ISSN: 0973-7510
E-ISSN: 2581-690X
Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae are the most common Gram-negative bacillus (GNB) isolates from clinical samples. These bacteria produce enzymes such as ESBL, AmpC β-lactamase, and carbapenemase as a resistance mechanism. Enzymes are responsible for resistance development across all cephalosporin and carbapenem antibiotic generations. In this study, we aimed to determine ESBL, AmpC-ESBL, and carbapenemase producer occurrences from clinical specimens from December 1, 2023, to February 1, 2024. We processed the clinical specimens in the Department of Bacteriology, Gurukrupa Laboratory, Pune, and performed the antibiotic susceptibility testing using an automated phenotypic method to identify organisms and their susceptibility profiles for screening ESBL, AmpC-ESBL, and carbapenemase producer organisms by an automated BD phoenix system. Carbapenemase producer organisms were reconfirmed by a modified carbapenemase inactivation method per CLSI guidelines, with 288 GNB strains isolated from 801 clinical specimens. Among all isolates, E. coli, P. aeruginosa, and K. pneumoniae (n = 90) exhibited high levels of drug resistance with a Multiple Antibiotic Resistance Index above 0.80. Therefore, these three organisms were considered for further characterization. P. aeruginosa was the highest ESBL producer at 27.7%, while the highest AmpC-ESBL coproduction was observed in E. coli, with 73.9%, the majority of which originated from urine. The highest Class B and D carbapenemase production was observed at 47.8% and 26.5% in E. coli and K. pneumoniae, respectively. We detected the highest correlation between AmpC and carbapenemase with a 0.81 correlation coefficient. Amikacin displayed good sensitivity among all antibiotics. The high occurrence of AmpC-ESBL producers and carbapenemase production from clinical samples indicates a need for strict antimicrobial policy and interventions. Carbapenem and colistin combination exhibited promising efficacy. Finally, several emerging therapeutic approaches could provide potential solutions for antibiotic use.
Extended-spectrum β-lactamase (ESBL), AmpC ESBL co-production, Carbapenemase
Over the past 70 years, antibiotics have made substantial contributions to global health development and have become essential for treating infectious diseases. However, antibiotic overuse and misuse have led to antibiotic resistance, which reduces the efficacy of each novel antibiotic within a few years of its introduction. Microorganisms adapted multiple mechanisms to develop drug resistance. Gram-negative bacteria such as E. coli, P. aeruginosa, and K. pneumoniae are frequently isolated from clinical specimens and are associated with nosocomial infections. Most Gram-negative organisms produce enzymes such as ESBL, AmpC β-lactamase, and carbapenemase. All three enzymes are responsible for developing resistance to most cephalosporin generations and carbapenem antibiotics.1 The European Centre for Disease Control and Centre of Disease Control established a standard terminology for PDR, MDR, and XDR organisms.2
Multidrug-resistant infections are generally treated by β-lactam antibiotics. If an organism is resistant to β-lactam drugs Carbapenem is considered a treatment option. Extended-spectrum β-lactam (ESBL) producers are typically resistant to multiple drugs. Bacterial genes are involved in mutations under conditions of antibiotic misuse and overuse.2,3 ESBL enzymes can hydrolyze β-lactam antibiotics, including penicillin, cephalosporins, and the oxy-amino group of β-lactamases, except for the cefoxitin and carbapenem groups of antibiotics. Apart from K. pneumoniae and E. coli, ESBL is also produced by Serratia spp., Citrobacter spp., Pseudomonas spp., and Morganella spp.4 Moreover, ESBL producers display co-resistance with other Fluoroquinolones, Aminoglycosides, and Sulfonamides.4
Class C β-lactamases (AmpC) are generally considered plasmid-mediated in K. pneumoniae, E. coli, Citrobacter freundii, Salmonella spp., and Proteus mirabilis, but they have also been observed to be chromosomal-mediated.5 The existence of these enzymes comprises ESBLs, and the treatment of these infection types is critical. Chromosomal-mediated AmpC ESBLs are mainly observed in Citrobacter, Serratia, Acinetobacter, Enterobacter, and Pseudomonas.6 Therefore, identifying proper treatments is particularly important. ESBL and AmpC-ESBL detection can be performed by conventional methods as well as by automated systems.7,8 ESBL- and AmpC-ESBL-associated infection treatment is particularly challenging and is associated both with mortality and morbidity.
