 0.00±0.00 | 0.00±0.00 | 603.75±86.25 | 1236.25±277.25 |
| 9 | E2 | 0.00±0.00 | 0.00±0.00 | 316.25±99.59 | 862.50±86.25 |
| 10 | W1 | 2242.50±480.22 | 5203.75±131.75 | 0.00±0.00 | 0.00±0.00 |
| 11 | W2 | 1351.25±131.75 | 3680.00±263.50 | 28.75±49.80 | 57.50±99.59 |
| 12 | W3 | 8337.50±248.98 | 9286.25±587.09 | 0.00±0.00 | 28.75±49.80 |
| 13 | W4 | 2300.55±248.98 | 2846.25±345.00 | 0.00±0.00 | 0.00±0.00 |
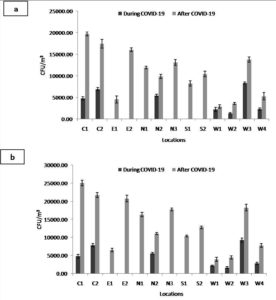
All samples were dominated by bacteria during and after the lockdown. There was no statistically significant difference (p>0.05) observed in the bacterial count between the 24-hour and 48-hour time points. The average outdoor total bacterial count varied (p<0.05) among different locations significantly. During COVID-19, it ranged between 0.00±0.00-9286.25±587.09 CFU/m3, compared with 2903.75±407.60-19722.50±475.03 CFU/m3 after the pandemic (Tables 1 & 2). During COVID-19, the western part of Madinah had the maximum average outdoor bacterial count, ranging between 3680.00±263.50 and 9286.25±587.09 CFU/m3. The districts located in the east, north and south had zero outdoor bacterial counts (Table 1 and Figures 2 & 3). In comparison, the central part had the highest average outdoor bacterial counts after the pandemic (21418.75±131.75-25070.00±777.85 CFU/m3). The outdoor bacterial average ranged between 6497.50±475.03-25472.50±623.95 CFU/m3 in the eastern part, 10407.50±217.06-12793.75±359.09 CFU/m3 in the southern part, 4283.75±733.55-16905.00±395.25 CFU/m3 in the western part and 14403.75±831.76-21390.00±395.25 CFU/m3 in the northern part (Table 2 and Figures 2 & 5).
Table (2):
Descriptive statistics of bacteria at different sampling locations after the COVID-19 pandemic
| No. | Location | Bacteria CFU/m3 | |||
|---|---|---|---|---|---|
| Nutrient agar | MacConkey agar | ||||
| 24 hours | 48 hours | 24 hours | 48 hours | ||
| 1 | C1 | 19722.50±475.03 | 25070.00±777.85 | 115.00±49.80 | 776.25±86.25 |
| 2 | C2 | 17451.25±1021.74 | 21418.75±131.75 | 201.25±49.80 | 287.50±99.59 |
| 3 | N1 | 11960.00±302.90 | 18313.75±587.09 | 57.50±99.59 | 143.75±248.98 |
| 4 | N2 | 9861.25±505.38 | 14403.75±831.76 | 0.00±0.00 | 28.75±49.80 |
| 5 | N3 | 13138.75±658.75 | 21390.00±395.25 | 0.00±0.00 | 0.00±0.00 |
| 6 | S1 | 8251.25±623.95 | 10407.50±217.06 | 0.00±0.00 | 57.50±99.59 |
| 7 | S2 | 10436.25±684.59 | 12793.75±359.09 | 0.00±0.00 | 28.75±49.80 |
| 8 | E1 | 4571.25±822.77 | 6497.50±475.03 | 0.00±0.00 | 0.00±0.00 |
| 9 | E2 | 16100.00±425.46 | 25472.50±623.95 | 0.00±0.00 | 460.00±131.75 |
| 10 | W1 | 2903.75±407.60 | 4283.75±733.55 | 0.00±0.00 | 57.50±99.59 |
| 11 | W2 | 3622.50±228.20 | 4485.00±456.39 | 0.00±0.00 | 0.00±0.00 |
| 12 | W3 | 13800.00±603.75 | 16905.00±395.25 | 28.75±49.80 | 28.75±49.80 |
| 13 | W4 | 5290.00±863.94 | 7791.25±527.00 | 0.00±0.00 | 0.00±0.00 |
Figure 2. The concentration of total airborne bacteria isolates on nutrient agar after incubation for (a) 24 hours and (b) 48 hours at different locations during and after the COVID-19 pandemic
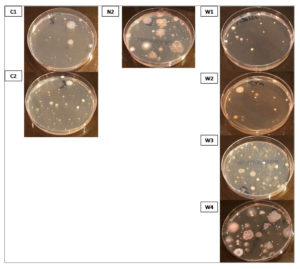
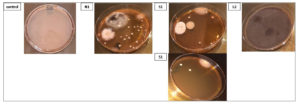
Figure 3. Representative plates of bacterial colonies isolated from air samples collected from different locations during the COVID-19 pandemic. The control showed zero colonies (data not shown)
Gram-positive bacteria were the most common in most of the samples across all sampling locations during and after the COVID-19 lockdown, with a statistically significant correlation (p<0.05). The outdoor average number of Gram-negative bacteria (lactose fermenters) grown on MacConkey agar ranged between 0.00±0.00-1236.25±302.90 CFU/m3. After COVID-19, the number decreased to 460.00±131.75-776.25±86.25 CFU/m3 (Table 1-2 and Figure 4 & 5).
