ISSN: 0973-7510
E-ISSN: 2581-690X
A novel organic acid-modified starch and carboxymethyl cellulose (CMC) based films plasticized with glycerol were prepared from unconventional tikhur starch (Curcuma angustifolia) by solution casting. Wet milling was used in the laboratory to extract starch from the tikhur rhizome. Carboxymethyl cellulose, at a concentration of (0.2 g−1 starch dry basis) was blended with the starch to improve its film-forming properties. Three different treatments with varying organic acids (lactic, citric, and acetic acid) with a concentration of 5% w/w of starch (2 ppm) in a film-forming solution were given. The effect of organic acid incorporation on the antibacterial, morphological, structural, thermal, and crystalline properties of developed films was studied. The minimum inhibitory concentration values of the three organic acids against gram-negative (E. coli) and gram-positive (S. aureus) bacteria were measured using the tube dilution method. The MIC results revealed that lactic acid and citric acid are effective against both gram-negative and gram-positive bacteria, while acetic acid showed more effectiveness against gram-negative bacteria (E. coli). MBC results revealed that organic acids have potent bactericidal activity. Citric acid resulted in higher inhibition for gram-positive bacteria (S. aureus) compared to gram-negative bacteria (E. coli.). While acetic acid showed higher inhibition for E. coli. than S. aureus. Lactic acid displayed similar inhibition against both S. aureus and E. coli. Among different organic acids, lactic acid incorporation resulted in a more homogeneous, transparent, and thermally stable film. As evidenced by the micrographs, the lactic acid incorporation resulted in a compact film structure without any visible cracks. While X-ray diffraction showed an increase in crystalline properties due to organic acid modification. In this study, it was indicated that modification with organic acids (polycarboxylic acids) effectively improved the overall properties of developed films depending on the type of organic acid used. The developed films have the potential to replace harmful synthetic films in food packaging.
Curcuma angustifolia, Carboxymethyl Cellulose (CMC), Lactic Acid, Citric Acid, Acetic Acid, Minimum Inhibitory Concentration (MIC)
Plastics are now widely used in packaging as a result of significant developments in the petrochemical industry. The packaging industry accounts for more than 42% of all plastic materials used worldwide. The food packaging industry is the major consumer of plastics due to their good mechanical capabilities and low moisture permeability.1,2 However, synthetic plastics are becoming a threat to the environment as they are petroleum-derived products that lead to the emission of CO2 into the atmosphere.3 Due to its high chemical and mechanical durability, plastic degradation in the environment is a slow process, and it takes years to convert into simple compounds. Biopolymers are now being researched as a possible replacement for synthetic polymers. The development of biodegradable packaging materials sparked the search for novel biodegradable film manufacturing sources.4,5 To address these concerns, renewable packaging materials with similar properties that can be used in food packaging must be developed. Numerous studies have been published in the literature utilising polysaccharides, primarily starch, for biodegradable films development from various sources, such as cereals (rice, corn, oats, etc.),6 tubers (tapioca, cassava, potato, yam, etc.),7,8, and rhizomes (lotus, ginger)9 because of their ability to form an uninterrupted matrix, low cost, nutritional value, biodegradability, and edibility with functional qualities.10,11 After grains and legumes, rhizomes and tubers are the third most important food crop, with starch as a key component.12,13
Curcuma angustifolia (Tikhur), which has around 50 types and 1000 species, is found in the tropics of Australia, Africa, and Asia. It is one of the less-known and valuable sources of starch, which is also known as “East Indian arrowroot” or “white turmeric”.14 However, due to their brittleness, strong hydrophilic character, limited stability under high relative humidity conditions, poor mechanical properties, poor water vapour permeability, and retrogradation phenomena, starch-based films are challenging to use for food packaging on an industrial scale.15,16 Moreover, retrogradation causes a change in crystallinity, thus a change in texture, color, etc.17 Several studies have attempted to overcome this issue, such as blending of two or more biopolymers, to maximise the benefits of two or more ingredients,18 modifications of starch and treatment combinations to improve starch properties.19,20 Thus, incorporating alternative natural and ecologically acceptable hydrophilic polymers into the starch films is an interesting way to improve their functional qualities.21 Carboxymethyl cellulose (CMC) can be used as a natural filler in a starch-based matrix due to its polymeric structure, high transparency, high molecular weight, high mechanical strength, non-toxicity, biodegradability, and improved synergy with the starch.22-24
Organic acid modification of starches can be another useful tool for adjusting the overall properties of starch films, particularly for maintaining or improving water stability and preventing retrogradation, in addition to imparting antibacterial functionality. Organic acids can be found naturally in fruits and vegetables or produced by biotechnological methods via fermentation. Organic acids including lactic, citric, acetic, malic, and ascorbic acids are inexpensive, non-toxic, and low weight poly-carboxylic acids. The antimicrobial properties of organic acid are thought to be caused by the reduction of the microbial cell’s internal pH due to the ionisation of undissociated acid molecules, reduction in proton motive force or due to disruption of substrate transport caused by changes in cell membrane permeability.25,26 Thus, organic acids can be incorporated into the film to provide antibacterial properties. It reduces the hydrophilic property of thermoplastic starch and prevents retrogradation by substituting hydrophobic ester groups for the hydroxyl groups. In this study, Curcuma angustifolia starch-CMC-based glycerol plasticized biodegradable films were modified with organic acids (lactic, citric, and acetic acid) and their strength, flexibility, crystallinity, morphology, thermal stability, biodegradability, and antibacterial activity were examined.