The purpose of this study was to provide insights into the occurrence of multidrug- and extensively drug-resistant organisms, including ESBL, AmpC-ESBL, and carbapenemase producers, and to observe their antibiotic susceptibility test reports. This study could potentially contribute to the development of a robust antibiotic stewardship and infection control program.
Additionally, the goal of this study was to identify multidrug- and extensively drug-resistant organisms, which are difficult to treat, especially in cases of AmpC-ESBL- and carbapenemase-associated drug-resistance. Future prospects of this study include the development of new therapeutic strategies, such as phage therapy, to target these resistant isolates.
A short-term observational study was carried out at the Department of Bacteriology, Gurukrupa Laboratory, Pune, from December 1, 2023, to February 1, 2024. We received different clinical specimens from various areas of Pune, such as urine, aerobic and anaerobic sets of blood bottles, sputum, endotracheal aspirates, endotracheal tips, bronchoalveolar lavage pus, and wound and tissue samples. We processed all clinical samples in selective, differential, and enrichment media. For urine samples, 0.001 mL of calibrated loop was used to culture the samples using a semi-quantitative method. Results were evaluated according to the Kass criteria, considering >105 colony-forming units of growth as significant bacteriuria. Surveillance specimens and sterility checks were excluded from this study. False-positive carbapenemase non-producers might be considered carbapenem-resistant organisms other than the carbapenemase enzymatic mechanism. Therefore, we excluded those from the study. Enterobacteriaceae group and P. aeruginosa isolates exhibiting resistance to more than one carbapenem antibiotic were used in this study. The isolates had an MIC of Ertapenem >1 µg/mL and MIC of Meropenem and Imipenem ranging from 2-4 µg/mL, identified as carbapenem-resistant by an automated system.9
Antibiotic susceptibility testing
Culture-positive and significant growth obtained specimens were processed further for antibiotic susceptibility tests using the automated BD Phoenix, minimum inhibitory concentration-based method. As per the guidelines of the Clinical Laboratory Standard Institute (CLSI),10 we used the three reference bacterial strains: E. coli ATCC 25922, S. aureus ATCC 25923, and P. aeruginosa ATCC 27853 for internal quality checks of the AST results.
Antibiotic selection and susceptibility testing were performed according to the CLSI guidelines,10 using the following antibiotics: Ciprofloxacin, Levofloxacin, Norfloxacin, Amikacin, Gentamycin, Amoxicillin-Clavulanate, Ampicillin-sulbactam, Ceftazidime-avibactam, Piperacillin-Tazobactam, Ampicillin, Trimethoprim-Sulfamethoxazole, Cefazolin, Cefepime, Cefotaxime, Cefoxitin, Ceftazidime, Ceftriaxone, Cefuroxime, Nitrofurantoin, Aztreonem, Imipenem, Ertapenem, Meropenem, Colistin, Minocycline, Tigecycline, and Fosfomycin. AST was performed according to the manufacturer’s instructions. Briefly, we inoculated the bacterial colonies in identification broth as per the 0.5-0.6 McFarland standard. We followed the same procedure for the AST broth and observed the results after 16 h of incubation. We flagged the results of multidrug-resistant organisms that were ESBL producers and ESBL-AmpC coproducers as well as carbapenemase producers in the analyzer.