Figure 4. The concentration of total airborne bacteria isolates on MacConkey agar after incubation for (a) 24 hours and (b) 48 hours at different locations during and after the COVID-19 pandemic
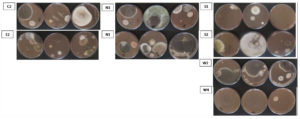
Figure 5. Representative plates of bacterial colonies isolated from air samples collected from different locations after the COVID-19 pandemic. The control showed zero colonies (data not shown)
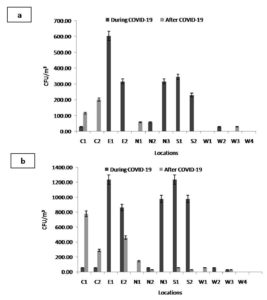
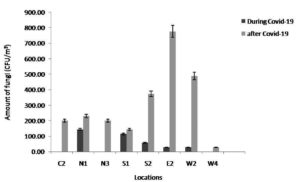
The outdoor average total fungal counts in the different locations showed no significant (p>0.05) differences. It was lower than that of bacteria and ranged between 0.00±0.00-143.75±131.75 CFU/m3 during the COVID-19 lockdown (Table 3 and Figure 6-8). The range increased significantly (p<0.05) after the pandemic to reach 28.75±49.80-776.25±298.78 CFU/m3. Among all districts in Madinah, C2, N3 and W4 had zero outdoor fungal counts, while N1 had the highest outdoor average fungal count (143.75±131.75 CFU/m3). After COVID-19, E2 illustrated the highest outdoor counts of fungi (776.25±298.78 CFU/m3), whereas W4 had the minimum average fungal count (28.75±49.80 CFU/m3).
Table (3):
Descriptive statistics of fungi at different sampling locations during and after the COVID-19 pandemic
| No. | Location | Fungi (CFU/m3) | |
|---|---|---|---|
| Sabouraud Dextrose agar | |||
| During COVID-19 | After COVID-19 | ||
| 1 | C2 | 0.00±0.00 | 201.25±131.75 |
| 2 | N1 | 143.75±131.75 | 230.00±131.75 |
| 3 | N3 | 0.00±0.00 | 201.25±131.75 |
| 4 | S1 | 115.00±99.59 | 143.75±131.75 |
| 5 | S2 | 57.50±49.80 | 373.75±179.54 |
| 6 | E2 | 28.75±49.80 | 776.25±298.78 |
| 7 | W2 | 28.75±49.80 | 488.75±263.50 |
| 8 | W4 | 0.00±0.00 | 28.75±49.80 |
Figure 6. The concentration of total airborne fungal isolates at different locations during and after the COVID-19 pandemic
Figure 7. Representative plates of fungal colonies isolated from air samples collected from different locations during the COVID-19 pandemic
Bacteria and fungus are widely distributed throughout the atmosphere near the earth’s surface, forming a vital component of atmospheric aerosols that could affect human health and atmospheric dynamics. They both display a range of differences in terms of their composition and spatiotemporal dynamics.45 Taking into account the possible role of airborne microorganisms in public health, many studies have investigated factors affecting airborne microbial composition and biodiversity, including meteorological conditions,46,47 as well as human activities.48
In March 2020, strict lockdown procedures were implemented by the Saudi authorities to stop the spread of the coronavirus. This lockdown has had a substantial influence on the mobility of individuals within the country. The implementation of social distancing measures among individuals has resulted in the disruption of the movement of aircraft, the suspension of industrial production and economic operations, and has had significant consequences on air quality.49 A review of the literature shows that most researchers have mainly focused only on indoor microbial contamination during COVID-19. Some air contaminants may be two to five times higher indoors than outdoors during the epidemic.41,42,50,51 Other researchers have investigated the levels of primary chemical contaminants in the atmosphere in numerous cities.47,52,53 However, based on the current body of information, there is no studies have been conducted to highlight the outdoor microbial load during the pandemic. The reason could be that people were not allowed to leave their houses at that time, but formal written permission was obtained for this study, in addition to their feelings of worry and fear from going outdoors. Hence, it is believed that none of the previous studies compared the outdoor microbial load during and after the pandemic. Therefore, this study intended to provide a glimpse into the influence of the pandemic on microbial air quality in a number of outdoor environments during and after the COVID-19 pandemic in Madinah, KSA. Madinah is regarded as Islam’s second sacred City. The Central Department of Statistics and Information (CDSI) of Saudi Arabia reports that Madinah receives millions of visitors annually.54 It has distinguished air environment, topography, and weather.