Materials
Curcuma angustifolia rhizomes for the harvest year 2020 were acquired from Baster, Chhattisgarh state of India. All the chemicals, viz., citric acid (C6H8O7), acetic acid (C2H4O2), and lactic acid (C3H6O3) (food grade), were obtained from Merck India Limited. Carboxymethyl cellulose (CMC), with a viscosity of 22,000, was purchased from ACS India. Glycerol (C3H8O3), sodium chloride (NaCl), potassium acetate (CH3COOK), and anhydrous calcium chloride (CaCl2) were procured from Rankem India Limited.
Bacterial strains, namely gram-negative Escherichia coli (Strain ATCC: 0018) and gram-positive Staphylococcus aureus (Strain ATCC: 0045) were procured from the Department of Industrial Microbiology, Sam Higginbottom University of Agriculture Technology and Sciences, Prayagraj, India, and preserved on Nutrient Agar slants (Hi-Media, India) at 4°C which were regularly subcultured at 15 days intervals.
Determination of the Minimum Inhibitory Concentration (MIC) of Organic Acids
The MIC of lactic, citric, and acetic acid against the test bacteria E. coli and S. aureus was evaluated using the traditional tube dilution method with nutritional broth as the growth media. To allow enough bacterial development, the test bacteria were transferred to the nutrient broth and incubated for 24 hours at 37°C. On the second day, 0.05 μL of the calibrated broth (108 CFU/ml) was transferred into 5 ml nutrient broth along with the decreasing order of organic acid concentration from 50 mg/ml to 0.78 mg/ml. The turbidity of the tubes was visualised, after incubation to confirm the MIC value.
Determination of the Minimum Bactericidal Concentration (MBC) of Organic Acids
MBC determination of the organic acids was carried out by transferring, 0.05 μL of suspension from the MIC tubes, which showed no obvious bacterial growth on nutrient agar plates.
Starch isolation
Starch from the Curcuma angustifolia rhizome was isolated using a wet milling scheme as described in literature13 with some modification. For film formation, extracted starch with a water content of 5.97%, protein of 0.92%, lipid of 0.54%, ash of 0.52%, and amylose of 31.9% on a dry basis was used.
Preparation of films
The solution casting of films was performed utilising the method as described in literature27 with suitable adaptations. Different films were prepared as tikhur starch film (TS), (Starch: CMC) film (SC), lactic acid modified films (SCL), citric acid modified film (SCC), and acetic acid modified film (SCAc).
For tikhur starch film (TS) preparation pre-weighed Curcuma angustifolia (tikhur) starch was stirred well with distilled water using a mechanical stirrer for 15 min. Glycerol (25% v/w of starch) was added and mixed by stirring with a mechanical stirrer for 5 min. This suspension was heated at 80°C for 30 min with stirring. The resulting viscous suspension was sonicated for 5 mins at 40°C with an ultrasonic bath sonicator (National Scientific, Varanasi, U.P., India). For solution casting of the film, 150 ml of prepared solution was transferred into the casting plate (412 cm2 area and 20 cm diameter) and dried for 16 hours in a hot air oven at 50°C.
For (starch: CMC) film (SC) preparation starch suspension containing glycerol was prepared as described above. CMC solution was prepared separately by dissolving CMC powder (0.2 g-1 starch dry basis) in distilled water at 90°C for 10 mins with mechanical stirring at 900 rpm. Prepared starch and CMC solutions were homogenised with a mechanical stirrer at 500 rpm for 30 mins at 80°C.
A modified starch filmogenic solution was prepared by incorporating citric acid, acetic acid, and, lactic acid 5% w/w starch into the starch: CMC solution after cooling to room temperature.
Film characterization
Mechanical Properties
The thickness of the developed films was measured by using a digital micrometre with a sensitivity of ± 0.01 mm. The films’ mechanical properties were evaluated with a texture analyzer (Stable microsystem with exponent software), according to ASTM D882-12. Tensile strength and percent elongation at break were determined for each conditioned film sample (9 cm x 2 cm) by determining the force and deformation during extension at 50 mm/min. The initial distance of 80 mm was maintained between the grips during measurement.
Moisture content (MC)
The Moisture content of preconditioned (23% RH, 25°C) film samples was measured according to the ASTM D644-99 (ASTM, 1999).
Moisture Content =[ (Mi-Mfi)/Mi ] x 100 …(1)
where Mi and Mfi refer to the initial and final weight of the film samples, respectively.
Moisture Absorption
Moisture absorption was performed according to28 with modifications. Dried film samples (2 cm2) were conditioned to 0% RH using a saturated solution of CaSO4. After weighing, the films were conditioned to 75% RH containing a saturated solution of NaCl as a desiccant. The samples were weighed hourly for the first 12 hours; after that, they were weighed every 12 hours until equilibrium was attained. MA was obtained from the following equation.
MA (%) = [(Ws-Wo)/Wo ] x 100 …(2)
Where Ws represents the weight of the film sample at 75% RH and Wo represents the initial weight of the film sample at 0% RH, respectively.