Reference Method
Carbapenem is a key antibiotic to treat multidrug-resistant organism-related infections. Carbapenem resistance means resistance to all β-lactam drugs. We identified as carbapenem-resistant those isolates resistant to one or more carbapenem antibiotic groups with a MIC of Imipenem and Meropenem – 2-4 µg/mL and of Ertapenem >1 µg/mL. We validated again these isolates using a modified carbapenemase inactivation method (mCIM) as per CLSI guidelines 2024.7 We identified carbapenemase producer isolates with different classes provided by BD panels phenotypically.11
We carried out mCIM by inoculating two to three pure colonies of fresh Enterobacteriaceae isolates and P. aeruginosa subcultures in 2 mL Trypticase soya broth in separate test tubes. We incubate all tubes for 4 h. After proper vortexing, Immerse meropenem disc (10 µg, Hi-media) into all the test tubes. After incubation time completion, we took out all the discs from all the test tubes. We prepared a lawn culture of E. coli ATCC on Mueller-Hinton Agar and placed a meropenem disc on all plates, then incubated all plates again at 37 °C for 18-24 h.12 We interpreted the results based on organisms that showed a 19 mm zone of inhibition; if the organism’s growth was found surrounding and inside the zone, it was considered positive for carbapenemase-producing organisms. The antibiotic sensitivity report was then compared for final interpretation.
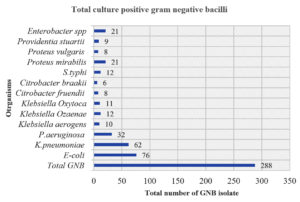
We isolated a total of 50.7% (n = 288) Gram-negative bacilli (GNB) out of 568 culture-positive samples from 801 clinical specimens. Out of the 288 GNB, we identified 31.2 0% (n = 90/288) as the most drug-resistant. By a magnitude of 90 clinical specimens, drug-resistant K. pneumoniae stood first with 45.3% (n = 49/62), while we identified 21.2% (n = 23/76) of drug-resistant E. coli. Apart from Enterobacteriaceae, we isolated 16.60% (n = 18/32) of drug-resistant P. aeruginosa (Figure 1).
Out of all the isolates, we identified 23 E. coli from 76 total isolates, 49 K. pneumoniae species from 62 total isolates, and 18 P. aeruginosa species from 32 total isolates, all exhibiting the highest multiple drug resistance. These organisms are common causes of direct and community-acquired infections. Therefore, this study has examined the magnitude of these three organisms at the level of antibiotic resistance, the occurrence of ESBL, AmpC-ESBL coproducer, and carbapenemase (Table 1 and Figure 2). A significant p-value was found less than 0.05 with reference to individual classes of antibiotics for organisms (Table 2).
Table (1):
Percentage of Occurrence of ESBL, AmpC and Carbapenemase producers Enterobacterales and Pseudomonas spp. from clinical samples
Characteristics of major drug resistant isolates |
E. coli (n = 23/76)% |
K. pneumoniae (n = 49/63)% |
P. aeruginosa (n = 18/32)% |
|---|---|---|---|
Total drug resistant species |
30.2% |
77.7% |
56.2% |
ESBL producer |
13.04% (n = 3) |
12.2% (n = 6) |
27.7% (n = 5) |
Atypical ESBL (AmpC) |
73.9% (n = 17) |
69.3% (n = 34) |
61.1% (n = 11) |
Atypical ESBL (AmpC) + Carbapenemase producer |
73.9% (n = 17) |
69.3% (n = 34) |
61.1% (n = 11) |
Class A carbapenemase |
0 |
0 |
0 |
Class B carbapenemase |
47.8% (n = 11) |
30.6% (n = 15) |
27.7% (n = 5) |
Class D carbapenemase |
17.3% (n = 4) |
26.5% (n = 13) |
5.5% (n = 1) |
Potential Carbapenemase producer (Not defined class) |
8.6% (n = 2) |
8.16% (n = 4) |
33.3% (n = 6) |
