ISSN: 0973-7510
E-ISSN: 2581-690X
The keratin degrading bacterial strain KRD1 was isolated from poultry farm soil by using enrichment technology. The strain was identified as Bacillus olironius on the basis of biochemical and 16S rRNA sequencing. The keratinase production was maximum in keratin medium under optimum condition (pH 7.5, time 96 h; temperature 37°C; and 5% inoculums). The KRD1 keratinase activity decreased by the addition of carbon sources and strain KRD1 produce high keratinase in the presence of 0.1% yeast extract. Therefore enzymatic hydrolysis of feather wastes could be a safe method for recycling of keratin materials.
Bacillus olironius, keratenase, poultry soil, optimization.
Keratin forms a major component of the epidermis and its appendages viz. hair, feathers, nails, horns, hoofs, scales and wool. On the basis of secondary structural confirmation, keratins have been classified into a (a-helix of hair and wool) and b (b-sheets of feather) (Akhtar et al., 1997). The high sulfur content in keratin is due to the presence of sulfur containing amino acids namely cysteine and methionine. The intensive cross-linkage in keratin hinders their degradation by commonly known proteolytic enzymes like trypsin, pepsin and papain (Papadopoulos, 1986). The degradation of keratin by microorganisms is performed by specific protease (keratinase) (Gessessse et al., 2003). The proteolytic enzymes like trypsin, pepsin and papain are largely produced in the presence of keratinous substrates (Gupta et al., 2002). The purified keratinases known till now cannot completely solubilize native keratin, their exact nature and uniqueness for keratinolysis is still an enigma in the world of proteases (Ignatova et al., 1999). The keratinases in nature have been continuously contributing to valorization of huge keratin containing wastes in the form of hair, feathers, dead birds and animals (Farag and Hassan, 2004). The feather hydrolysates of Bacillus licheniformis PWD-1 and Vibrio sp. strain kr2 can be used as feed additives, while the keratinase from Bacillus subtilis S14 exhibits remarkable dehairing capabilities (Grazziotin et al., 2006; Macedo et al., 2005). Moreover, keratinase from B. licheniformis PWD-1 can degrade the infectious form of prion, in the presence of detergents and heat treatment, which is very important for the utilization of animal meal as feed (Langeveld et al., 2003). It is important to improve the enzyme yield, so various methods like optimization of culture conditions, medium composition and heterologous gene expression have been applied (Ramnani and Gupta, 2004; Anbu et al., 2005). In place of using such modern, expensive and time consuming techniques, proper selection of wild microbial isolate can provide stable enzymes that can easily serve the purpose without any additional requirements. In this study, our aim was to isolate efficient keratinase producing bacteria strain form poultry farm soil.
Chemical and preparation of chicken feather powder
The media ingredients, chemicals and dialysis membrane were procured from Hi Media. Chicken feathers and soil sample were collected from poultry farm, kurukshetra. The feathers were washed properly with distilled water and partially denatured by autoclave method. The dried feathers were grind in electrical grinder, passed through small mesh and kept for further study.
Isolation, screening identification, heavy metal tolerance and antibiotic resistance of keratin degrading bacterial strain
The keratin degrading bacterial strains were isolated by enrichment culture technique. The 5 g poultry farm soil sample was added in 100 ml keratin media (10 gl-1 feather powder; 0.5 gl-1 NaCl; 0.3 gl-1K2HPO4; 0.4 gl-1 KH2PO4; 0.1 gl-1 MgSO4 6H2O; pH 7.5) and incubated for one week at 28°C. The 10 ml culture broth from enriched flask was transferred into fresh keratin medium up to 4 weeks. The serially diluted enriched medium was spread over nutrient agar plates and incubated at 30°C for 2 days. The isolated strains were streaked on milk agar plates for 24 h at 37°C. The strains produce clear zone on milk agar plates were selected for keratinolytic activity (Zerdani et al., 2004). The selected strains were spotted on keratin agar for 72 h at 37°C to check their keratinolytic activity. The bacterial strains produce clear zone on keratin agar was selected for further study. The identification of bacterial strain was done by using biochemical and molecular characterization. The 16S rRNA sequencing was carried out from Institute of Microbial Technology, Chandigarh, India. The bacterial strain was grown in the presence of heavy metals (Co2+, Cu2+, Mg2+, Hg2+ Zn2+ Mo2+, Ni2+ and Pb2+) at different concentrations (8, 12, 16 and 20 µg/ml) in LB broth for 24 h and growth was measured. Further, the antibiotic resistant pattern was determined by using disc diffusion method (Bauer et al., 1966), using antibiotic discs (chloromphenicol, oxacillin, ampicillin, clarithromycin, gentamycin, amoxyclav, vancomycin, cephalothinamikacin, novobiocin, erythromycin, teicoplanin, co-trimoxazole, penicillin, azithromycin, ofloxacin, methicillin, linezolid, clindamycin and tetracycline).