Water vapour permeability (WVP)
Water vapour permeability was performed according to ASTM E 96-95 with modifications. Samples were cut into a square shape (3 cm2) and their thickness was measured. Films were conditioned in saturated potassium acetate solution for 24 h (RH = 23%) at 25 ± 0.2°C. 3 g of dry CaSO4 (RH = 0%) powder was kept in the vials (4.5 cm x 2.5 cm) and conditioned film pieces were kept over the outlet head of the vials and sealed with the help of silicon grease tightly to prevent leakage of water vapour. After determining the initial weight of the vials along with CaSO4 and film, they were kept in a desiccator containing saturated NaCl solution at 25 ± 0.2°C (RH = 75%). An increase in the weight of the vials due to the absorption of moisture that permeated through the film by CaSO4 was recorded every 1h for 8h and their curves were plotted and then the slope was calculated. The water vapour transmission rate (g./m2h) and water vapour permeability (g.mm/KPa.m2.h) were obtained by using the following equations.
WVTR=(Slope (g/h))/(Film transfer area (m2) …(3)
WVP= [WVTR/P (R1 ‒ R2)] ×T …(4)
Where P represents the saturated vapour pressure of water at test temperature (25°C), R1 represents the RH in a desiccator (75 %), R2 represents the RH in the vial (0%), and T is the film thickness (mm).
Water solubility (WS)
Film samples (4 cm2) were conditioned at 0% RH using dry calcium sulphate as desiccant till the constant weight was obtained and weighed (W1). Conditioned film samples were mixed in 50ml of distilled water for 24 h at 4, 25 and 50°C±0.5°C in a shaker at 150 rpm. After 24 hours, the samples of film that didn’t dissolve in water were taken back and put in a desiccator until they reached a constant weight (W2).
WS% = [ (W1-W2)/W1 ] x 100 …(5)
Internal transmittance and opacity
The internal transmittance and opacity of the films of the film samples were determined using a Shimadzu UV-visible spectrophotometer model UV2250 by measuring percent transmittance at a wavelength of 600 and 280 nm, respectively. Air was taken as a reference.
Morphological characteristics
FESEM was used to get the results of the morphological characteristics of the developed films. Film samples were fixed on FESEM stubs covered with double-sided carbon adhesive tape with a gold layer in an ion sputter to increase their electrical conductivity. The samples were then scanned in a FESEM (Zeiss Gemini 300) at 50x magnification and 20 kV acceleration voltage.
Fourier transform infrared spectroscopy
FTIR spectra were recorded by a (Bruker, ATR-FTIR) spectrometer in attenuated total reflectance mode. Films were scanned in the 4000-700 cm-1 range with 32 scans per sample and a 4 cm-1 spectral resolution.
X-ray diffraction (XRD)
The crystalline nature of the films was verified with the help of an X-ray diffraction pattern (Smartlab, Rigaku Technologies, Japan) at a step width of 1.2°/min over the 2θ between 5 to 40°. The experiment was carried out at 23 ± 2°C.
Thermal properties
The thermal profile of the samples was obtained on an (EXSTAR TG/DTA, model 6300). Samples weighing about 10 mg were heated from 25°C to 650°C at a rate of 10°C/min in a standard alumina crucible. The measurements were performed under nitrogen with a flow rate of 100 ml/min, which had previously been calibrated with an alumina standard. The TGA curve was used to measure the amount of char and the percentage of weight loss. The maximum temperature at which the films decompose was determined using the DTG curve.
Biodegradability test
The biodegradability of developed films was determined using the soil burial method reported by Nguyen et al.29 with slight modifications. The film samples (2 cm2) were buried at a depth of 5 cm in the soil. After every two days, water was sprinkled on the soil to maintain a humidity of 40-45%. The degradation of film samples was conducted at intervals of 5 days by carefully removing the samples, brushing them gently to remove soil, drying them at 50°C for 10 h, and measuring the final weight. The weight loss of the film sample during degradation was used to indicate the degradation rate. The percentage weight loss during degradation was calculated as:
Weight loss (%)= [ (m0-m1)/m0 ] x 100 …(6)
Where m0 is the initial film weight, and m1 is post degradation weight.
Antibacterial effect of developed films (clear inhibition zone assay)
The antibacterial effect of developed films was determined according to30 with slight modifications. A 0.1 mL aliquot of pathogenic strain suspension was transferred to a pre-prepared agar surface, spread uniformly, and kept for drying. Film discs of 0.5 cm in diameter, with or without organic acid, were placed on the surface of a dried plate to determine antibacterial activity.
Antibacterial assay of organic acids
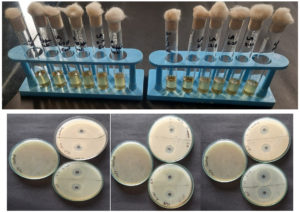
The MIC and MBC results displayed that organic acids have effective antibacterial properties against tested bacteria (Table 1 and 2). The MIC value was 12.5 ppm both against E. coli and S. aureus for lactic, citric, and acetic acid. As shown by the zone of inhibition in Figure 1, organic acids have potent bactericidal activity. Citric acid resulted in higher inhibition for gram-positive bacteria (S. aureus) compared to gram-negative bacteria (E. coli.) bacteria. While acetic acid showed higher inhibition for E. coli. than S. aureus. Lactic acid displayed similar inhibition against both S. aureus and E. coli. Because weak organic acids are lipophilic and can easily enter the plasma membrane, as well as intracellular acidification, they are more inhibitory than strong mineral acids.31,32
Table (1):
Minimum inhibitory concentration for different concentrations of organic acids.