Table (2):
Antibiotics Resistance Profile of Enterobacterales
| Antibiotics | E. coli (n = 23) | K. pneumoniae (n = 49) | ||||||
|---|---|---|---|---|---|---|---|---|
| Group of tested Antibiotics | Antibiotic | Range of Test MIC (µg/ml) | Resistant Isolates (n) | SD (Resistant) | p-value (Resistant) | Resistant isolates (n) | Standard Deviation (SD) | p-value |
| 5-Fluoroquinolone | Ciprofloxacin | 0.25-4 | 87% (n = 20) | 0.41 | 0.001 | 93% (n = 46) | 2.5 | 0.001 |
| Levofloxacin | 0.25-8 | 87% (n = 20) | 0.37 | 0.002 | 95% (n = 47) | 2.45 | 0.002 | |
| Norfloxacin | 0.25-16 | 83% (n = 19) | 0.45 | 0.0025 | 85% (n = 42) | 2.35 | 0.003 | |
| Aminoglycosides | Amikacin | 0.5-64 | 30.4% (n = 7) | 2.13 | 0.0005 | 53% (n = 26) | 2.6 | 0.004 |
| Gentamycin | 0.25-16 | 65.2% (n = 15) | 1.78 | 0.002 | 69% (n = 34) | 2.5 | 0.002 | |
| β-Lactam/β-Lactamase Inhibitors | Amoxicillin-Clavulanate | 0.5/0.25-32/16 | 100% (n = 23) | 3.13 | 0.0001 | 97% (n = 48) | 2.7 | 0.005 |
| Ampicillin-salbactam | 0.5/0.25-32/16 | 83% (n = 19) | 2.45 | 0.0015 | 83% (n = 41) | 2.5 | 0.003 | |
| Ceftazidime-avibactam | 0.125/4-8/4 | 83% (n = 19) | 1.87 | 0.002 | 93% (n = 46) | 2.4 | 0.004 | |
| Piperacillin-Tazobactam | 0.5/4-128/4 | 83% (n = 19) | 1.67 | 0.0025 | 100% (n = 49) | 2.7 | 0.002 | |
| β-Lactam Penicillin | Ampicillin | 0.5-32 | 91.3% (n = 21) | 2.09 | 0.001 | 85% (n = 42) | 2.45 | 0.004 |
| Folate Antagonist | Trimethoprim-Sulfamethoxazole | 0.5/9.5-16/304 | 78.2% (n = 18) | 2.14 | 0.0015 | 100% (n = 49) | 2.7 | 0.003 |
| Cephalosporin | Cefazolin | 0.5-32 | 91.3% (n = 21) | 1.98 | 0.0015 | 97% (n = 48) | 2.65 | 0.002 |
| Cefepime | 0.5-64 | 91.3% (n = 21) | 1.87 | 0.002 | 97% (n = 48) | 2.6 | 0.004 | |
| Cefotaxime | 0.5-64 | 91.3% (n = 21) | 2.13 | 0.002 | 97% (n = 48) | 2.55 | 0.005 | |
| Cefoxitin | 0.5-64 | 87% (n = 20) | 2.03 | 0.002 | 97% (n = 48) | 2.6 | 0.002 | |
| Ceftazidime | 0.5-64 | 87% (n = 20) | 1.94 | 0.002 | 97% (n = 48) | 2.5 | 0.004 | |
| Ceftriaxone | 0.5-64 | 87% (n = 20) | 2.16 | 0.002 | 100% (n = 49) | 2.65 | 0.003 | |
| Cefuroxime | 1-16 | 91.3% (n = 21) | 2.08 | 0.0015 | 79% (n = 39) | 2.4 | 0.004 | |
| Nitrofurantoin | Nitrofurantoin | 8-512 | 39.1% (n = 9) | 2.32 | 0.002 | 93% (n = 46) | 2.5 | 0.002 |
| Monobactam | Aztreonem | 0.5-64 | 87% (n = 20) | 2.45 | 0.001 | 85% (n = 42) | 2.35 | 0.003 |
| Carbapenem | Imipenem | 1-16 | 83% (n = 19) | 2.34 | 0.0015 | 97% (n = 48) | 2.6 | 0.005 |
| Ertapenem | 0.25-2 | 91.3% (n = 21) | 2.56 | 0.0005 | 93% (n = 46) | 2.55 | 0.004 | |
| Meropenem | 0.25-16 | 83% (n = 19) | 2.45 | 0.001 | 10% (n = 5) | 2.4 | 0.002 | |
| Polymyxin B | Colistin | 0.25-1 | 4.3% (n = 1) | 1.12 | 0.003 | 73% (n = 36) | 2.35 | 0.004 |
| Tetracycline | Minocycline | 1-32 | 39.1% (n = 9) | 2.01 | 0.004 | 45% (n = 22) | 2.5 | 0.003 |
| phosphonic antibiotics | Fosfomycin | ≤16->64 | 26% (n = 6) | 2.45 | 0.001 | 77% (n = 38) | 2.55 | 0.002 |
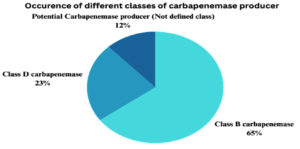
Figure 2. Occurrence of different classes of carbapenemase producers among Enterobacterales and P. aeruginosa
The largest source of multidrug-resistant organisms was urine, accounting for 54.4% (n = 90/49), followed by pus at 18% (n = 90/16), blood at 11.1% (n = 10/90), respiratory samples at 10% (n = 9/90), tissue at 2.2% (n = 2/90), and body fluids at 2.2% (n = 2/90). Respiratory samples included sputum, bronchoalveolar lavage, and endotracheal secretions. Blood and body fluids accounted for 4% (n = 29).
