Human monkeypox (MPX), a multi-country re-emerging disease, is rapidly spreading around the world. The etiological agent of this disease, Monkeypox virus (MPXV), is a DNA virus classified into three genetic types (West Africa, Congo Basin clade, and one new clade-3). Atypical or unusual symptoms as well as asymptomatic infection of MPXV has also been reported. Transmission among humans is possible by droplets, contact, sexual intercourse, and fomites. Secondary transmission of this disease has been reported to occur in less than 10% of cases where it was found 35%–88% of smallpox. Mother-to-fetus transmission by vertical route is also possible for this disease. Modern equipment, biosafety level-3 laboratory facilities, and trained expert persons are needed to diagnose this disease. Previous data support that ~85% clinical protection is provided by smallpox vaccines for monkeypox, although initially non-human primates models were used for various experiments, and also side-effects of this vaccine have been notably mentioned in various studies. Limited research findings of JYNNEOS vaccine has supported the comparatively lower prevalence of MPX cases with vaccination. Few drugs, including cidofovir, tecovirimat, brincidofovir, and vaccinia immune globulin intravenous are preferable against this disease, although clinical trial data is limited and FDA-approval is also pending. This review-based study presents an overall scenario of Monkeypox disease (MPXD) based on previously published studies. Recommended clinical treatment and vaccination, appropriate infection prevention and control strategies, adopting one health approach, and quick identification of hotspots using a wastewater-based surveillance system need to be followed to check the further spread of MPX outbreaks.
Monkeypox (MPX), Monkeypox Virus (MPXV), Epidemiology, Virology, Prevention and Control, Treatment, Vaccine, JYNNEOS Vaccine
Viral zoonotic diseases appear one after another while coronavirus disease 2019 (COVID-19)-positive cases still increasing after nearly three years of the pandemic in several parts of the world.1-3 The most devastative coronavirus disease 2019 (COVID-19) pandemic disease has attacked the world economy, where people lost about 6.6 million lives including 640 million total positive cases as of December 5, 2022.3-7 The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has drawn high public health attention against zoonotic infections,3 and novel, emerging or re-emerging pathogens, to develop new vaccines and therapeutic regimens but recently during this 2022 year another zoonotic viral disease, namely Monkeypox (MPX) caused by Monkeypox virus (MPXV), has also re-emerged in multiple countries with its cases increasing day after day without control that paused global public health emergency of international emergency.8-11 MPXV is an enveloped DNA virus under the Poxviridae family and genus Orthopox virus.12,13 Based on global experts discussion, WHO preferred a new term “mpox” for Monkeypox for synchronizing as the synonym of this disease. According to WHO, Mpox and monkeypox, both of these names will be used simultaneously after a transition while “monkeypox” is phased out.14 It assists to lessen the concerns that rose by subject experts and complete the International Classification of Diseases (ICD). However, the International Committee on Taxonomy of Viruses (ICTV) has shown an unwillingness to change it to “mpox”, raising concerns regarding the continuity of literature.15 Most of the viruses in the viral family of Poxviridae are not as deadly as SARS-CoV-2, and MPX symptoms observed are commonly cutaneous.7,16,17 As a result, doctors especially dermatologists can diagnose MPXV infection easily. The natural host of MPXV has not been yet identified clearly. Large number of animals including non-human primates and other species have been identified as vulnerable to this disease such as rope or tree squirrels, Gambian pouched rats, and dormice.18 However, still, uncertainty is also found for identifying a natural host of this virus, as a result, more researches need to be performed for detecting the exact reservoir(s). The mortality rate of this self-curable disease is typically 1 to 11%.19 Young children are mostly affected by this disease who are multi-sexual, bi-sexual, and without smallpox vaccination. Newly found old monkeypox disease has changed its biological nature due to climate, patient immunity, smallpox vaccination, and other unknown reasons.20 Although two genetic clades (West Africa, Congo Basin clade) of this virus were familiar but phylogenetic study support that another clade of MPXV that is responsible for the current outbreak belongs to clade 3.21 The case fatality rate (CFR) of this disease was 8.7% previously, and our data-based analysis has observed 4% CFR (Manuscript under submission). A recently published multicountry-based study found 99% of MPXV-infected patients were men (n= 528; 527 men and 1 woman) from 5 continents and 16 countries.22 According to this study, the average age of positive patients were 38 years (range 18–68 years) where almost all were gay or bisexual (98%), white observed more than 70%, and 41% had HIV infection.22
The mode of transmission of MPXV of current outbreaks is being explored based on analysis of recent outbreaks, and apart from clinical disease manifestations, unusual and atypical symptoms and asymptomatic MPXV infections have also been documented. This virus or viral particles have also been detected in wastewater and various environmental samples that prove virus persistence in the environment.18 As a message from the world’s largest SARS-CoV-2 / COVID-19 pandemic disease, zoonotic disease prevention and control strategies should be strengthened by following one health approach rule and a proper education and awareness system along with proactive control measures and preparedness plans to be adopted timely. The present article highlights various aspects of MPXV and MPX including epidemiology, virology, clinicopathology, and advances in diagnosis, vaccination, and treatment as well as important prevention and control measures to be adopted for limiting the further spread of MPX outbreaks.
Epidemiology
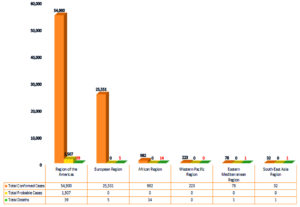
According to our world in data, a total of 81,351 Monkeypox (MPX) positive cases were confirmed from more than 115 countries including 20 deaths at the time of writing this manuscript (December 5, 2022) (Figure 1).23-25 In the beginning of this year of 2022 few MPX cases were detected in non-endemic countries firstly wherein on 6 May, 2022, United Kingdom (UK) first reported a monkeypox (MPX) outbreak which originated from a British travel history.26,27,28 The MPXV positive patient was urgently admitted in the hospital and isolated, followed by contact tracing of all possible suspected cases that confirmed either positive or negative case by diagnosis tests. Portugal has reported the first five positive MPX cases where all of the infected men had sex with men and Spain also identified the first 07 cases in Madrid including 22 suspected cases. On May 21, 2022, 13 countries reported the first cases including the United States of America, the United Kingdom, Australia, Belgium, Canada, France, Germany, Italy, Netherlands, Portugal, Spain, and Sweden.29 On May 24, 2022 The United Arab Emirates (UAE) announced the first MPXV case.30 India first reported four positive cases on 24 July 2022 from Asian countries.