| Pathogenic Microorganism | Organic acid | Antibacterial (%) ppm | ||||||
|---|---|---|---|---|---|---|---|---|
| 50 | 25 | 12.5 | 6.25 | 3.12 | 1.56 | 0.78 | ||
| S. aureus | Lactic acid | – | – | + | + | + | + | + |
| Citric acid | – | – | + | + | + | + | + | |
| Acetic acid | – | – | + | + | + | + | + | |
| E. coli | Lactic acid | – | – | + | + | + | + | + |
| Citric acid | – | – | + | + | + | + | + | |
| Acetic acid | – | – | + | + | + | + | + | |
Table (2):
Minimum Bactericidal Concentrations for different concentrations of organic acids.
| Pathogenic Microorganism | Organic acid | Antibacterial (%)ppm | |
|---|---|---|---|
| 50 | 25 | ||
| S. aureus | Lactic acid | – | – |
| Citric acid | – | – | |
| Acetic acid | – | – | |
| E. coli | Lactic acid | – | – |
| Citric acid | – | – | |
| Acetic acid | – | – | |
The positive (+) sign indicates growth while the Negative (–) indicates an absence of growth.
Figure 1. Photographs of Antibacterial test of organic acids (lactic, citric, and acetic acid) on gram-negative (Escherichia coli) and gram-positive (Staphylococcus aureus) bacteria (a) MIC by tube dilution method (b) MBC by Disk diffusion method
Characterization of Films
Thickness
The thickness of the starch, starch/CMC, and modified starch/CMC films was shown in Table 3. Since thickness has an impact on barrier qualities like WVP, it is a crucial aspect of food packing. Due to variations in the water vapour pressure and the accumulation of moisture on the film-air interface, the film thickness has a special impact on the water vapour permeability. The thickness increased with the blending of CMC due to the higher molecular weight and viscosity of CMC.24 While acid incorporation leads to a decrease in the thickness due to the hydrolysis of starch with acid and a reduction in molecular weight.33
Table (3):
Mechanical Properties and Thickness of films.
Thickness mm |
Tensile strength Mega pascals (MPa) |
Elongation at break % |
|
|---|---|---|---|
TS |
0.170±0.013 |
6.15± 0.59 |
58.87±1.34 |
SC |
0.181±0.020 |
8.80±0.31 |
77.97±1.72 |
SCL |
0.167±0.031 |
10.61±0.94 |
80.03±2.76 |
SCC |
0.171±0.031 |
8.96±0.89 |
82.01±1.27 |
SCAc |
0.174±0.011 |
9.21±0.23 |
71.49±1.31 |
Mechanical properties
Mechanical properties of starch-based bioactive films were revealed in Table 3 and Figure 2. The tensile strength of films improved slightly from 6.15 MPa (TS) to 8.8 MPa (SC), whereas elongation at break increased significantly from 58.87% (TS) to 77.97% (SC) by incorporating CMC into the starch matrix. The above results shows that the incorporation of CMC not only improved the strength of starch-based films but also reduced their brittleness while improving flexibility. This was possibly due to the chemical similarity of starch and CMC. Depending on their unique nature and impact on the film microstructure, different organic acids exhibited varying effects on mechanical parameters. Lactic acid (SCL) enhanced the mechanical properties of the films and also increased their stretchability. Acetic acid (SCAc) incorporation also strengthens the film, along with an improvement in elongation. While the incorporation of citric acid (SCC) led to an increase in the elongation at break but a decrease in the tensile strength compared to other organic acid-modified starch films. As a result of citric acid’s plasticizing effect, as described earlier, this may occur.34 Acid treatment caused polymer chains to hydrolyze, lowering the average molecular weight of linear chains.35 These properties are required for the easy handling of the starch suspension in the development of starch films.36-38
Physico-chemical properties
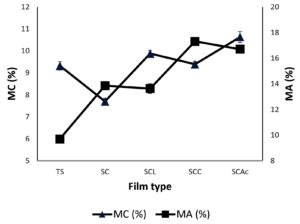
Moisture content (MC) and Moisture absorption (MA)
The film’s ability to absorb moisture from the environment over time is measured by its moisture absorption. SC film showed a lower moisture content than starch film while still having substantial water absorption as shown in Figure 3 and Table 4. Due to the development of a compact structure, the moisture content is reduced when CMC is added to the starch matrix, as can be seen from FESEM images (Figure 6). But due to the hydrophilicity of CMC, it leads to higher water affinity compared to pure starch film. While the water affinity of the films diminished with the addition of lactic acid. The decrease of water affinity in lactic acid-treated films (SCL) may be due to crosslinking and the consequential decrease of free OH groups.39,40 On the other hand, the incorporation of citric acid and acetic acid increases the moisture content along with the moisture absorption of films. The multi-carboxyl structure of citric acid allows it to possibly function as a plasticizing agent. Plasticizing of starch macromolecules weakened the intermolecular hydrogen bonding that complements natural intermolecular amylose bonding to increase the water affinity. It may also be due to the porous nature of the films, which is evidenced by the FESEM images (Figure 6).
Table (4):
Water vapour permeability, Moisture content and Moisture absorption of films.