The resistance mechanisms showed a strong correlation between AmpC and carbapenemase producers, with a correlation coefficient of 0.81. In contrast, ESBL production showed a relatively weaker correlation with AmpC (0.72) and carbapenemase (0.58) production.
ESBL and AmpC-ESBL coproduction prevalence
In the present study, ESBL was detected at 17.7%, with a high prevalence in P. aeruginosa at 27.70% (n = 5/18), followed by K. pneumoniae at 12.2% (n = 6/49) out of 49 drug-resistant species. Among the 18 drug-resistant species, the highest drug resistance was observed in organisms displaying ESBL and AmpC enzyme coproduction, accounting for 73.9% in E. coli, 69.3% (n = 34/49) in K. pneumoniae, and 61.1% (n = 11/18) in P. aeruginosa. Throughout the study, the highest ESBL-AmpC enzyme coproduction was observed in E. coli, with the majority of cases originating from urine samples.
Prevalence of carbapenemase-producer organisms
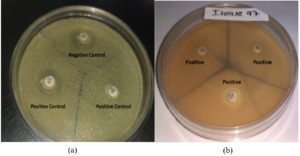
Modified carbapenemase inactivation method (mCIM) was performed on the isolates that were flagged as resistant to carbapenem antibiotics. The results were compared by checking the zone diameter, where a zone inhibition larger than 19 mm surrounding the colony was recorded accordingly (Figure 3).13
Figure 3. Result of the modified carbapenemase inactivation method. (a) Positive & Negative Control, (b) Resulted of tested organisms
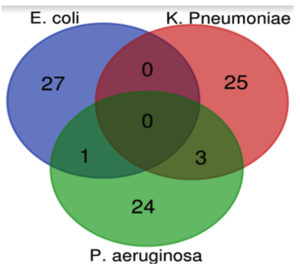
Venn diagram reveals the percentage of antibiotic resistance among E. coli, K. pneumoniae, and P. aeruginosa, showing overlap in the percentage of resistant species isolates across all three groups (Figure 4).
Figure 4. Venn diagram showing the percentage of antibiotic resistance between E. coli, K. pneumoniae, and P. aeruginosa
P. aeruginosa has the highest Antibiotic Resistance Index (ARI) and Multiple Antibiotic Resistance (MAR) Index, indicating that this organism is the most resistant of the three. All three organisms exhibit extremely high levels of multi-drug resistance, as evidenced by their MAR indices exceeding 0.80 (Tables 3 and 4).