Figure 1. Total Monkeypox cases in the WHO region from 1 Jan 2022 to 13 Nov 2022.31
The bars represent the total confirmed cases, probable cases and death case of monkeypox in the region of America, European, Africa, the Western Pacific Region, Eastern Mediterranean Region, and South East Asia Region.
There after during June and July months of 2022, this disease rapidly spread to multiple countries and hence WHO declared this disease as a public health emergency of international concern (PHEIC) on July 23, 2022. Although this virus was first detected in 1958 in Singapore where some monkeys were carried to the country Denmark for scientific research goals and called the disease as “monkeypox”. However, In 1970, the first human case was confirmed to be caused by MPXV and after a few years, numerous countries reported human MPX in western and central Africa, where the disease is endemic.
In the past, MPX outbreaks have been associated with the animal-to-human (zoonotic) transmission, and the extensive spread of human-to-human transmission was not observed. In contrast, the present 2022 MPX outbreak is marked by substantial human-to-human transmission, which has caused rise in both the number of cases and the geographic spread (100+ countries) to be unusual.
Virology
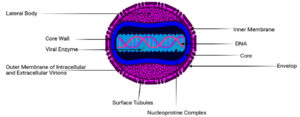
The size of Orthopoxviruses (OPXV) ranges from 140–450 nm with a structure similar to brick with a genome containing 200-500 kbp which encodes over 200 genes and these viruses infect a wide range of host. It is found that maximum genes of this OPXV genome are not required for virus multiplication, rather they play a vital role in the host antiviral response.32,33 The replication cycle of MPXV is not different from other poxviruses where all of the members of Poxviridae family accomplish their life cycle in the cytoplasm of infected cells following molecular pathways.34 The intracellular stage is known for the Vaccinia virus and following that strategy the vaccine was developed to eradicate smallpox. Intracellular mature virion is one of the first stages of the infection cycle and the extracellular enveloped virion is another stage that differs by surface glycoproteins. MPXV is an enveloped, completed by lipoprotein membrane, ds DNA virus with a ~190 kb genome, and has enclosed pleomorphic dumbbell-shaped core of 140 to 260 nm diameter, giving a brick-shaped virion (Figure 2A).19 This genome has closed hairpins on both ends and several open reading frames (ORF) that cover more than 180 nucleotide sizes. There is a highly conserved central coding region of approximately 56 to 120 kb that is flanked by variable regions and terminal repeats, these containing four additional ORFs that are mainly involved in immunomodulation for host range determination and pathogenicity.30 Although the poxviruses genome contains all the required proteins to replicate, transcribe, assemble and exit, it needs infected individuals’ ribosomes to translate mRNA.21
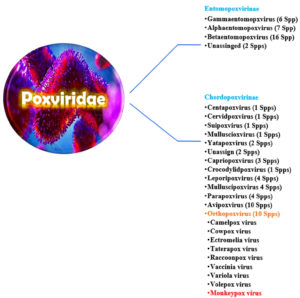
Poxviruses are a distinct, sophisticated, and diversified class of massive DNA viruses that produce both RNA and DNA inside the cytoplasm of infected cells. All viruses under Poxviridae family divided into two major subfamilies with 16 genera (Figure 2B).35 Based on the range of hosts they may infect different species, the two subclasses are called Entomopoxvirinae and Chordopoxvirinae, respectively. In the Chordopoxvirinae, category, some viruses, including cowpox, monkeypox, and taterapox, infect birds and other animals, including cows and monkeys, and sporadically spread to people and cause illness.36 MPXV was first found in 1958 and officially joined the Poxviridae family in 1978, as well as the Chordopoxvirinae subfamily and the genus Orthopoxvirus.34
The physicochemical characteristics of MPXV showed that it is resistant to conventional phenolic disinfectants, can be inactivated by polar lipophilic solvents like chloroform, and at low pH.37 The closely similar vaccinia virus is completely inactivated within 2-3 hours at 60°C or within minutes after exposure to 20 nM caprylate at 22°C; however, MPXV is much more resistant to solvent-detergent treatment than vaccinia.37 Phylogenetic analysis identifies two clades of MPXV that are linked to human disease: The West African clade, which is associated with 1% to 4% mortality, and the Congo Basin (Central African) another high mortality clade which a mortality rate of 10%. A comparatively less number of mutations are observed in MPXV due to the high stability of dsDNA and having the 3′–5′ proof-reading exonuclease. It was found that the evolutionary changing percentage counted at 1–2 nucleotide per year.
A very recently discovered the third clade of MPXV, is known as Clade 3; where Clades 2 and 3 are both members of the older Western Africa clade. Clade 3 was in charge of the 2017–2019 pandemic, and it had several changes that appeared to show adaption to humans. The strain that is currently in circulation and the strain from 2017–2019 that belongs to clade 3 differ by 47 nucleotides. As a DNA virus, monkeypox does not exhibit high mutational alteration compared to SARS-CoV-2 or others RNA viruses like HIV. Interestingly, the analysis of 2022 outbreak genomes showed more mutation rather than the previous (40 mutations).38
Phylogenetic analysis
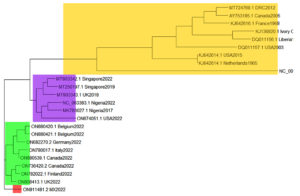
Sequence alignment, analysis, and phylogeny were carried out with sequins, adegenet, ape, viridis, msa and ggtree packages of R-studio. The tree was calculated using the genetic distance model and a tree build method of neighbour joining neighbor. The pairwise evaluation was used to rank the MPXV according to their phylogenetic connexion; a comparison of percentage identity with genetic distance. The diagonal in the comparison chart is an intercept where no comparisons were carried out. Further elaboration of the phylogeny was carried out in a meta tree (https://itol.embl.de) in order to picture the evolutionary relationships of the MPXV genomes. The MPXV sequences for the current and earlier outbreaks are denoted in red and blue colours respectively. The studio syntax for the analysis is shown below (Figure 4).