The present investigation employed the open plate method (sedimentation technique) to collect the samples. Although this method provides an imprecise estimation of the quantities and varieties of airborne organisms, it is still regarded as a cost-effective and scientific practical technique.55 In our study, assessment of microbial air pollutant levels in the outdoor environment of a variety of locations in Madinah City indicated that the numbers of bacteria and fungi were significantly lower in the outdoors during COVID-19 than after the pandemic. The reason behind this was that during the COVID-19 pandemic, 90% of a person’s waking hours were spent in a contained space, mostly at home. Prior research has demonstrated that the primary causes of micro-contaminants are human activity and habitation in residential areas.56,57,58 In addition, dust is considered one of the most microbial sources, and its composition significantly affects the survival of the attached microorganisms. Moreover, dust on surfaces exists in different sizes and is periodically resuspended in air due to human activities.13,59 In a study conducted by Jiang et al.,60 using a meta-analysis of air samples (3226 samples), it was revealed that samples in categories linked to anthropogenic activities displayed higher co-occurrence network complexity, higher microbial diversity, and higher relative prevalence of pathogens. Gao et al.61 reported that atmospheric pollutants from human activities were the main factors in shaping bacterial community structure. Cordeiro et al.62 demonstrated that outdoor microbial quality is related to the natural and anthropogenic air pollution, besides climatic and geographical factors.
Bacterial presence is widespread in the atmosphere, exhibiting a density ranging from 104-108 cells per cubic meter.63,64 The results of the study showed that Gram-positive bacteria were detected in a large number of the samples in all the sampling areas. Indeed, Gram-positive bacterial species exhibited greater prevalence and resistance to environmental influences than Gram-negative bacteria. A similar finding was discovered in a study carried out in Madinah by Bakutis et al.,65 since a higher prevalence of Gram-positive bacteria was observed in farms raising cattle and poultry. The difference may be explained by the Gram-negative bacteria’s susceptibility to Atom Environs and the cell wall structure of the Gram-positive bacteria.66 Other studies reported that microorganism viability can be greatly impacted by temperature and sun radiation intensity, with Gram-positive bacteria being more resistant to elevated solar radiation than Gram-negative bacteria.67,68
In terms of airborne bacterial counts, they varied significantly between the studied outdoor sites. The differences in bacterial concentrations indicate that sources of this bio-contamination are diverse in various locations of Madinah City. Of the twelve sampling locations, western part of Madinah had the maximum average outdoor bacterial count during COVID-19. This may be related to the poor infrastructure of this location and the standards of living and socioeconomic status. The east and south districts had zero bacterial counts since Saudi Arabia’s interior ministry imposed a complete lockdown in these neighbourhoods to prevent the spread of coronavirus. All movement was restricted in those areas, and residents were prohibited from circulating outside their homes. After the pandemic, the central part had the highest average outdoor bacterial count. The main reason for the high numbers of microbes found in this location might be due to overcrowding with people. This time of year, millions of Muslims visit the two holy mosques in KSA. The Prophet’s Mosque and the Grand Mosque in Makkah are important sites for the Hajj and Umrah. The Prophet’s Mosque (Al-Masjid al-Nabawi) is located in the centre of the City of Madinah and all these visitors stay in there for some days. However, this location had a lower microbial load during COVID-19, due to the lockdown at these sacred locations and the suspension of Umrah and Ziyarah by the Saudi Arabian authorities.69 The western region (W1) had the lowest bacterial count, which may be explained by the fact that it has lowered human occupancy compared with other locations. It has been demonstrated that human occupancy and pollutant levels in the outdoors have a proportional and direct relationship.13