Film type |
WVP (g.mm/kPa.m2.h) |
MC (%) |
MA (%) |
|---|---|---|---|
TS |
1.15±0.12 |
9.32±0.19 |
9.71±0.28 |
SC |
1.42±0.01 |
7.70±0.14 |
13.86±0.18 |
SCL |
1.27±0.18 |
9.88±0.15 |
13.63±0.37 |
SCC |
2.72±0.21 |
9.39±0.13 |
17.31±0.24 |
SCAc |
2.61±0.11 |
10.63±0.25 |
16.70±0.22 |
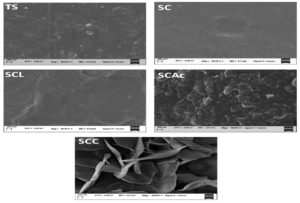
Figure 6. Field emission scanning electron microscopy(FESEM) micrographs of developed films at 50,000 magnification
Water vapour permeability (WVP)
The film should reduce or prevent moisture transfer between food and the environment in applications involving food packing. As shown in Table 4, the WVP of the SC film without organic acid was 1.42 ± 0.01 g.mm/kPa.m2.h and the incorporation of lactic acid resulted in a reduction in the water vapour permeability (WVP) of the films. This may be associated with the replacement of the hydrophilic hydroxyl group with hydrophobic ester groups. Since a more compact structure was created by the addition of lactic acid, the water molecules’ ability to transport through it was likely made more difficult. In addition to high crystallinity, which results in reduced intermolecular space, lactic acid-treated films may have lower water vapour permeability. Conversely, the incorporation of citric acid and acetic acid increased the water vapour permeability due to the formation of the porous structure, as evidenced by the FESEM images.
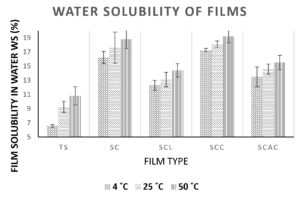
Film solubility in water (WS)
The water solubility of developed films plays a significant part in improving film integrity, biodegradability, shelf life, and resistance to water for food packaging applications. Low solubility percentages are required for water resistance and food integrity. The water solubility results are presented in Figure 4. The water solubility of films was lowest in Curcuma angustifolia starch films, which increased significantly with the incorporation of CMC. The incorporation of organic acids had variable effects on water solubility, which depended on the type of organic acid and its interaction with the base matrix. The incorporation of lactic acid and acetic acid was able to decrease the water solubility of the control films. By forming hydrogen bonds between the hydroxyl groups on starch and the carboxylic acid groups on lactic acid, molecular interactions are enhanced and water solubility is reduced.41
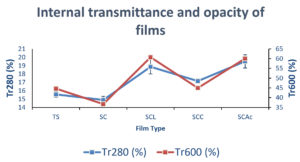
Internal transmittance and opacity
Film translucency is determined by the spectral distribution of its internal transmittance. Due to the incorporation of organic acids (lactic, citric, and acetic acid) into the base matrix, the internal transmittance was increased as shown in Figure 5 and Table 5. This was due to changes in the refractive index of the film structure caused by the creation of a more homogeneous structure with acid hydrolysis. As transparency is correlated with internal transmittance.42 Due to an increase in internal transmittance, the opacity of the acid-modified films was decreased.
Table (5):
Internal transmittance and opacity of films.
Film type |
Tr280 (%) |
Tr600 (%) |
Opacity |
|---|---|---|---|
TS |
15.56±0.35 |
44.61±0.07 |
2.06±0.06 |
SC |
14.89±0.45 |
36.67±0.31 |
1.89±0.31 |
SCL |
18.86±0.86 |
60.74±0.49 |
1.37±0.49 |
SCC |
17.15±0.13 |
44.95±0.8 |
0.96±0.8 |
SCAc |
19.51±0.79 |
60.02±0.62 |
0.83±0.62 |
Morphological characteristics
The microstructure of the films was qualitatively determined, to observe the effect of different organic acids on the morphology of the film. FESEM micrographs (Figure 6) of the film surface without organic acids TS and SC showed an ordered, compact, and homogeneous structure, which shows that tikhur starch and CMC are compatible polymers.34,22 The modification of starch with organic acids, which represent almost 5% w/w of the starch used, brought about some structural modifications due to the nature of organic acids. With the addition of lactic acid into the base matrix, the surface smoothed down and became more uniform with fewer bumps. The acetic acid films (SCAc) had a surface with a small area of agglomerates that might be attributed to the crosslinking between starch and acetic acid. The granular structures on the surface of the SCAc film can also be due to the presence of recrystallized particles formed by acidolysis. In films containing citric acid (SCC), phase separation was observed due to its plasticizing properties. Thus, citric acid promoted the fragmentation and plasticization of starch granules. According to,33 the roughness of the cross-linked potato starch/chitosan composite films with citric acid was higher than that of the uncross-linked films because of the plasticizing effect of the acid.
Fourier transform infrared spectroscopy
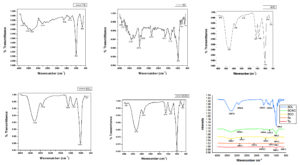
In Figure 7, FTIR spectra are shown for developed films, including TS films containing pure starch. TS film showed prominent absorption peaks at 669 (C-H bending) of 1, 2, 3-trisubstituted alkene, 989 (C=C bending) alkene, and 1651 cm-1 (C=C stretching) alkene. With starch/CMC (SC) film, a prominent peak at 2934 cm-1 that corresponded to C─H stretching due to the presence of CMC was shown, but there is no such peak in TS film that indicates the change in surface morphology. Bands observed at 3300 cm-1 correspond to stretching modes of the different -OH groups. It is known that a strong hydrogen bond shifts the band to a higher wavenumber. The addition of organic acid to the starch/CMC matrix resulted in the shifting of bands associated with hydroxyl groups. It was observed in lactic acid (3300 cm-1) containing films. A broader hydroxyl peak was observed in citric acid (SCC) and acetic acid (SCAc) films, indicating higher water affinity of citric acid (3302 cm-1) and acetic acid (3310 cm-1) containing films.