Although the number of sequence data in this study are small, our findings could provide meaningful basic information MPXV in Asia. Notably, the bulk of the sequences from the monkeypox outbreak in Asia (Taiwan, Israel and Singapore) were polyphyletically clustered. The Asian strains shared a polyphyletic cluster while the relationship with African strains were largely paraphyletic. The observation that the OP012849 singleton from Asian phylogeny shared a cluster with ON927248.1. This depicts MPXV’s previous cross-continent activity.
Sing and Symptoms of MPXV
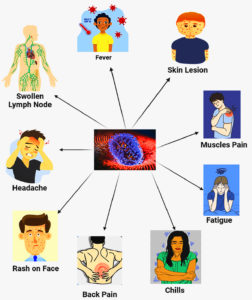
According to CDC, the signs and symptoms of the MPXV infection appear within three weeks after viral exposure from the contaminated substance or direct contact with MPX patient.39 The basic dissimilarity of these signs with smallpox, chickenpox, and other pox viruses is the lymphadenopathy that is observed in MPX. The incubation period of this virus is typically one week to three weeks. Few early symptoms are found such as headaches, backaches, myodynia, and fainting.23 Skin rash is prominent in this disease which usually starts from the mouth, and moves to the face, palms, and soles. There are different stages of this lesion, commences as maculae form, then develop to papules shape, vesicles, pustules, and scabs.40 About 10 to 150 lesions can be found in the patients body with pain. In a few cases, MPX positive patients face severe situations rarely like confection by other pathogenic bacteria, encephalitis, pneumonitis, and conjunctivitis/keratitis.41 About 98% skin rashes are observed in the patient’s skin face, 95% of palms and soles, 70% in oral mucous membranes, 28% in genitalia, and 20% conjunctiva.42
MPXV can be devastating for some groups of people like as women who are pregnant, children, or those with impaired immune systems.47 The infection period is categorizes by two phases which are the invasion period and the skin eruption period. The invasion period is of 0 to 5 days marked by fever, severe headache, and back pain. lymphadenopathy, chills, tiredness, asthenia, and myalgia.48 Typically, it starts within 1-3 days following the emergence of a fever. Previous research and studies conducted during this epidemic suggested that a rash might precede a fever.49 The rash frequently appears on the face and rapidly spreads centrifugally around the body, with a concentration on the face & extremities rather than the abdomen. The infected person has a rash on their face after the onset of fever, which then spreads to other body areas.46 Most of the MPXV outbreak case analyses have observed signs and symptoms of MPXV during illness, fatigue or malaise, chills, lymphadenopathy, inguinal, cervical, headache, nausea or vomiting, fever, body ache, back pain sore throat or cough, and sweat. Myalgia, pruritus or skin lesions, rash, headache, fever, and skin rash are observed as the most common symptoms (Figure 3).47,48
Transmission
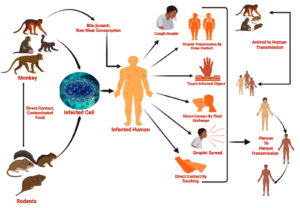
MPXV is zoonotic in nature, and can spread either from humans to humans or from animals to humans by bite/scratch, close contact, and by eating inadequately cooked meat of infected animals (Figure 5).49 The virus can directly spread from a person’s droplet, close contact or infected fluid exchange with non-infected, but most of the previously published studies observed male-to-male transmission by sex as potential route in the 2022 MPX outbreak.45 MPXV can also be transmitted from animals to humans through infected animal meat or fruits (eaten by affected animals) or touching infected droplets, and has been considered as an emerging zoonotic pathogen.50 More recently, the first report of human to dog transmission of MPXV has emphasized exploration of the reverse zoonotic event.51 The virus will become more threatening when human to human transmission occurs via an infected human droplet of fluid passing with a non-infected community. Asymptomatic transmission of MPXV has also been reported recently.52 Figure 5 depicts an overview of the MPXV transmission events.49
Diagnosis
Identification of MPX can be done using a differential diagnosis procedure. Laboratory tests must be used to confirm the positive clinical case. Diagnostic testing for MPXV should be carried out in a well-equipped lab that is properly set up by skilled personnel who have received technical and safety training.53 WHO recommends that the sample types for MPX consist of skin lesion materials, such as swabs of the lesion’s surface, secretions, or crust.54 The additional types of samples can be the patient’s urine, semen, rectal, and genital swabs which depend on signs of lesion location.55 These specimen types depend on the research study design or concern on research investigation board strategy.56 According to a previous study it was found that the additional specimen is more helpful for further investigation of disease sources and for investing other phenomena.57 After collection, samples need to be transported within 1 hour following cold conditions (2-8°C) or freeze (-20°C or below) and for long-term storage, specimens should be kept at -20°C or lower. According to the guideline of the WHO (World Health Organization) and PAHO (Pan American Health Organization) for long-term storage or more than 60 days, samples should be kept -70°C after specimen collection. The quality of the specimens and viral load may be affected by frequent freeze-thaw cycles, thus it should be avoided. Monkeypox may be suspected if a person exhibits the aforementioned symptoms, especially if there has been previous interaction with MPXV affected person or travel to monkeypox-endemic regions.23,58 For confirmation of a suspected case of MPXV molecular test (PCR, RT-PCR), viral Isolation (cell culture), antibody test (ELISA), and electron microscopic examination can be performed.30,59 The gold standard method for MPXV diagnosing is PCR using a specific primer. Experts suggest PCR should be done first, and after the PCR test other methods might be performed if the PCR test results were negative.60 Monkeypox can also be validated by sequencing from the PCR product results for MPXV-specific genes. Viral isolation can be used as a confirmatory test; virus isolation requires techniques and facilities with a Biosafety level-3 or above. Usually, antibody tests may be done for studies on surveillance and suspected cases after skin lesions have healed. Another important test is the electron microscopic test which can be performed for morphological analysis of MPXV.