The dispersion of spores from various fungal species in the atmosphere contributes significantly to air pollution, highlighting the crucial role of airborne fungus in this environmental concern,70 which can change environmental biotic and/or abiotic elements, which has an impact on human health.71 The abundance and composition of aerial fungal populations within a certain region are contingent upon a variety of factors, including environmental parameters, human interventions, and the presence of suitable substrates conducive to fungal proliferation.72 In this study, few fungal species were detected compared to bacteria before and after the pandemic, since sufficient moisture is necessary for fungi to be active.73 During COVID-19, The northern region (N1) had the highest number of fungi, since farms are common in this area. Various studies have earlier reported that the concentration of airborne fungi was strongly correlated with the amount of vegetation cover in outdoor environments. A research conducted in Beijing by Fang et al.,74 showed that the diversity of fungi in green regions was found to be greater in comparison to those observed in heavily inhabited and polluted areas. Previous research has indicated that regions exhibiting a greater prevalence of verdant vegetation were associated with elevated levels of aerial fungal spores.74-77 Lymperopoulou et al.,78 illustrated that local vegetation strongly influences the composition of outdoor air locally. The concentration of airborne microbial particles was up to ten times greater close to plants than in the bulk air distant from the plants. After the pandemic, the eastern region (E2) recorded the highest level of fungi among all the districts. This district is crowded with four hospitals and several shopping centres located there. A large number of studies have revealed that diversity and concentration of fungi in outdoor environments are primarily influenced by factors such as traffic flow and human activities.13,79,80
One drawback of this study was the wind speed during sample collection. Prior research has demonstrated that several environmental parameters, temperature, atmospheric pressure, wind velocity, and humidity, have the potential to influence the variety of airborne microbes across diverse habitats.81 However, it is fairly known that the results of these studies vary significantly from one study to another. The difficulty in comparing the microbial load is related to the influence of a number of factors apart from the location, such as changes in atmospheric conditions (temperature, wind speed and direction, humidity, rainfall and solar radiation), sampling technique, and social and economic conditions.
Both the productivity of naturally occurring and managed ecosystems and human health can be significantly impacted by airborne microorganisms. Air and microbial pollutants research are a crucial component in comprehending public health policies. Evidence shows airborne microorganisms play a significant but understudied, role in air quality, and their inclusion in models and measurements of atmospheric contaminants has to be improved. Governments worldwide imposed lockdowns in early 2020 due to the sudden increase in COVID-19 cases. This provided a unique opportunity to learn more about how human activities affect air quality. The scientific community used this global experiment as a natural laboratory to gain knowledge and reconstruct their findings. In this study, the microbial load of the outdoor environments of Madinah City, KSA, for the period of the COVID-19 lockdown was investigated. It provides the first report on the bacterial and fungal diversity in outdoor air during COVID-19 pandemic. In this regard, all studied locations had significantly lower biological pollutants during the pandemic due to lockdown limitations. Mostly Gram-positive were detected, with few fungal species. Different studied outdoor locations showed varied microbial counts due to particular conditions related to these locations. The results confirmed the strong influence of human activities as a primary source of microbes in air. The understanding of factors that determine the composition of microbes in outdoor air might contribute to our understanding of indoor air quality. In many cases, outside air significantly contributes to the bacterial and fungal populations that are found in interior spaces, including homes. However, it is important to be careful when comparing short-term data during lockdown because seasonal changes can be significant and may mask any actual changes caused by the lockdown.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SMA designed the study, collected the samples and produced the lab work. HMA achieved the lab work and wrote the manuscript. SMA revised the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Dacarro C, Picco AM, Grisoli P, Rodolfi M. Determination of aerial microbiological contamination in scholastic sports environments. J Appl Microbiol. 2003;95(5):904-912.