X-Ray Diffraction (XRD) Analysis
The percentage crystallinity of the film samples was investigated using the X-ray diffraction technique. XRD can characterise the compatibility of films. The amorphous section of starch is made up of amylose molecules with long branches, while the crystalline region is made up of amylopectin molecules with short branches. As higher crystallinity is associated with higher amylopectin content in starch granules, consequently tikhur starch films are more crystalline than starch/CMC blend films (Table 6 and Figure 8). The peak at 17.02° could be assigned to amylose, while the peak at 21.94° to Vh crystallinity. Because of interactions between starch and CMC, the blend peaks have shifted slightly. The CMC structure is linked to the low crystallinity that has been reported previously by.22 Tikhur starch-CMC mix films had a broad peak at 22.79°. This means that there was not much crystalline structure left in the starch-CMC mix film.
Table (6):
Crystallinity Index of films.
Sample name |
Crystallinity index |
|---|---|
TS |
56.23 |
SC |
37.85 |
SCAc |
53.04 |
SCC |
52.22 |
SCL |
54.9 |
Figure 11. Photographs of disk-diffusion Antibacterial test of organic acids (lactic, citric, and acetic acid) modified starch/CMC film samples on E. coli and S. aureus bacteria
CMC damaged the tikhur starch film’s original crystalline domains, lowering the crystallinity percentage from 56.23 to 37.85. The crystallinity of two biopolymer ingredients in the films becomes lower than that of a single-crystalline ingredient when they have good miscibility. New diffraction peaks occurred at 2θ=14.90°, demonstrating that starch and CMC are miscible at the molecular level. The cross-linking process between starch and lactic acid stopped molecular chains from moving and improved crystallinity. While in the case of citric acid and acetic acid, hydrolysis caused the accumulated short-chain fragments to recrystallize and create a quasi-crystalline structure, increasing crystallinity. Similarly, in starch-chitosan ferulic acid-containing films, a more amorphous pattern appeared, indicating the miscibility of film components and delaying the occurrence of retrogradation.39
Thermal properties
The results of the thermal properties of developed films were studied, and the Figure 9 and Table 7 shows the TGA/DTG analysis of developed films in the range of 25–650°C under a nitrogen atmosphere. The developed films went through three stages of thermal disintegration in the form of weight loss, similar to other thermoplastic starch-based films. The evaporation of water and glycerol causes an initial steady-state weight loss between 25 and 150°C. Thermal pyrolysis of the starch backbone at 300°C caused a sudden weight loss of 41–50% in the second stage between 200 – 380°C. At 300°C, the final stage of fast depolymerization of carbon residues was caused by the breakdown of amylopectin.43,44 At 650°C, films had a higher char content and decomposed only by 73-79% in the nitrogen atmosphere.
Table (7):
Weight loss percentage of developed films during TGA analysis.
°C |
TS |
SC |
SCL |
SCAc |
SCC |
|---|---|---|---|---|---|
23- 100 |
5.6 |
9.6 |
9.3 |
6.9 |
9.5 |
100-200 |
6.3 |
6.4 |
7 |
6.5 |
6.6 |
200-300 |
42.7 |
44.4 |
43.7 |
41.7 |
50.4 |
300-400 |
14.7 |
13.9 |
13.8 |
16.1 |
6.6 |
400-500 |
3.9 |
3.8 |
3.8 |
5.1 |
3.9 |
500-650 |
1.3 |
1.3 |
1.1 |
1.7 |
1.4 |
Biodegradability
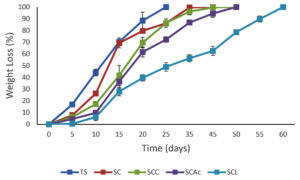
The weight loss during the degradation of bioactive starch-based films in the soil is shown in (Figure 10). A variety of bacteria may easily break down starch to produce fermentation products like ethanol and methane. When it is buried in soil, microbes attack it swiftly. Increased water sorption encourages soil microorganisms to enter, which utilise the starch films for their growth as a source of energy. There is consensus in the literature that crosslinking modifies water sorption.39 The delayed breakdown rate of citric acid cross-linked starch films was also observed by. 17 Although it is anticipated that citric acid cross-linked or esterified starch does not break down excessively slowly, no research has examined this issue. All starch-based films showed altered tonality and breakdowns after 5-day storage, indicating hydrolysis reactions that take place during early deterioration. In general, the use of lactic acid led to the longest degradation times (>55 days) compared to films produced with citric acid and acetic acid. The highest percentage of weight loss was observed for TS film, followed by SC. Lactic acid incorporation in the starch/CMC matrix not only improves the film’s water insolubility but also enhances the film’s resistance to degradation.
Antibacterial activity
A comparison of the antibacterial activities of organic acid-modified films and controls (without organic acid) of gram-positive (Staphylococcus aureus) and gram-negative (E. coli) organisms is shown in Figure 11 and Table 8. TS and SC films without organic acid showed no antibacterial activity against test organisms, but the films with organic acid showed potent antibacterial activity against both gram-positive and gram-negative bacteria. In general, the effect of lactic acid was more pronounced than that of citric and acetic acid. Staphylococcus aureus, a gram-positive bacteria, was more vulnerable to the organic acid-modified films than gram-negative bacteria (E. coli). However, because gram-positive and gram-negative bacteria have different cell walls, the antibacterial action may change across the two types of bacteria. It has been recognised that the antibacterial action of organic acids is due to damage to the bacterial cell membrane caused by failure of the synthesis system between protein and cell membrane, which inhibits the propagation of bacteria and releases cytoplasmic contents, resulting in the bacterium’s death. In addition, organic acid provides an acidity that has anti-bacterial properties.