Prevention and Control
Primary prevention of MPXV infection includes isolation of an infected person from other person and their pets, and avoiding close contact and sexual activity with infected individuals. Patients who are hospitalized need to be kept in a room with negative pressure and under strict airborne isolation. Before coming into contact with infected patients, medical professionals should wear correctly N95 masks, gloves, and eye protection goggles until the lesions have crusted and scabs have come off.60
Monkeypox virus prevention is extremely challenging as the spread of MPXV in endemic areas is very tough, it involves avoiding all contact with rodents, and primates, and restricting direct contact with blood and undercooked meat.61 It was observed that the prevalence areas of zoonotic disease used to consume wild animal meat as major protein source which need to inspect properly to control such types of disease.62 Human-to-human MPXV transmission prevention in healthcare depends on infection control techniques. Improved isolation procedures and nursing techniques require sufficient resources for staff.63 Healthcare providers, as well as others related to treating or coming in contact with patients who have monkeypox, should be immunized against Monkeypox by national health authorities.62 Smallpox virus vaccine programs might effectively control MPXV infection. Patient contact tracing, detection of hotspots, isolation, and waste management of positive confirmed cases are important.63 Interview using phone call or maintaining proper PPE direct interview can be taken. Healthcare personnel who are contacted with patients directly need to take proper precautions. Isolation need to continue until total recovery of lesions. Wastewater is a useful tool to monitor this disease with clinical tests.64–66 It offers early detection of hotspots and prediction of patient numbers using Monte Carlo simulation.67,68
Treatment
The majority of MPX patients recover without medical therapy.69 In the primary stage of MPXV infection, experts may suggest primary supportive care (Table 1).70 Primary care consists of supportive, symptom-directed care until the condition is clinically resolved. Clinicians should be on the lookout for secondary bacterial infections and start direct antibiotic therapy as soon as possible. Children, women who are pregnant or nursing mother, immunosuppressed people, and individuals with extensive skin or mucosal infections may all benefit from a particular therapy. In some cases patients gastrointestinal fluid is decreased, and those experiencing gastrointestinal symptoms (e.g., vomiting, diarrhea) will require oral/intravenous rehydration.71,72
Table (1):
According to US CDC Antiviral treatment patterns based on the primary, secondary, and tertiary stage of patient group.44-46
| Stage of treatment | Antiviral agent Name | Treatment received group | Doses |
|---|---|---|---|
| Primary | Tecovirimat | children <13 kg body weight | consult specialist for guidance on oral dose |
| children 13 to <25 kg body weight | 200 mg orally twice daily for 14 days | ||
| children 25 to <40 kg body weight | 400 mg orally twice daily for 14 days | ||
| children and adults 40 to <120 kg body weight | 600 mg orally twice daily for 14 days | ||
| children and adults ≥120 kg body weight | 600 mg orally three times daily for 14 days | ||
| Secondary | brincidofovir | children <10 kg body weight | 6 mg/kg orally once weekly for 2 doses |
| children and adults 10 to <48 kg body weight: | 4 mg/kg orally once weekly for 2 doses | ||
| children and adults ≥48 kg body weight | 200 mg orally once weekly for 2 doses | ||
| Tertiary | Cidofovir | children and adults | consult specialist for guidance on dose |
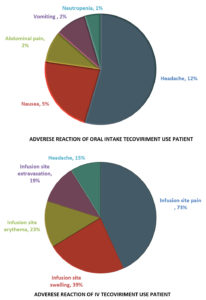
There are no specific or particular therapies for monkeypox infection at the moment. But smallpox experience indicates that such vaccinia vaccine, cidofovir, tecovirimat, and vaccinia immune globulin (VIG) may be useful in treating monkeypox.73,74 Two antiviral medicines, tecovirimat (TPOXX) and brincidofovir, are among these treatments.75 Adverse reaction noticed of tecovirmat for pateints include headache, vomiting, abdominal pain, nausea (Figure 6).
Figure 6. Adverse reaction pattern of oral intake Tecovirimat use Patient According to US CDC Study43
These antiviral drugs are also effective against MPXV. Tecovirimat was authorized by the FDA in 2018, and oral brincidofovir will be approved in 2021.76 Tecovirimat was also authorized by the EMA in 2021.77 Tecovirimat is the first antipox viral medication to obtain FDA approval in the U. S. It is available in tablet and injectable form. Tecovirimat is an antiviral drug that inhibits the replication of Orthopoxviruses like as the MPXV.78 It is an orthopoxvirus VP37 envelope-wrapping protein inhibitor. Tecovirimat has been found to be the most used drug and revealed promising beneficial effects in ameliorating aggravated and severe MPX cases.79 In laboratory tests, tecovirimat was effective. It has been demonstrated that it protects animals against monkeypox and rabbit pox while causing no major negative effects in humans. It has been demonstrated to shield animals against monkeypox and rabbitpox and has no negative effects on humans.80 Tecovirimat was initially used to treat a laboratory-acquired vaccinia virus infection in December 2018. According to the Food and Drug Administration, the most prevalent adverse effects of tecovirimat in humans were headache, nausea, and stomach aches.
Cidofovir, another active antiviral that has been used for decades, is available in both topical and intravenous forms and found useful against MPX.74,81 Brincidofovir (Tembexa) is an antiviral medication used to treat smallpox an inhibitor of viral DNA polymerase, the equivalent of the IV drug cidofovir (Table 2).82 It was given FDA approval in the United States in June 2021 to treat the sickness caused by human smallpox in adults and children, including neonates. In vitro and animal, investigations have demonstrated its effectiveness against Orthopoxviruses.83 Liver function tests must be performed both before and during therapy. In UK, case series of infections with MPXV showed that brincidofovir may elevate blood transaminases and serum bilirubin.84 The substance, which is conjugated to a lipid, is made to release cidofovir intracellularly. This results in higher intracellular and decreased plasma concentrations of cidofovir, which increases both the drug’s action against dsDNA viruses and its oral bioavailability.84 Brincidofovir ‘s efficacy has only been investigated in animals suffering from orthopoxvirus illnesses. There were no human investigations in patients with smallpox.85 The most common side effects include diarrhea, nausea, vomiting, and abdominal pain.86
Table (2):
Updates of Monkeypox vaccination with short description.