Crossref - Vermani M, Vijayan VK, Kausar MA, Agarwal MK. Quantification of Airborne Aspergillus Allergens: Redefining The Approach. J Asthma. 2010;47(7):754-761.
Crossref - Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann Occup Hyg. 2003;47(3):187-200.
Crossref - Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525(7569):367-371.
Crossref - Landrigan PJ, Fuller R, Acosta NJR,et al. The Lancet Commission on pollution and health. Lancet . 2018;391(10119):462-512.
Crossref - Jaenicke R. Abundance of Cellular Material and Proteins in the Atmosphere. Science. 2005;308(5718):73-73.
Crossref - Maki T, Puspitasari F, Hara K, Yamada M, Kobayashi F, Hasegawa H, Iwasaka Y. Variations in the structure of airborne bacterial communities in a downwind area during an Asian dust (Kosa) event. Sci Total Environ. 2014;488- 489:75-84.
Crossref - Barberian A, Ladau J, Leff JW, et al. Continental-scale distributions of dust-associated bacteria and fungi. Proc Natl Acad Sci U S A . 2015;112(18):5756-5761.
Crossref - Li K, Dong S, Wu Y, Yao M. Comparison of the biological content of air samples collected at ground level and at higher elevation. Aerobiologia. 2010;26(3):233-244.
Crossref - Rajasekar A, Balasubramanian R. Assessment of airborne bacteria and fungi in food courts. Building and Environment. 2011;46(10):2081-2087.
Crossref - Madureira J, Paciencia I, Rufo J, et al. Indoor air quality in schools and its relationship with children’s respiratory symptoms. Atom Environ. 2015;118:145-156.
Crossref - Crawford JA, Rosenbaum PF, Anagnost SE, Hunt A, Abraham JL. Indicators of airborne fungal concentrations in urban homes: Understanding the conditions that affect indoor fungal exposures. Sci Total Environ. 2015;517:113- 124.
Crossref - Awad AHA. Environmental Study in Subway Metro Stations in Cairo, Egypt. J Occup Health. 2002;44(2):112- 118.
Crossref - Yassin MF, Almouqatea S. Assessment of airborne bacteria and fungi in an indoor and outdoor environment. Int J Environ Sci Technol. 2010 7(3):535-544.
Crossref - Gladyszewska-Fiedoruk K. Concentrations of carbon dioxide in the cabin of a small passenger car. Transportation Research Part D: Transport and Environment. 2011;16(4):327-331.
Crossref - Mashat B. Indoor and outdoor microbial aerosols at the holy mosque: A case study. Atmospheric Pollution Research. 2015;6(6):990-996.
Crossref - Whon TW, Kim M-S, Roh SW, Shin N-R, Lee H-W, Bae J-W. Metagenomic Characterization of Airborne Viral DNA Diversity in the Near-Surface Atmosphere. J Virol. 2012;86(15):8221-8231.
Crossref - Bertolini V, Gandolfi I, Ambrosini R, et al. Temporal variability and effect of environmental variables on airborne bacterial communities in an urban area of Northern Italy. Appl Microbiol Biotechnol. 2013;97(14):6561-6570.
Crossref - Robertson CE, Baumgartner LK, Harris JK, et al. Culture- Independent Analysis of Aerosol Microbiology in a Metropolitan Subway System. Appl Environm Microbiol. 2013;79(11):3485-3493.
Crossref - Cao C, Jiang W, Wang B, et al. Inhalable Microorganisms in Beijing’s PM 2.5 and PM 10 Pollutants during a Severe Smog Event. Environ Sci Technol. 2014;48(3):1499-1507.
Crossref - Gataa R, Ajmi T, Bougmiza I, Mtiraoui A. Morbidity patterns in general practice settings of the province of Sousse, Tunisia. Pan Afr Med J. 2009;3(1).