Table (3):
Antibiotics Resistance Profile of P. aeruginosa (n = 18)
| Group of tested antibiotics | Antibiotics | Number of Resistant isolates | Standard Deviation (SD) | p-value |
|---|---|---|---|---|
| Aminoglycosides | Amikacin | 67% (n = 12) | 2.5 | 0.001 |
| Gentamycin | 68% (n = 13) | 2.45 | 0.002 | |
| Carbapenem | Imipenem | 94.4% (n = 17) | 2.6 | 0.002 |
| Meropenem | 100% ( n = 18) | 2.7 | 0.002 | |
| Cephalosporin | Ceftazidime | 100% ( n = 18) | 2.7 | 0.003 |
| β-Lac/β-Lac inhibitor | Ceftazidime-avibactam | 94.4% (n = 17) | 2.65 | 0.003 |
| Cephalosporin | Ceftriaxone | 94.4% (n = 17) | 2.6 | 0.002 |
| Cefepime | 94.4% (n = 17) | 2.65 | 0.003 | |
| Monobactam | Aztreonem | 94.4% (n = 17) | 2.55 | 0.004 |
| β-Lac Penicillin | Ampicillin | 100% ( n = 18) | 2.7 | 0.002 |
| β-Lac/β-Lac inhibitor | Ampicillin-salbactam | 100% ( n = 18) | 2.7 | 0.003 |
| Piperacillin-Tazobactam | 67% (n = 12) | 2.5 | 0.002 | |
| Polymyxin B | Colistin | 17% (n = 3) | 2.35 | 0.003 |
| Folate antagonist | Trimethoprim-Sulfamethoxazole | 94.4% (n = 17) | 2.65 | 0.002 |
| 5-Fluoroquinolone | Ciprofloxacin | 94.4% (n = 17) | 2.6 | 0.004 |
| Levofloxacin | 94.4% (n = 17) | 2.65 | 0.003 | |
| Norfloxacin | 94.4% (n = 17) | 2.55 | 0.004 |
Table (4):
Antibiotic Resistance Index (ARI) and Multiple Antibiotic Resistance (MAR) index
Organisms |
ARI |
MAR index |
SD |
p-value |
|---|---|---|---|---|
E. coli |
0.71 |
0.82 |
0.08 |
0.001 |
K. pneumoniae |
0.86 |
0.91 |
0.04 |
0.0005 |
P. aeruginosa |
0.89 |
0.94 |
0.03 |
0.0002 |
The percentage of antibiotic resistance for these three organisms was generally high, with P. aeruginosa bacteria having the highest resistance. The high correlation values among several forms of resistance indicate the different resistance mechanisms in Enterobacterales and P. aeruginosa. The results reveal that combination therapies, especially those involving carbapenems and Polymyxin, are more successful than single antibiotics. However, the reasonable MAR indices for these two combinations suggest that they may still face challenges in handling infections due to these MDRBs.
In this study, aminoglycosides group, Amikacin, demonstrated good sensitivity across all Enterobacterales strains, with 30.4% and 67% resistance in P. aeruginosa compared to all tested antibiotics. In the 5-Fluoroquinolone group of antibiotics, the highest resistance was shown by K. pneumoniae (93%) out of 49 drug-resistant strains, while P. aeruginosa exhibited 94.4% resistance. Fluoroquinolone resistance is most commonly observed in Enterobacteriaceae due to the extensive use of drugs to treat bacterial infection. Several mechanisms are associated with this resistance, with the most prominent being mutations of chromosomes at quinolone-determining regions, which are encoded by topoisomerase IV and DNA gyrase. In 1998, quinolone resistance, originally plasmid-mediated, was first observed in clinical isolate K. pneumoniae.14 If a bacterium is resistant to three or more antibiotics, it is considered to have a MAR Index, which is the ratio of the number of bacteria resistant to antibiotics to the total number of antibiotics tested. This method is valid for tracking the source of bacteria and contamination, with a MAR index value greater than 0.2 indicating a high risk. In our study, P. aeruginosa has shown the highest ARI and MAR indices of 0.89 and 0.94, respectively.15 These values indicate the urgent need to establish guidelines for antibiotic use and proper disposal of infected sewage to reduce the risk of contamination.15
Ambler Class B carbapenemase enzymes are a major concern for plasmid-mediated carbapenem drug-resistant Enterobacteriaceae. In our study, carbapenemase class B was most commonly observed in 47.8% of cases, while class A was not observed. When comparing resistance patterns, Cephalosporin and carbapenem resistance trends remain low for all three organisms. Among the studied bacteria, P. aeruginosa had the highest resistance rate to all the antibiotic classes, followed by K. pneumoniae, and the least resistant was E. coli (Table 5).