Keratinase assay and Optimization keratinase production
The production medium was inoculated with 1% inoculum and incubated at 37°C for 48 h under 120 rpm. The culture was centrifuged at 8,000 rpm for 20 min at 4°C. The cell free extract was used as crude preparation to measure the protease activity. The reaction mixture was filtered through whatman no.1 filter paper. The colour development assay of tyrosine in filtrate was done by adding the following reagent. The reaction mixture was incubate at room temperature for 30 min and absorbance was measured at 660 nm.
Reaction |
Test |
Control |
|---|---|---|
Test filtrate |
2 ml |
– |
Control filtrate |
– |
2 ml |
0.4 M Sodium carbonate |
5 ml |
5 ml |
Folin & Ciocalteu’s Phenol Reagent (1N) |
1 ml |
1 ml |
The concentration of L-tyrosine released in the filtrate was determined by comparing against standard curve of L-tyrosine. One unit of keratinase activity was defined as the amount of enzyme that releases 1 µg of tyrosine per ml per minute under the above assay conditions. The keratinase activity in terms of U/ml was derived as [µg of Tyrosine equivalents released X Total volume (ml) of assay]
[Volume of enzyme (ml) X Time of assay (min) X Volume used in colorimeter (ml)]
The protein concentration was determined, using bovine serum albumin as standard (Lowry et al., 1951).
The optimization of keratinase production was done by using different factors i.e. inoculum size, different time, pH, temperature, carbon source and nitrogen source.
Isolation, identification and heavy metal tolerance of keratin degrading strain KRD1
The proteolytic enzymes have ample utilization in industrial processes, such as the detergent, food and leather industries (Gupta et al., 2002). The Bacillus licheniformis, Bacillus subtilis, Bacillus pumilus and Bacillus cereus are reported to produce keratinase (Cai et al., 2008; Ghosh et al., 2008). In this study, 30 morphologically different bacterial strains were isolated by using enrichment culture technique. The isolated cultures were screened for their proteolytic activity, only 12 showed the formation of clear zone on skim milk agar plate. Out of 12 proteolytic bacterial strains, only 4 showed zone formation on keratin agar plate. The bacterial strain KRD1 was selected for further study on the basis of zone formation. The morphological, physiological and biochemical characteristics of strain KRD1 is shown in Table 1. The strain KRD1 showed (starch and gelatin hydrolysing capability) and utilized (glucose, mannitol, xylose, inositol, sorbitol and sucrose, salicin). The 16S rRNA sequencing showed that strain KRD1 have 100% homology with Bacillus oleronius. The 16S rRNA sequence and phylogenetic tree of strain KRD1 was constructed by using clustalW2 program as shown in Fig 1. The strain KRD1 was able to grow in the presence of heavy metals (Co2+, Cu2+, Mg2+, Zn2+, Mo2+, Ni2+ and Pb2+) even at high concentration 20 µg/ml. In case of Hg2+ grow only up to 12 µg/ml concentration. The antibiotic resistant pattern of strain was determined by using disc diffusion method and found sensitive against all used antibiotics.
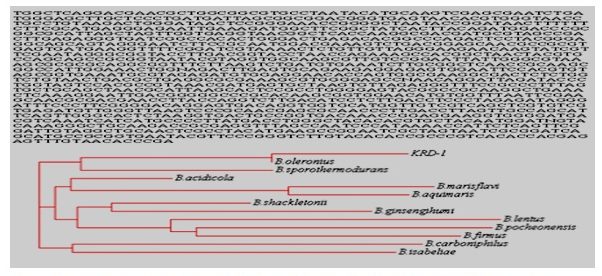 Fig 1. The 16S rRNA sequences and phylogenetic tree of strain KRD1 by using clustalW2
Fig 1. The 16S rRNA sequences and phylogenetic tree of strain KRD1 by using clustalW2
Table (1):
The morphological, physiological and the biochemical characteristics of strain KRD1.