Trial ID and Title |
Type, Model, Time Prospective |
Description |
Actual Study Start Date |
Estimated Study Completion Date |
|---|---|---|---|---|
NCT05522296, Break-through Infection Following Monkeypox vaccination: Target Trial Emulation |
observational cohort prospective |
Close follow-up study of close contacts of MPX confirmed cases. Evaluation of secondary attack rate of MPXV infection in contacts, defined by PCR positivity on any sample; clinical and serological evaluation. |
September 13, 2022 |
September 13, 2023
|
NCT05562323, Characterization of Vaccine-induced Responses Against Monkeypox (MoVIHvax) An Observational Prospective Cohort Study |
observational cohort prospective |
study designed to characterize humoral and cellular immune responses after vaccination against monkeypox (MKP) in HIV positive and negative individuals at high risk of MKP infection during the vaccination campaign in the current monkeypox outbreak. |
September 14, 2022 |
April23,2023
|
NCT05443867, Monkeypox Asymptomatic Shedding: Evaluation by Self-Sampling MPX-ASSESS |
observational cohort prospective |
Close follow-up study of close contacts of MPX confirmed cases. Evaluation of secondary attack rate of MPXV infection in contacts, defined by PCR positivity on any sample; clinical and serological evaluation. |
June 22, 2022 |
December 31, 2022 |
NCT05058898, A One Health Study of Monkeypox Human Infection, Animal Reservoir, Disease Ecology and Diagnostic Tools |
Observational, case-control, prospective. |
Proportion of monkeypox cases occurring following interhuman exposures through a quantitative case-control study with an odds ratio of >3 for an exposure factor for human-to-human transmission. |
September 9, 2021 |
December 2023 |
NCT05438953, Follow-up of Contact at Risk of Monkeypox Infection: A Prospective Cohort |
Interventional (Clinical Trial), Parallel Assignment, Prospective. |
Estimation the failure rate of a post-exposure vaccination by the VMA vaccine in MPX contact case participants at risk (within 14 days after the last contact) after one dose |
July 12, 2022 |
July 2023 |
NCT02977715, An Open-Label Prospective Cohort Study of IMVAMUNE® Smallpox Vaccine in Adult Healthcare Personnel at Risk for Monkeypox in the Democratic Republic of the Congo |
Interventional (Clinical Trial), Single Group Assignment, Open-Label prospective. |
Description of monkeypox exposure and infection in eligible healthcare workers at risk of monkeypox infection, evaluating the immunogenicity and safety MVA vaccine. Evaluation of proportion of participants who after being vaccinated develop suspected or confirmed monkeypox infection, and experience exposure to monkeypox virus |
February 23, 2017 |
August 2022 |
NCT02080767, Clinical Protocol to Treat Individuals with Tecovirimat (ST-246) After Exposure to Orthopox Viruses
|
observational cohort |
Evaluation of efficacy and safety of tecovirimat in personnel (including US civilian employees, contractors and other US personnel and dependents, as well as allied military forces and local nationals) of any age exposed to or infected with Orthopox viruses or developed serious complications from vaccinia vaccination |
Estimated March 6, 2014 |
Not mentioned |
Vaccination Update
According to Centers for Disease Control and Prevention and National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), JYNNEOS and ACAM2000, two vaccines are approved for use in the US to prevent monkeypox, and these two smallpox vaccines have been suggested to be used against MPX.12,87 JYNNEOS two-dose vaccination can protect against both monkeypox and smallpox.88 The ACAM2000 vaccine is licensed to help prevent smallpox and has been made accessible to help prevent MPX, however it is not advised for use in pregnant women, babies younger than 12 months of age, people with certain medical disorders, such as a compromised immune system, or certain other illnesses or situations, such as heart issues and specific skin conditions.89 Both vaccinations are intended to offer adequate protection against monkeypox. The importance of ring vaccination has been suggested to play critical role in combating and limiting the spread of the virus during MPX outbreak and at mass gathering events.87,90,91
Monkeypox is a re-emerging viral disease, which has spread rapidly in multiple countries and posed the ongoing global public health emergency. It is high time to prevent and control outbreaks of this disease following strict public health counteracting measures and one health approach. If it is not possible to tackle this disease, this situation may convert into another new pandemic disease like COVID-19. Multi-departmental and collaborative international level combined action need to play critical role for preventing and controlling MPX. More literature review, data, and research-based explorative and investigative studies should be conducted to understand more about MPXV and MPX. Designing of newer MPX specific vaccines, antiviral drugs, therapeutics and treatment options need to be explored as such developments and advances will aid in counteracting MPX specifically and alleviate the fears of global health emergency and any feasible pandemic situation of the future.
ACKNOWLEDGMENTS
The authors would like to thank President Abdul Hamid Medical College Hospital, NSTU COVID-19 Diagnostic Lab, and all the institutions denoted in this manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by President Abdul Medical College Hospital, Noakhali Science and Technology University, and KTH Royal Institution, Sweden. PB would gratefully acknowledge the Life Science Technology Platform, Science for Life Laboratory for the seed funding to initiate the wastewater-based epidemiological studies for SARS-CoV-2 in Bangladesh.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Ahmed F, Islam MA, Kumar M, et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation centre through wastewater surveillance in Bangladesh. medRxiv. 2020;2020.09.14.20194696.
Crossref - Islam MA, Adeiza SS, Amin MR, Kaifa FH, Lorenzo JM, Bhattacharya P and Dhama K. A bibliometric study on Marburg virus research with prevention and control strategies. Front. Trop. Dis. 2022;3:1068364.