Crossref - Pellissier L, Oppliger A, Hirzel AH, et al. Airborne and Grain Dust Fungal Community Compositions Are Shaped Regionally by Plant Genotypes and Farming Practices. Appl Environ Microbiol. 2016;82(7):2121-2131.
Crossref - Chew FT, Lim SH, Shang HS, et al. Evaluation of the allergenicity of tropical pollen and airborne spores in Singapore. Allergy. 2000;55(4):340-347.
Crossref - Pastuszka JS, Paw UKT, Lis DO, Wlazlo A, Ulfig K. Bacterial and fungal aerosol in indoor environment in Upper Silesia, Poland. Atom Environ. 2000;34(22):3833-3842.
Crossref - Meklin T, Potus T, Pekkanen J, Hyvarinen A, Hirvonen M-R, Nevalainen A. Effects of moisture-damage repairs on microbial exposure and symptoms in schoolchildren. Indoor Air. 2005;15(Suppl 10):40-47.
Crossref - Gutarowska B, Sulyok M, Krska R. A Study of the Toxicity of Moulds Isolated from Dwellings. Indoor and Built Environment. 2010;19(6):668-675.
Crossref - Moon HJ, Yoon YR. Investigation of Physical Characteristics of Houses and Occupants’ Behavioural Factors for Mould Infestation in Residential Buildings. Indoor Built Environ. 2010;19(1):57-64.
Crossref - Pasquarella C, Albertini R, Dall’aglio P, Saccani E, Sansebastiano GE, Signorelli C. Air microbial sampling: the state of the art. Igiene e Sanita Pubblica. 2008;64(1):79-120.
- Ely G. ISO 14698 or EN 17141: Is There a Choice for Cleanroom Compliance? Biomed Instrum Technol. 2023;57(s1):15-17.
Crossref - Contini D, Costabile F. Does Air Pollution Influence COVID-19 Outbreaks? Atmosphere (Basel). 2020;11(4):377.
Crossref - Khalifa SAM, Swilam MM, El-Wahed AAA, et al. Beyond the Pandemic: COVID-19 Pandemic Changed the Face of Life. Int J Environ Res Public Health. 2021;18(11):5645.
Crossref - Alsharif SM. Why Children survive from Corona Complications, COVID-19 and MERS. 2020; 25. https:// www.researchgate.net/publication/341322370
- Mohammedsaeed W, Alrashidi H, Alsharif SM, Aljardi O, Al-Sehli A. COVID-19 Vaccine Impacts in Saudi Arabia: A Cross-Sectional Study. Cureus. 2023;15 (6):e40460.
Crossref - Venter ZS, Aunan K, Chowdhury S, Lelieveld J. COVID-19 lockdowns cause global air pollution declines. Proc Natl Acad Sci U S A . 2020;117(32):18984-18990.
Crossref - Dantas G, Siciliano B, Franca BB, da Silva CM, Arbilla G. The impact of COVID-19 partial lockdown on the air quality of the city of Rio de Janeiro, Brazil. Sci Total Environ. 2020;729:139085.
Crossref - Mahato S, Pal S, Ghosh KG. Effect of lockdown amid COVID-19 pandemic on air quality of the megacity Delhi, India. Sci Total Environ. 2020;730:139086.
Crossref - Wang Q, Su M. A preliminary assessment of the impact of COVID-19 on environment – A case study of China. Sci Total Environ. 2020;728:138915.
Crossref - Heinrich J, Hölscher B, Douwes J, et al. Reproducibility of allergen, endotoxin and fungi measurements in the indoor environment. J Expo Sci Environ Epidemiol. 2003;13(2):152-160.
Crossref - Viani I, Colucci ME, Pergreffi M, et al. Passive air sampling: the use of the index of microbial air contamination. Acta Biomed. 2020;91(3-S):92-105.
Crossref - Minimalist Modern Map of Medina, Saudi Arabia 3. https://fineartamerica.com/featured/minimalist-modern-map-of-medina-saudi-arabia-3-celestial-images.html. Accessed 17 July, 2023.
- Bhat MA, Eraslan FN, Awad A, et al. Investigation of indoor and outdoor air quality in a university campus during COVID-19 lock down period. Build Environ. 2022;219:109176.