Table (8):
Inhibitory activity of different biofilm against selected bacterial pathogens.
| S. No. | Samples | Zone of Inhibition (mm diameter) * | |
|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | ||
| 1. | TS | – | – |
| 2. | SC | – | – |
| 3. | SCL | 8 | 12 |
| 4. | SCC | 7 | 9 |
| 5. | SCAc | 7 | 8 |
* Includes disk diameter of 5 mm
Non-conventional starch from Curcuma angustifolia (tikhur) rhizome was extracted through wet milling and used for the development of biodegradable films. The CMC was incorporated into the starch-based matrix to improve the physicochemical properties of the blended film. Organic acids (lactic, citric, and acetic acid) were incorporated to modify the starch/CMC blended film. Organic acid incorporation affected the functional properties of the starch/CMC matrix. The addition of organic acids produced films with better qualities that might potentially replace non-biodegradable films in addition to displaying antibacterial properties both against gram-positive and gram-negative bacteria. Lactic acid films led to the longest degradation times (> 55 days) compared to films produced with citric acid and acetic acid. The study found that organic acids can be used to change the properties of starch-based bioplastics for food packaging applications.
ACKNOWLEDGMENTS
The authors would like to thank Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj, for supporting this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
PK conceptualized the study. PK, HS, JJ collected the resources. PK, AAB, TA, HS, JJ, GS performed formal analysis. PK, AAB, TA, HS, JJ performed visualization. PK investigated the study and prepared original draft. PK, JJ wrote the manuscript. AAB, TA, HS, GS, JJ reviewed and edited the manuscript. AAB, TA, HS, GS performed supervision. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Hosseini SN, Pirsa S, Farzi J. Biodegradable nano composite film based on modified starch-albumin/MgO; antibacterial, antioxidant and structural properties. Polym Test. 2021;97:107182.
Crossref - Sganzerla WG, Rosa GB, Ferreira ALA, et al. Bioactive food packaging based on starch, citric pectin and functionalized with Acca sellowiana waste by-product: Characterization and application in the postharvest conservation of apple. Int J Biol Macromol. 2020;147:295-303.
Crossref - Shankar S, Rhim J-W. Bionanocomposite Films for Food Packaging Applications. Ref Modul Food Sci. 2018.
Crossref - Daudt RM, Avena-Bustillos RJ, Williams T, et al. Comparative study on properties of edible films based on pinhao (Araucaria angustifolia) starch and flour. Food Hydrocoll. 2016;60:279-287.
Crossref - Chen H, Wang J, Cheng Y, et al. Application of protein-based films and coatings for food packaging: A review. Polymers (Basel). 2019;11(12):2039.
Crossref - Yurong G, Dapeng L. Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ϵ-polylysine as a novel antimicrobial packaging material. E-Polymers. 2020;20(1):154-161.
Crossref - Franklin MEE, Pushpadass HA, Kumar B, et al. Physicochemical, thermal, pasting and microstructural characterization of commercial Curcuma anustgifolia starch. Food Hydrocoll. 2017;67:27-36.
Crossref - Gopi S, Amalraj A, Jude S, Thomas S, Guo Q. Bionanocomposite films based on potato, tapioca starch and chitosan reinforced with cellulose nanofiber isolated from turmeric spent. J Taiwan Inst Chem Eng. 2019;96:664-671.
Crossref - Sukhija S, Singh S, Riar CS. Isolation of starches from different tubers and study of their physicochemical, thermal, rheological and morphological characteristics. Starch/Staerke. 2016;68(1-2):160-168.
Crossref - Ahmad S, Manzoor K, Ahmad M, Purwar R, Ikram S. Starch-Based Bionanocomposites. INC. 2020:157-171.
Crossref - Gadhave RV, Das A, Mahanwar PA, Gadekar PT. Starch Based Bio-Plastics: The Future of Sustainable Packaging. Open J Polym Chem. 2018;08(02):21-33.
Crossref - Daiuto E, Cereda M, Sarmento S, Vilpoux O. Effects of extraction methods on Yam (Dioscorea alata) starch characteristics. Starch/Staerke. 2005;57(3-4):153-160.
Crossref - Man J, Cai J, Cai C, Xu B, Huai H, Wei C. Comparison of physicochemical properties of starches from seed and rhizome of lotus. Carbohydr Polym. 2012;88(2):676-683.
Crossref - Das D, Jha S, Kumar KJ. Isolation and release characteristics of starch from the rhizome of Indian Palo. Int J Biol Macromol. 2015;72:341-346.
Crossref - Molavi H, Behfar S, Shariati MA, Kaviani M, Atarod S. A review on biodegradable starch based film. J Microbiol Biotech Food Sci. 2015;(2011):456-461.
Crossref - Diyana ZN, Jumaidin R, Selamat MZ, et al. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends : A Review. ;13(9):1-20.
Crossref - Seligra PG, Medina Jaramillo C, Fama L, Goyanes S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr Polym. 2016;138:66-74.
Crossref - Silva-Weiss A, Bifani V, Ihl M, Sobral PJA, Gomez-Guillen MC. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013;31(2):458-466.
Crossref - Kumar R, Ghoshal G, Goyal M. Biodegradable composite films/coatings of modified corn starch/gelatin for shelf life improvement of cucumber. J Food Sci Technol. 2021;58(4):1227-1237.