Crossref - Islam MA, Hasan MN, Ahammed T, et al. Association of household fuel with acute respiratory infection (ARI) under-five years children in Bangladesh. Front Public Heal. 2022;10.
Crossref - Sakib MMH, Nishat AA, Islam MT, et al. Computational screening of 645 antiviral peptides against the receptor-binding domain of the spike protein in SARS-CoV-2. Comput Biol Med. 2021;136:104759.
Crossref - Islam MA, Marzan AA, Islam MS, et al. Sex-specific epidemiological and clinical characteristics of COVID-19 patients in the southeast region of Bangladesh 2021. MedRxiv. 2021.
Crossref - Islam MA, Haque MA, Rahman MA, et al. A Review on Measures to Rejuvenate Immune System: Natural Mode of Protection Against Coronavirus Infection. Front Immunol. 2022;13:837290.
Crossref - Hossain M, Huq TS, Rahman A, Islam A, Tabassum N. Novel mutations identified from whole-genome sequencing of SARS-CoV-2 isolated from Noakhali, Bangladesh. Research Square. 2021.
Crossref - Dhama K, Chandran D, Chakraborty S, et al. Zoonotic concerns of Marburg virus: Current knowledge and counteracting strategies including One Health approach to limit animal-human interface: An update. Int J Surg. 2022;106:106941.
Crossref - Chakraborty S, Chandran D, Mohapatra RK, et al. Langya virus, a newly identified Henipavirus in China – Zoonotic pathogen causing febrile illness in humans, and its health concerns: Current knowledge and counteracting strategies – Correspondence. Int J Surg. 2022;105:106882.
Crossref - Chakraborty C, Bhattacharya M, Sharma AR, et al. Deep learning research should be encouraged for diagnosis and treatment of antibiotic resistance of microbial infections in treatment associated emergencies in hospitals. Int J Surg. 2022;105:106857.
Crossref - Chakraborty S, Chandran D, Mohapatra RK, et al. Marburg Virus Disease – A Mini-Review. J Exp Biol Agric Sci. 2022;10(4):689-696.
Crossref - Chakraborty S, Mohapatra RK, Chandran D, et al. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. An update – Correspondence. Int J Surg. 2022;105:106869.
Crossref - Chandran D, Dhama K, K MAM, et al. Monkeypox : An Update on Current Knowledge and Research Advances. J Exp Biol Agric Sci. 2022;10(2320):679-688.
Crossref - WHO. 2022a; Available from: (https://www.who.int/news/item/28-11-2022-who-recommends-new-namefor-monkeypox disease#:~:text=Following%20a%20series%20of%20consultations,%E2%80%9
Cmonkeypox%E2%80%9D %20is%20phased%20out.) Accessed on: December 5, 2022. - Graham F. Daily briefing: Mpox – a new name for monkeypox. Nature. 2022.
Crossref - Rakib SH, Masoom SM, Islam A, Islam F, Reza T. Design of a cost-effective Ultraviolet Disinfection unit to minimize the cross-contamination of COVID-19 in transport. International Conference on Advancement in Electrical and Electronic Engineering (ICAEEE) 2022;2-7.
Crossref - Rakib SH, Masum S, Patwari MRI, Fahima RA, Farhana A, Islam MA. Design and Development of a low cost Ultraviolet Disinfection system to reduce the cross infection of SARS-CoV-2 in ambulances. In: 2021. International Conference on Electronics, Communications and Information Technology (ICECIT). IEEE; 2021:1-4. https://ieeexplore.ieee.org/document/9641131/
- Tiwari A, Adhikari S, Kaya D, et al. Monkeypox outbreak: Wastewater and environmental surveillance perspective. Sci Total Environ. 2023;856(Pt 2):159166.
Crossref - Islam MA, Sangkham S, Tiwari A, et al. Association between Global Monkeypox Cases and Meteorological Factors. Int J Environ Res Public Health. 2022;19(23):15638.
Crossref - Quarleri J, Delpino MV, Galvan V. Monkeypox: considerations for the understanding and containment of the current outbreak in non-endemic countries. GeroScience. 2022;44(4):2095-2103. https://link.springer.com/10.1007/s11357-022-00611-6
- Mohapatra RK, Tuli HS, Sarangi AK, et al. Unexpected sudden rise of human monkeypox cases in multiple non-endemic countries amid COVID-19 pandemic and salient counteracting strategies: Another potential global threat? Int J Surg. 2022;103:106705. https://linkinghub.elsevier.com/retrieve/pii/S1743919122004824
- Thornhill J, Barkati S, Walmsley S, et al. Monkeypox Virus Infection in Humans across 16 Countries – April-June 2022. N Engl J Med. 2022;387(8):679-91.
Crossref - Peiro-Mestres A, Fuertes I, Camprubi-Ferrer D, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27(28):2200503.
Crossref - Petersen E, Abubakar I, Ihekweazu C, et al. Monkeypox – Enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019;78:78-84.
Crossref - Rahman MS, Hoque MN, Islam MR, et al. Epitope-based chimeric peptide vaccine design against S, M and E proteins of SARS-CoV-2 etiologic agent of global pandemic COVID-19: an in silico approach. PeerJ. 2020;8:e9572.
Crossref - Zumla A, Valdoleiros SR, Haider N, et al. Monkeypox outbreaks outside endemic regions: scientific and social priorities. Lancet Infect Dis. 2022;22(7):929-931.
Crossref - Harapan H, Setiawan AM, Yufika A, et al. Knowledge of human monkeypox viral infection among general practitioners: a cross-sectional study in Indonesia. Pathog Glob Health. 2020;114(2):68-75.
Crossref - Sallam M, Al-Mahzoum K, Dardas LA, et al. Knowledge of Human Monkeypox and Its Relation to Conspiracy Beliefs among Students in Jordanian Health Schools: Filling the Knowledge Gap on Emerging Zoonotic Viruses. Medicina. 2022;58(7):924.
Crossref - Giorgi J, Bader G, Wang B. A sequence-to-sequence approach for document-level relation extraction. In: Proceedings of the 21st Workshop on Biomedical Language Processing. Stroudsburg, PA, USA: Association for Computational Linguistics; 2022:10-25.