Table (5):
Comparison of resistance patterns across bacterial species
Antibiotic Class |
E. coli (%) |
K. pneumoniae (%) |
P. aeruginosa (%) |
SD |
p-value |
|---|---|---|---|---|---|
Fluoroquinolones |
85.7 |
91.0 |
94.4 |
4.4 |
0.018 |
Aminoglycosides |
47.8 |
61.0 |
67.5 |
10.1 |
0.032 |
Carbapenems |
88.5 |
91.7 |
98.1 |
4.9 |
0.009 |
Cephalosporins |
89.3 |
98.0 |
97.2 |
4.7 |
0.007 |
Therefore, we could conclude that, in terms of antibacterial agent efficacy, the highest combined efficacy of carbapenem and Polymyxin was observed for all three species. The efficiency of E. coli in β-lactam and β-lactamase inhibitor combination was higher than that of the other species (Table 6).
Table (6):
Effectiveness of Antibiotic Combination
Antibiotic Combination |
E. coli (%) |
K. pneumoniae (%) |
P. aeruginosa (%) |
SD |
p-value |
|---|---|---|---|---|---|
β-Lactam + β-Lactamase inhibitor |
82.7 |
93.3 |
87.0 |
5.3 |
0.011 |
Carbapenem + Polymyxin |
91.3 |
95.9 |
100.0 |
4.4 |
0.006 |
Aminoglycoside + Fluoroquinolones |
78.3 |
85.7 |
88.9 |
5.4 |
0.021 |
We discovered that AmpC ESBL coproduction led to a high chance of carbapenem resistance development through porin channel formation.16 In our study, the correlation coefficient value of 0.81 suggests a high degree of correlation between AmpC and carbapenemase producers. ESBL production is relatively weakly correlated with AmpC (0.72) and carbapenemase (0.58) production (Table 7).
Table (7):
Correlation between different resistance mechanisms
Resistance Mechanism |
ESBL |
AmpC |
Carbapenemase |
SD |
p-value |
|---|---|---|---|---|---|
ESBL |
1.00 |
0.72 |
0.58 |
0.21 |
0.003 |
AmpC |
0.72 |
1.00 |
0.81 |
0.14 |
0.001 |
Carbapenemase |
0.58 |
0.81 |
1.00 |
0.22 |
0.002 |
Recently, the increase of carbapenem-resistant Enterobacteriaceae has left limited treatment options, mainly relying on older antibiotics, except for “Polymyxin”. In 2015, a meta-analysis of 19 controlled and 6 single-arm cohort studies of 1086 patients was conducted. The study divided the patients into two groups: one group received only colistin as monotherapy (p < 0.01, 95% CI, 0.19-0.68; OR, 0.36), while the second group received a colistin and carbapenems combination therapy (p < 0.01, 95% CI, 0.31-0.75, OR, 0.49, lowers 28-30 days mortality rate), with the combination therapy showing significant results.17
Overall, the gradual rise of antibiotic resistant, coupled with the dwindling availability of last-line antibiotics, poses a major threat to public health. ESKAPE Pathogens like Enterococcus faecium, Staphylococcus aureus, K. pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter spp. remain major challenges despite of introduction of new antibiotics and adjuvants. There is an urgent need to develop new potential therapies. Strategies such as the application of Lytic bacteriophage,18,19 antimicrobial peptides, and the development of vaccines against multidrug-resistant infections could provide possible and potential therapeutic options.
Limitations of the study
Our study has some limitations, such as the lack of patient information and presentation centers, especially for inpatient cases. Another limitation was the absence of ESBL confirmation through susceptibility test, as well as genotypic confirmation of carbapenem producers. Although automated ESBL detection allows for rapid identification, certain experts recommended performing confirmatory tests. The data presented in the study was based solely on the BD Phoenix automated system, which has several limitations. Several performance evaluation studies have been performed globally and the majority have demonstrated good results for ESBL, atypical ESBL, and carbapenemase detection. Although this study suggests an additional confirmatory test to reduce false positivity, the CPO panel of the BD Phoenix automated system could correctly identify and classify carbapenem classes B and D with accuracies of 35.5% and 16.4%, respectively. In this study, we did not detect any carbapenem misclassification as per the Ambler classification.11,18
Continuous changes in antibiotic susceptibility pattern trends for major and last-resort antibiotics urgently necessitate an increase in the frequency of antibiograms and empirical therapeutic assurance. Our study helps identify common isolates, changing antibiotic sensitivity trends, and the most prevalent resistance mechanisms. As scientists, this study enabled us to identify novel therapeutic approaches that could provide alternatives and protect antibiotics from the growing threat of antimicrobial-resistance.