Morphological tests |
Colony was circular, entire, flat, smooth, white and cells were gram negative and rod shape |
Physiological tests |
Growth was not present at (4,10,15, 25, 70 °C) and (pH 4.0) Growth was present at (30, 37, 42 °C), (pH 5.0, 6.0, 8.0, 10.0) and (NaCl 2.0, 4.0, 6.0 %) |
Biochemical tests |
Strain not to show indole, phenyl-amine deamination, ONPG, urea hydrolysis and raffinose, lactose, arabinose, adonitol, rhamnose metabolism |
Strain show |
lysine, ornithine, arginine, malonate utilization, protease, skim milk, gelatin and starch hydrolysis, nitrate reduction, catalase test and glucose, mannitol, xylose, inositol, sorbitol, sucrose, salicin metabolism |
Optimization of keratinase production
The keratinase production is greatly influenced by the nutritional and environmental factors (Zambare et al., 2007). The keratinase production by strain KRD1 at different time, carbon sourecs, temperature, pH and nitrogen sources is shown in Fig 2. The maximum keratinase production by KRD1 was observed at 4 days of incubation, prolonged incubation caused activity reduction. Similar trend was reported in Bacillus licheniformis and Bacillus subtilis (Rao, and Narasu, 2007; Younis et al., 2009). The maximum keratinase production by strain KRD1 was found with 5% (v/v) inoculum. The keratinase level increased from 15 µg/ml/min to 28 µg/ml/min, when inoculums size increased from 1 to 5% (v/v) and decreased with further increase in inoculums size. Similar results reported in Bacillus subtilis with 5% (v/v) inoculum size (Abusham et al., 2009). The optimum inoculum size for protease production by Bacillus cereus was 4.0% (v/v) (Shafee et al., 2005). A higher inoculum of 10% (v/v) was found to reduce the production of keratinase more than lower inoculum size of 1% (v/v). Therefore, high inoculum sizes do not necessarily give higher keratinase yield. The increase in the production of keratinase using small inoculum size has been suggested due to thehigher surface area to volume ratio (Rahman et al., 2005. The fermentation process is influenced by the pH, as microorganisms are sensitive to the changes in the hydrogen ion concentration of their environment (Sharmin et al., 2005). The production of keratinase by KRD1 increased with increase in pH of the medium and peaked to 31 µg/ml/min at 7.5 pH. The maximum keretinase production by strain KRD1 was observed at pH 7.5. The Bacillus lichniformis, Bacillus subtilis, and Bacillus subtilis has been reported to produce maximum enzyme in production medium at pH 7.5 (Olajuyigbe and Ajele, 2005; Das and Parsad, 2010). The temperature is very critical parameter which needs to be controlled with organisms (Kumar and Takagi, 1999). The keratinase production was optimum at 37°C and decreased beyond 37°C. The optimum temperature for keratinase production by Bacillus sp. was reported at 45°C (Kainoor and Naik, 2010). In extra-cellular enzymes, temperature was found to influence their secretion, possibly by changing the physical properties of the cell membrane (Rahman et al., 2005). The significant decline in keratinase production was observed beyond 40°C. It is a well-known fact that protein conformation changes at higher temperatures, causes a decrease in the protease activity (Johnvesly and Naik, 2001). The keratinase production is dependent on both carbon and nitrogen sources (Moon and Parulekar, 1991). The KRD1 keratinase activity decreased by the addition of carbon sources, this shows that enzyme production has catabolite repression system, support earlier study (Kainoor and Naik, 2010). Nitrogen plays an important role in regulating pH of the medium and may be crucial to maintain the activity and stability of the enzyme. In this study, strain KRD1 produced high keratinase in the presence of 0.1% yeast extract.
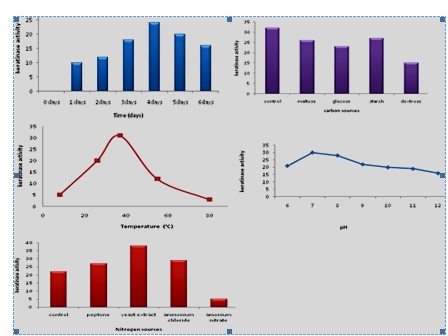 Fig 2. The keratinase production by KRD1 at different time, carbon sources, temperature, pH and nitrogen source
Fig 2. The keratinase production by KRD1 at different time, carbon sources, temperature, pH and nitrogen source- Abusham, R. A., Rahman, R. N. Z. R. A., Salleh, A. B. and Basri, M. Optimization of physical factors affecting the production of thermo-stable organic solvent-tolerant protease from a newly isolated halo tolerant Bacillus subtilis strain Rand. Microb. Cell Fact., 2009; 8: 20-28
- Akhtar, W. and Edwards H.G.M. Fourier-transform Raman spectroscopy of mammalian and avian keratotic biopolymers. Spectrochim Acta, 1997; 53:81–90.
- Anbu, P., Gopinath, S.C.B., Hilda, A., Priya, L.T. and Annadurai, G., Purification of keratinase from poultry farm isolatesScopulariopsis brevicaulis and statistical optimization.Enzyme Microb Technol, 2005; 36: 639–647.
- Bauer, A.W., Kirby, M.M. and Sherris, J.C. M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol., 1966; 45: 493-496.
- Cai, C. G., Lou, B. G. and Zheng, X. D. Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. J Zhejiang Univ Sci B., 2008; 9:60–67.