Crossref - Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):P1153-1162. https://linkinghub.elsevier.com/retrieve/pii/S1473309922002286
- WHO 2022b; Available from: (https://www.who.int/emergencies/situations/monkeypox-oubreak-2022). Accesed from: December 12, 2022
- Jain N, Lansiaux E, Simanis R. The new face of monkeypox virus: an emerging global emergency. New Microbes New Infect. 2022;47:100989.
Crossref - Barnewall RE, Fisher DA, Robertson AB, Vales PA, Knostman KA, Bigger JE. Inhalational monkeypox virus infection in cynomolgus macaques. Front Cell Infect Microbiol. 2012;2:117.
Crossref - Ahammed T, Anjum A, Rahman MM, Haider N, Kock R, Uddin MJ. Estimation of novel coronavirus (<scp>COVID</scp> -19) reproduction number and case fatality rate: A systematic review and meta-analysis. Heal Sci Reports. 2021;4(2):e274.
Crossref - Haider N, Hasan MN, Khan RA, et al. The Global case-fatality rate of COVID-19 has been declining disproportionately between top vaccinated countries and the rest of the world. medRxiv. 2022;2022.01.19.22269493.
Crossref - Mauldin MR, McCollum AM, Nakazawa YJ, et al. Exportation of Monkeypox Virus From the African Continent. J Infect Dis. 2022;225(8):1367-1376.
Crossref - Kampf G. Efficacy of biocidal agents and disinfectants against the monkeypox virus and other orthopoxviruses. J Hosp Infect. 2022;127:101-10.
Crossref - Kannan SR, Sachdev S, Reddy AS, et al. Mutations in the monkeypox virus replication complex: Potential contributing factors to the 2022 outbreak. J Autoimmun. 2022;133:102928.
Crossref - CDC 2021; Available from: (https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html). Accessed on: 12 December, 2022.
- Bragazzi NL, Kong JD, Mahroum N, et al. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review. J Med Virol. 2022.
Crossref - Sah R, Abdelaal A, Reda A, et al. Monkeypox and Its Possible Sexual Transmission: Where Are We Now with Its Evidence? Pathogens. 2022;11(8):924.
Crossref - Johnson RF, Dyall J, Ragland DR, et al. Comparative Analysis of Monkeypox Virus Infection of Cynomolgus Macaques by the Intravenous or Intrabronchial Inoculation Route. J Virol. 2011;85(5):2112-2125.
Crossref - Tambo E, Al-Nazawi AM. Combating the global spread of poverty-related Monkeypox outbreaks and beyond. Infect Dis Poverty. 2022;11(1):80.
Crossref - Sbrana E, Jordan R, Hruby DE, et al. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am J Trop Med Hyg. 2007;76(4):768-773.
Crossref - Adegboye OA, Castellanos ME, Alele FO, et al. Travel-Related Monkeypox Outbreaks in the Era of COVID-19 Pandemic : Are We Prepared? Viruses. 2022;14(6):1283..
Crossref - Parker S, Nuara A, Buller RML, Schultz DA. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2(1):17-34.
Crossref - Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020;12(11):1257.
Crossref - Saxena SK, Ansari S, Maurya VK, et al. Re-emerging human monkeypox: A major public-health debacle. J Med Virol. 2022:e27902.
Crossref - Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical Manifestations of Human Monkeypox Influenced by Route of Infection. J Infect Dis. 2006;194(6):773-80.
Crossref - Beig M, Mohammadi M, Nafe Monfared F, Nasereslami S. Monkeypox: An emerging zoonotic pathogen. World J Virol. 2022;11(6):426-434.
Crossref - Mohapatra RK, Mishra S, Kandi V, et al. Monkeypox plays a similar role like SARS-CoV-2; intensive animal screening is crucial after the first human-to-dog transmission report – Correspondence. Int J Surg. 2022;106:106925.
Crossref - Farahat RA, Khan SH, Dergaa I, Rabaan AA, Dhama K, Memish ZA. Asymptomatic transmission of monkeypox: Implications for mass gatherings? New Microbes New Infect. 2022;49-50:101056.
Crossref - van Furth AMT, van der Kuip M, van Els AL, et al. Paediatric monkeypox patient with unknown source of infection, the Netherlands, June 2022. Euro Surveill. 2022;27(29):2200552.
Crossref - WHO 2022c; Available from: (https://www.who.int/news-room/fact-sheets/detail/monkeypox). Accessed on: December 16, 2022
- Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51(1):23-32.
Crossref - Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38):180059.
Crossref - Yong SEF, Ng OT, Ho ZJM, et al. Imported Monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826-1830.
Crossref - Wolfe MK, Duong D, Hughes B, et al. Detection of monkeypox viral DNA in a routine wastewater monitoring program. medRxiv. 2022:1-23.
Crossref - Hobson G, Adamson J, Adler H, et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021;26(32):100745.
Crossref - McCollum AM, Damon IK. Human Monkeypox. Clin Infect Dis. 2014;58(2):260-267.
Crossref - Otu A, Ebenso B, Walley J, Barcelo JM, Ochu CL. Global human monkeypox outbreak: atypical presentation demanding urgent public health action. The Lancet Microbe. 2022;3(8):e554-e555.
Crossref - Simpson K, Heymann D, Brown CS, et al. Human monkeypox – After 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38(33):5077-5081.
Crossref - Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. Gromowski G, editor. PLoS Negl Trop Dis. 2022;16(2):e0010141.
Crossref - Islam A, Rahman A, Jakariya M, et al. A 30-day follow-up study on the prevalence of SARS-COV-2 genetic markers in wastewater from the residence of COVID-19 patient and comparison with clinical positivity. Sci Total Environ. 2022;858(3):159350.
Crossref - Jakariya M, Ahmed F, Islam MA, et al. Wastewater-based epidemiological surveillance to monitor the prevalence of SARS-CoV-2 in developing countries with onsite sanitation facilities. Environ Pollut. 2022;311:119679.