Crossref - Tavares KM, Campos A de, Luchesi BR, Resende AA, Oliveira JE de, Marconcini JM. Effect of carboxymethyl cellulose concentration on mechanical and water vapor barrier properties of corn starch films. Carbohydr Polym. 2020;246:116521.
Crossref - Sagnelli D, Kirkensgaard JJK, Giosafatto CVL, et al. All-natural bio-plastics using starch-betaglucan composites. Carbohydr Polym. 2017;172:237-245.
Crossref - Tavares KM, de Campos A, Mitsuyuki MC, Luchesi BR, Marconcini JM. Corn and cassava starch with carboxymethyl cellulose films and its mechanical and hydrophobic properties. Carbohydr Polym. 2019;223:115055.
Crossref - Tongdeesoontorn W, Mauer LJ, Wongruong S, Sriburi P, Rachtanapun P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem Cent J. 2011;5(1):6.
Crossref - Ghanbarzadeh B, Almasi H, Entezami AA. Physical properties of edible modified starch/carboxymethyl cellulose films. Innov Food Sci Emerg Technol. 2010;11(4):697-702.
Crossref - Eswaranandam S, Hettiarachchy NS, Johnson MG. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara. J Food Sci. 2004;69(3):79-84.
Crossref - Zhong Y, Song X, Li Y. Antimicrobial, physical and mechanical properties of kudzu starch-chitosan composite films as a function of acid solvent types. Carbohydr Polym. 2011;84(1):335-342.
Crossref - Ahmadi R, Ghanbarzadeh B, Ayaseh A, et al. The antimicrobial bio-nanocomposite containing non-hydrolyzed cellulose nanofiber (CNF) and Miswak (Salvadora persica L.) extract. Carbohydr Polym. 2019;214:15-25.
Crossref - Ghanbarzadeh B, Almasi H, Entezami AA. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind Crops Prod. 2011;33(1):229-235.
Crossref - Nguyen DM, Do TVV, Grillet AC, Ha Thuc H, Ha Thuc CN. Biodegradability of polymer film based on low density polyethylene and cassava starch. Int Biodeterior Biodegrad. 2016;115:257-265.
Crossref - Wu J, Chen S, Ge S, Miao J, Li J, Zhang Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013;32(1):42-51.
Crossref - Trcek J, Mira NP, Jarboe LR. Adaptation and tolerance of bacteria against acetic acid. Appl Microbiol Biotechnol. 2015;99(15):6215-6229.
Crossref - Akbas MY, Olmez H. Inactivation of Escherichia coli and Listeria monocytogenes on iceberg lettuce by dip wash treatments with organic acids. Lett Appl Microbiol. 2007;44(6):619-624.
Crossref - Wu H, Lei Y, Lu J, et al. Effect of citric acid induced crosslinking on the structure and properties of potato starch/chitosan composite films. Food Hydrocoll. 2019;97:105208.
Crossref - Bonilla J, Talon E, Atares L, Vargas M, Chiralt A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch-chitosan films. J Food Eng. 2013;118(3):271-278.
Crossref - Chan-Matu DI, Toledo-Lopez VM, Vargas M de LV y, Rincon-Arriaga S, Rodriguez-Felix A, Madera-Santana TJ. Preparation and characterization of chitosan-based bioactive films incorporating Moringa oleifera leaves extract. J Food Meas Charact. 2021;15(5):4813-4824.
Crossref - Luchese CL, Frick JM, Patzer VL, Spada JC, Tessaro IC. Synthesis and characterization of biofilms using native and modified pinhao starch. Food Hydrocoll. 2015;45:203-210.
Crossref - Zhang H, Hou H, Liu P, Wang W, Dong H. Effects of acid hydrolysis on the physicochemical properties of pea starch and its film forming capacity. Food Hydrocoll. 2019;87:173-179.
Crossref - Majzoobi M, Beparva P. Effects of acetic acid and lactic acid on physicochemical characteristics of native and cross-linked wheat starches. Food Chem. 2014;147:312-317.
Crossref - Qin Y, Wang W, Zhang H, Dai Y, Hou H, Dong H. Effects of citric acid on structures and properties of thermoplastic hydroxypropyl amylomaize starch films. Materials (Basel). 2019;12(9):1-13.
Crossref - Gutierrez TJ, Tapia MS, Perez E, Fama L. Structural and mechanical properties of edible films made from native and modified cush-cush yam and cassava starch. Food Hydrocoll. 2015;45:211-217.
Crossref - Olivato JB, Grossmann MVE, Yamashita F, Eiras D, Pessan LA. Citric acid and maleic anhydride as compatibilizers in starch/poly(butylene adipate-co-terephthalate) blends by one-step reactive extrusion. Carbohydr Polym. 2012;87(4):2614-2618.
Crossref - Arafat Y, Altemimi A, Pratap-Singh A, Badwaik LS. Active biodegradable films based on sweet lime peel residue and its effect on quality of fish fillets. Polymers (Basel). 2021;13(8):1240.
Crossref - Mendes JF, Norcino LB, Martins HHA, et al. Correlating emulsion characteristics with the properties of active starch films loaded with lemongrass essential oil. Food Hydrocoll. 2020;100:105428.
Crossref - Yousefi AR, Savadkoohi B, Zahedi Y, Hatami M, Ako K. Fabrication and characterization of hybrid sodium montmorillonite/TiO 2 reinforced cross-linked wheat starch-based nanocomposites. Int J Biol Macromol. 2019;131:253-263.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.