- Das, G. and Parsad, M. P. Isolation, purification & mass production of protease enzyme from Bacillus subtilis. Int. Res. J. Microbiol., 2010; 1: 26-31.
- Farag, A. M. and Hassan, M.A. Purification, characterization and immobilization of a keratinase from Aspergillus oryzae. Enzyme Microb Technol., 2004; 34:85–93.
- Gessessse, A., Kaul, R.H., Gashe, B.A. and Mattiasson, B. Novel alkaline proteases from alkalophilic bacteria grown on chicken feather. Enzyme Microb Technol., 2003; 32:519–524.
- Ghosh, A., Chakrabarti , A. and Chattopadhyay, D. Degradation of raw feather by a novel high molecular weight extracellular protease from newly isolated Bacillus cereus DCUW. J Ind Microbiol Biotechnol, 2008; 35:825–834.
- Grazziotin, A., Pimentel, F. A., de Jong, E. V. and Brandelli, A., Nutritional improvement of feather protein by treatment with microbial keratinase. Anim. Feed Sci. Technol., 2006; 126(1-2):135-144.
- Gupta, R., Beg, Q. K. and Lorenz, P. Bacterial alkaline proteases: molecular approaches and industrial applications. Appl. Microbiol. Biotechnol., 2002; 59: 15-32.
- Ignatova, Z., Gousterova, A., Spassov, G. and Nedkov, P. Isolation and partial characterization of extracellular keratinase from a wool degrading thermophilic actinomycete strain Thermoactinomyces candidus. Can J Microbiol., 1999; 45: 217–222.
- Johnvesly, B. and Naik, G. R. Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochem., 2001; 37: 139-144.
- Kainoor, P. S. and Naik G. R. Production and characterization of feather degrading keratinase from Bacillus sp. JB99. Indian J. biotechnol., 2010; 9:384-390.
- Kumar, C. G. and Takagi, H. Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol. Adv., 1999; 17: 561-594.
- Langeveld, J. P. M., Wang, J. J., van de Wiel, D. F. M., Shih, G. C., Garssen, G. J., Bossers, A. and Shih, J. C. H., Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J. Infect. Dis. 2003; 188(11):1782-1789.
- Lowry, O. H., Roserbrough, N. J., Fair A. L. and Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951; 193: 255-275.
- Macedo, A. J., da Silva, W. O. B., Gava, R., Driemeier, D., Henriques, J.A.P. and Termignoni, C., Novel keratinasefrom Bacillus subtilis S14 exhibiting remarkable dehairing capabilities. Appl. Environ. Microbiol. 2005; 71(1): 594-596.
- Moon, S. H. and Parulekar, S. J. A parametric study of protease production in batch and fed-batch cultures of Bacillus firmus. Biotechnol. Bioeng., 1991; 37: 467-483.
- Olajuyigbe, F. M. and Ajele, J. O. Production dynamics of extracellular protease from Bacillus species. Afr. J. Biotechnol., 2005; 4: 776-779.
- Papadopoulos, M. C. The effect of enzymatic treatment on amino acid content and nitrogen characteristics of feather meal. Anim Feed SciTechnol, 1986; 16:151–156.
- Rahman, R. N. Z. R., Geok, L. P., Basri, M. and Salleh, A. B. Physical factors affecting the production of organic solvent-tolerant protease by Pseudomonas aeruginosa strain K. Bioresour. Technol. 2005; 96: 429-436.
- Ramnani, P. and Gupta, R. Optimization of medium composition for keratinase production on feather by Bacillus licheniformis RG1 using statistical methods involving response surface methodology. Biotechnol Appl Biochem., 2004; 40:491–496.
- Rao, K. and Narasu, M. L. Alkaline protease from Bacillus firmus 7728. Afr. J.Biotechnol, 2007; 6: 2493-2496.
- Sharmin, S., Hossain, M. T. and Anwar, M. N. Isolation and characterization of a protease producing bacteria Bacillus amovivorus and optimization of some factors of culture conditions for protease production. J. Biol. Sci., 2005; 5: 358-362.
- Younis, M. A. M., Hezayen, F. F., Eldein, M. A. N. and Shabeb, M. S. A. Production of protease in low-cost medium by Bacillus subtilis KO strain. Global J. Biotechnol. Biochem. 2009; 4: 132-137.
- Zambare, V. P., Nilegaonkar, S. S. and Kanekar, P. P. Production of an alkaline protease by Bacillus cereus MCM B-326 and its application as a dehairing agent. World J. Microbiol. Biotechnol. 2007; 23: 1569–1574.
- Zerdani, I., Faid, M., and Malki, A. Feather wastes digestion by new isolated strains Bacillus sp. in Morocco. Afr J Biotechnol. 2004; 3:67–70.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