Crossref - Islam A, Hossen F, Rahman A, et al. An opinion on Wastewater-Based Epidemiological Monitoring (WBEM) with Clinical Diagnostic Test (CDT) for detecting high-prevalence areas of community COVID-19 Infections. Curr Opin Environ Sci Heal. 2022:100396.
Crossref - Jakariya M, Ahmed F, Islam MA, et al. Wastewater based surveillance system to detect SARS-CoV-2 genetic material for countries with on-site sanitation facilities: an experience from Bangladesh. medRxiv. 2021;8852000:2021.07.30.21261347.
Crossref - Ahmed F, Islam MA, Kumar M, et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation Centre in Bangladesh: Variation along the sewer network. Sci Total Environ. 2021;776:145724.
Crossref - Kumar S, Subramaniam G, Karuppanan K. Human monkeypox outbreak in 2022. J Med Virol. 2022:e27894.
Crossref - Usman S, Isa Adamu I. Modeling the Transmission Dynamics of the Monkeypox Virus Infection with Treatment and Vaccination Interventions. J Appl Math Phys. 2017;05(12):2335-2353.
Crossref - Roy S, Ripon MAR, Begum R, et al. Arachidonic acid supplementation attenuates adipocyte inflammation but not adiposity in high fat diet induced obese mice. Biochem Biophys Res Commun. 2022;608:90-5.
Crossref - Roy S, Bhowmik DR, Begum R, et al. Aspirin attenuates the expression of adhesion molecules, risk of obesity, and adipose tissue inflammation in high-fat diet-induced obese mice. Prostaglandins Other Lipid Mediat. 2022;162:106664.
Crossref - Chakraborty S, Chandran D, Mohapatra RK, et al. Clinical management, antiviral drugs and immunotherapeutics for treating monkeypox. An update on current knowledge and futuristic prospects. Int J Surg. 2022;105:106847.
Crossref - Ortiz-Saavedra B, Leon-Figueroa DA, Montes-Madariaga ES, et al. Antiviral Treatment against Monkeypox: A Scoping Review. Trop Med Infect Dis. 2022;7(11):369.
Crossref - Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human Monkeypox: Clinical Features of 282 Patients. J Infect Dis. 1987;156(2):293-298.
Crossref - Russo AT, Grosenbach DW, Brasel TL, et al. Effects of Treatment Delay on Efficacy of Tecovirimat Following Lethal Aerosol Monkeypox Virus Challenge in Cynomolgus Macaques. J Infect Dis. 2018;218(9):1490-1499.
Crossref - Ogoina D, Iroezindu M, James HI, et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210-4e214.
Crossref - Tecovirimat – Wikipedia. Available from: (https://en.wikipedia.org/wiki/Tecovirimat). Accessed on: December 16, 2022.
- Shamim MA, Padhi BK, Satapathy P, et al. The Use of Antivirals in the Treatment of Human Monkeypox Outbreaks: A Systematic Review. Int J Infect Dis. 2022.
Crossref - Drug to Treat Smallpox Approved by F.D.A., a Move Against Bioterrorism – The New York Times. Available from: (https://www.nytimes.com/2018/07/13/health/smallpox-drug-fda-bioterrorism.html#:~:text=The%20antiviral%20pill%2C%20tecovirimat%2C%20
also,after%20the%20last%20known%20case.). Accessed on: December 16,2022. - Sobral-Costas TG, Escudero-Tornero R, Servera-Negre G, et al. Human monkeypox outbreak: epidemiological data and therapeutic potential of topical cidofovir in a prospective cohort study. J Am Acad Dermatol. 2022.
Crossref - Brincidofovir – Wikipedia. Available from: (https://en.wikipedia.org/wiki/Brincidofovir). Accessed on: December 16,2022.
- Hutson CL, Kondas AV, Mauldin MR, et al. Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model. mSphere. 2021;6(1):e00927-20.
Crossref - Tiecco G, Degli Antoni M, Storti S, Tomasoni LR, Castelli F, Quiros-Roldan E. Monkeypox, a Literature Review: What Is New and Where Does This concerning Virus Come From? Viruses. 2022;14(9):1894.
Crossref - Tembexa (Brincidofovir Tablets): Uses, Dosage, Side Effects, Interactions, Warning. Available from: (https://www.google.com/search?q=Tembexa+ (Brincidofovir+Tablets)% 3A+Uses%2C+Dosage%2C+Side+ Effects%2C+Intera ctions%2C+Warning.&rlz=1C1BNSD_enBD994BD994&oq=Tembexa +(Brincidofovir+Tablets)% 3A+Uses%2C+Dosage %2C+Side+Effects%2C+Interactions %2C+Warning.&aqs=chrome..69i57.616j0j 7&sourceid=chrome&ie=UTF-8). Accessed on: December 16, 2022.
- Brincidofovir – Chimerix/Emergent Biosolutions; Available from: (https://adisinsight.springer.com/drugs/800019605). Accessed on: 16 December,2022
- Sah R, Humayun M, Baig E, et al. FDA’s authorized “JYNNEOS” vaccine for counteracting monkeypox global public health emergency; an update – Correspondence. Int J Surg. 2022;107:106971.
Crossref - JYNNEOS Vaccine; Monkeypox; Poxvirus; CDC. Available from: (https://www.cdc.gov/vaccines/hcp/vis/vis-statements/smallpox-monkeypox.pdf). Accessed on: December 16,2022
- ACAM2000 Vaccine; Monkeypox; Poxvirus; CDC. Available from: (https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/acam2000-vaccine.html#:~:text=It%20has%20been%20made%20available,for%
20infection%20to%20prevent%20mpox.). Accessed on: December 16, 2022 - Yuan P, Tan Y, Yang L, et al. Modeling vaccination and control strategies for outbreaks of monkeypox at gatherings. Front Public Heal. 2022;10:1026489.
Crossref - Monkeypox – List Results – ClinicalTrials.gov. Available from: (https://www.clinicaltrials.gov/ct2/show/NCT05559099). Accessed on: December 16, 2022.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.