Crossref - Asril M, Sugiarto S, Zurfi A. Airborne Microbial Quality Assessment in the Educational Buildings during the COVID-19 Pandemic. Civil Engineering Journal. 2023;9(1):114-126.
Crossref - Omelyansky VL. Manual in microbiology. USSR academy of sciences, Moscow. 1940.
- Ljaljević-Grbić M, Stupar M, Vukojević J, Maričić I, Bungur N. Molds in museum environments: biodeterioration of art photographs and wooden sculptures. Archives of Biological Sciences. 2013;65(3):955-962.
Crossref - Bowers RM, Clements N, Emerson JB, Wiedinmyer C, Hannigan MP, Fierer N. Seasonal Variability in Bacterial and Fungal Diversity of the Near-Surface Atmosphere. Environ Sci Technol. 2013;47(21):12097-12106.
Crossref - Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211-224.
Crossref - Rao Y, Li H, Chen M, et al. Community Structure and Influencing Factors of Airborne Microbial Aerosols over Three Chinese Cities with Contrasting Social-Economic Levels. Atmosphere. 2020;11(4):317.
Crossref - Brusseau ML, Anderson RH, Guo B. PFAS concentrations in soils: Background levels versus contaminated sites. Sci Total Environ. 2020;740:140017.
Crossref - Yezli S, Khan A. COVID-19 social distancing in the Kingdom of Saudi Arabia: Bold measures in the face of political, economic, social and religious challenges. Travel Med Infect Dis. 2020;37:101692.
Crossref - Kuddus M, Khatoon F, Saleem M, et al. Assessment of bio-contaminants during COVID-19 outbreak from the indoor environment of Hail city, Kingdom of Saudi Arabia. Bioinformation. 2021;17(5):541-449.
Crossref - Yang JIL, Lee BG, Park J, Yeo M. Airborne fungal and bacterial microbiome in classrooms of elementary schools during the <scp>COVID</scp> -19 pandemic period: Effects of school disinfection and other environmental factors. Indoor Air. 2022;32(9):e13107.
Crossref - Ginzburg AS, Semenov VA, Semutnikova EG, Aleshina MA, Zakharova PV, Lezina EA. Impact of COVID-19 Lockdown on Air Quality in Moscow. Doklady Earth Sciences. 2020;495(1):862-866.
Crossref - Le T, Wang Y, Liu L, et al. Unexpected air pollution with marked emission reductions during the COVID-19 outbreak in China. Science. 2020;369(6504):702-706.
Crossref - Alananbeh KM, Boquellah N, Al Kaff N, Al Ahmadi M. Evaluation of aerial microbial pollutants in Al-Haram Al-Nabawi during pilgrimage of 2013. Saudi J Biol Sci. 2017;24(1):217-225.
Crossref - Atlas R, Bartha R. Microbial Ecology: Fundamentals and Applications. Benjamin/Cummings Sci. Pub., Menlo Park, CA. 1998:99-103.
- Goh I, Obbard JP, Viswanathan S, Huang Y. Airborne bacteria and fungal spores in the indoor environment. A case study in Singapore. Acta Biotechnologica. 2000;20(1):67-73.
Crossref - Meadow JF, Altrichter AE, Kembel SW, et al. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source. Indoor Air. 2014;24(1):41-48.
Crossref - Rai S, Singh DK, Kumar A. Microbial, environmental and anthropogenic factors influencing the indoor microbiome of the built environment. J Basic Microbiol. 2021;61(4):267-292.
Crossref - Thorne PS, Kiekhaefer MS, Whitten P, Donham KJ. Comparison of bioaerosol sampling methods in barns housing swine. Appl Environ Microbiol. 1992, 58(8):2543- 2551.
Crossref - Jiang X, Wang C, Guo J, et al. Global Meta-analysis of Airborne Bacterial Communities and Associations with Anthropogenic Activities. Environ Sci Technol. 2022;56(14):9891-9902.
Crossref - Gao M, Jia R, Qiu T, Han M, Song Y, Wang X. Seasonal size distribution of airborne culturable bacteria and fungi and preliminary estimation of their deposition in human lungs during non-haze and haze days. Atom Environ. 2015;118:203-210.
Crossref - Cordeiro RA, Brilhante RS, Pantoja LD, et al. Isolation of pathogenic yeasts in the air from hospital environments in the city of Fortaleza, northeast Brazil. Braz J Infect Dis. 2010;14(1):30-34.