ACKNOWLEDGMENTS
The authors would like to thank Atmiya University for its technical and infrastructure support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Amer J Med. 2006;119(6 Suppl 1):S20-S8.
Crossref - Washington CW Jr, Stephen DA, William MJ, et al. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, Lippincott Williams & Wilkins, Philadelphia, Pa, USA, 6th edition, 2006.
- Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug resistant, extensively drug-resistant and pan drug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-281.
Crossref - Mackie TJ MJ: Laboratory strategy in the diagnosis of infective syndromes. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Practical Medical Microbiology 14th ed Edinburg: Churchill Livingstone 2006:p. 796.
- Grover N, Sahni AK, Bhattacharya S. Therapeutic challenges of ESBLS and AmpC beta-lactamase. Med J Armed Forces India. 2013;69(1):4-10.
Crossref - Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161-182.
Crossref - Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC type b-lactamases. Antimicrob Agents Chemother. 2002;46(1):1-11.
Crossref - Rao MJ, Harle S, Padmavathy M. Prevalence of Extended Spectrum Beta-lactamases and AmpC beta-lactamases in clinical isolates of gram-negative bacilli at a tertiary care hospital. J Evol Med Dent. 2018;7(39):2278-4748.
Crossref - Albichr IS, Anantharajah A, Dodemont M, Hallin M, Verroken A, Rodriguez-Villalobos H. Evaluation of the automated BD Phoenix CPO Detect test for detection and classification of carbapenemases in Gram negatives. Diagn Microbiol Infect Dis. 2020;96(2):114911.
Crossref - Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 34th ed. CLSI supplement M100. CLSI. 2024.
- Gatermann SG. Tropley and Wilson’s microbiology and microbial infections. London: Hoddder Arnold; 2005.
- Codjoe FS, Donkor ES. Carbapenem Resistance: A Review. Med Sci (Basel). 2017;6(1):1.
Crossref - Chavan SS, Angadi KM, Dave RP. Occurrence and types of carbapenamase enzymes amongst enterobacterales and Pseudomonas spp. using automated phenotypic method. IP Int J Med Microbiol Trop Dis. 2024;10(2):138-144.
Crossref - Geetha PV, Aishwarya KVL, Mariappan S, Sekar U. Fluoroquinolone Resistance in Clinical Isolates of Klebsiella Pneumoniae. J Lab Physicians. 2020;12(2):121-125.
Crossref - Sandhu R, Dahiya S, Sayal P. Evaluation of multiple antibiotic resistance (MAR) index and Doxycycline susceptibility of Acinetobacter species among inpatients. Indian J Microbiol Res. 2016;3(3):299-304.
Crossref - Shaaban M, Elshaer SL, Abd El-Rahman OA. Prevalence of extended-spectrum β-lactamases, AmpC, and carbapenemases in Proteus mirabilis clinical isolates. BMC Microbiol. 2022;22:247.
Crossref - Ni W, Cai X, Wei C, et al. Efficacy of Polymyxin in the treatment of carbapenem-resistant Enterobacteriaceae infections: a systematic review and meta-analysis. Braz J Infect Dis. 2015;19(2):170-180.
Crossref - Johri AV, Johri P, Hoyle N, Nadareishvili L, Pipia L, Nizharadze D. Case report: Successful treatment of recurrent E. coli infection with bacteriophage therapy for patient suffering from chronic bacterial prostatitis. Front Pharmacol. 2023;14:1243824.
Crossref - Patel, DR, Bhartiya SK , Kumar R, Shukla VK , Nath G. Use of customized bacteriophages in the treatment of chronic nonhealing wounds: a prospective study. Int J Low Extrem Wounds. 2021;20(1):37-46.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.