Crossref - Bowers RM, McLetchie S, Knight R, Fierer N. Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. ISME Journal. 2011;5(4):601-612.
Crossref - Bowers RM, Lauber CL, Wiedinmyer C, et al. Characterization of Airborne Microbial Communities at a High-Elevation Site and Their Potential To Act as Atmospheric Ice Nuclei. Appl Environ Microbiol. 2009;75(15):5121-5130.
Crossref - Bakutis B, Monstviliene E, Januskeviciene G. Analyses of Airborne Contamination with Bacteria, Endotoxins and Dust in Livestock Barns and Poultry Houses. Acta Veterinaria Brno. 2004;73(2):283-289.
Crossref - Perron GG, Gonzalez A, Buckling A. Source-sink dynamics shape the evolution of antibiotic resistance and its pleiotropic fitness cost. Proc R Soc Lond B Biol Sci. 2007;274(1623):2351-2356.
Crossref - Huang H-C, Lee C-L, Lai C-H, Fang M-D, Lai I-C. Transboundary movement of polycyclic aromatic hydrocarbons (PAHs) in the Kuroshio Sphere of the western Pacific Ocean. Atom Environ. 2012;54:470-479.
Crossref - Soleimani Z, Goudarzi G, Sorooshian A, Marzouni MB, Maleki H. Impact of Middle Eastern dust storms on indoor and outdoor composition of bioaerosol. Atom Environ. 2016;138:135-143.
Crossref - Perveen S, Orfali R, Ul Azam MS, et al Coronavirus nCOVID-19: A pandemic disease and the Saudi precautions. Saudi Pharm J. 2020;28(7):888-897.
Crossref - Pei-Chih W, Huey-Jen S, Chia-Yin L. Characteristics of indoor and outdoor airborne fungi at suburban and urban homes in two seasons. Sci Total Environ. 2000;253(1- 3):111-118.
Crossref - Sivagnanasundaram P, Amarasekara RWK, Madegedara RMD, Ekanayake A, Magana-Arachchi DN. Assessment of Airborne Bacterial and Fungal Communities in Selected Areas of Teaching Hospital, Kandy, Sri Lanka. BioMed Res Int. 2019;1-11.
Crossref - Kurup VP, Shen H-D, Banerjee B. Respiratory fungal allergy. Microbes Infect. 2000;2(9):1101-1110.
Crossref - Lacey J, Dutkiewicz J. Bioaerosols and occupational lung disease. J Aerosol Sci. 1994;25(8):1371-1404.
Crossref - Fang Z, Ouyang Z, Hu L, Wang X, Zheng H, Lin X. Culturable airborne fungi in outdoor environments in Beijing, China. Sci Total Environ. 2005;350(1-3):47-58.
Crossref - Picco AM, Rodolfi M. Airborne fungi as biocontaminants at two Milan underground stations. Int Biodeterior Biodegrad . 2000;45(1-2):43-47.
Crossref - Fang Z, Tang Q, Gong C, et al. Profile and distribution characteristics of culturable airborne fungi in residential homes with children in Beijing, China. Indoor Built Environ. 2017;26(9):1232-1242.
Crossref - Fang Z, Guo W, Zhang J, Lou X. Assemblages of Culturable Airborne Fungi in a Typical Urban, Tourism-driven Center of Southeast China. Aerosol and Air Quality Research. 2019;19(4):820-831.
Crossref - Lymperopoulou DS, Adams RI, Lindow SE. Contribution of vegetation to the microbial composition of nearby outdoor air. Appl Environ Microbiol. 2016;82(13):3822-3833.
Crossref - di Giorgio C, Krempff A, Guiraud H, Binder P, Tiret C, Dumenil G. Atmospheric pollution by airborne microorganisms in the city of Marseilles. Atmos Environ. 1996;30(1):155-160.
Crossref - Pasanen A-L, Lappalainen S, Pasanen P. Volatile organic metabolites associated with some toxic fungi and their mycotoxins. The Analyst. 1996;121(12):1949-1953.
Crossref - Larsen LS. A Three-Year-Survey of Microfungi in the Air of Copenhagen 1977-79. Allergy. 1981;36(1):15-22.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.