ISSN: 0973-7510
E-ISSN: 2581-690X
Oral candidiasis is the main symptom that often appears in patients with Human Immunodeficiency Virus (HIV) / Acquired Immune Syndrome (AIDS). Recent studies reported that some bacteria causing oral candidiasis are resistant to antifungal drugs. Describing nystatin profile against candida species in HIV / AIDS patients with oral candidiasis. Twenty-nine subjects were divided into 2 groups based on sex (23 male subjects and 6 female subjects). Subjects carried out tissue culture procedure and were tested for sensitivity to fluconazole and nystatin. The analysis was conducted by comparing sex and type of infecting bacteria. Statistical analysis used chi-square test, fisher, or ANOVA with 95% CI with p <0.05. The average age of male and female subjects was 43.15 ± 3.67 years and 40.02 ± 10.23 years, respectively, with age range of 18-65 years. Recurrent oral candidiasis in male and female patients was 65.22% and 83.33%, respectively (p = 0.079). Subjects were resistant to fluconazole as much as 77.50% in men and 61.54% in women (p = 0.823). On the other hand, subjects sensitive to nystatin were 92.50% in men and 92.31% in women (p = 0.167). Fluconazole was resistant to Candida albicans (68.00%) and non-Candida albicans (78.57%) (p = 0.048), while nystatin was sensitive to Candida albicans (92.00%) and non-Candida albicans (92.86%) (p = 0.791). Most subjects were resistant to fluconazole, while the majority of subjects were sensitive to nystatin.
Nystatin, fluconazole, oral candidiasis, Candida albicans, HIV/AIDS.
Oral candidiasis is one of the first clinical signs of AIDS found in 50% to 95% of HIV/AIDS patients. The condition is mostly caused by Candida albicans, which number is around 2-69.1% found in adult’s oral cavity1,2. Candida albicans is not the only species causing candidiasis, but also other species including Candida glabrata, Candida krusei, Candida tropicalis, Candida parapsilosis and Candida dubliniensis. Candida species is a commensal microorganism found in oral mucosa. However, this species becomes a predisposition factor causing oral candidiasis3,4. Early treatment of oral candidiasis, according to WHO, includes administration of topical antifungal agents, such as nystatin, amphotericin B, miconazole, and clotrimazole. Those agents can be given in oral candidiasis case without complication2,5.
In Indonesia, nystatin is an effective and affordable choice of antifungal for oral candidiasis3. The available doses of nystatin are 100.000 U/mL, and 400.000 – 600.000 U/mL for adults for 4 times a day for 7 – 14 days6,5. In 2017, there were 261 out of 1020 patients with HIV/AIDS treated in Dr. Soetomo General Hospital, Surabaya, Indonesia, who experienced oral candidiasis. The number increased to 273 patients in 2018. From June – July 2018, there were 20 oral candidiasis patients treated with oral nystatin, but 30% of which returned to the hospital with the similar case.
Based on the above explanation, the authors conducted an in vitro test to measure nystatin resistance in oral candidiasis patients with HIV/AIDS.
The subjects of this research were HIV/AIDS patients treated in Dr. Soetomo General Hospital Surabaya, Indonesia. The inclusion criteria were: HIV/AIDS patients diagnosed with rapid test/3 HIV testing methods7,8, having diagnosed with oral candidiasis by clinical examination and 10-20% KOH test9,10, and male or female patients aged >18 years. This study excluded subjects taking antifungal drugs in 2 weeks before test, and no colony growth found on culture examination with candida Sabouraud Dextrose Agar (SDA). The subjects must fulfill the informed consent.
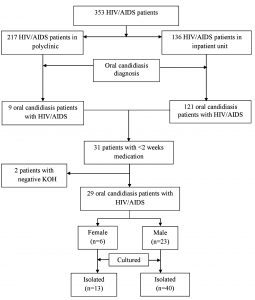
This study employed an observational descriptive design carried out from August 2018 to February 2019. The process of culture extraction was conducted in Dr. Soetomo General Hospital, Surabaya, Indonesia, and followed by culture examination that was carried out in Surabaya Health Laboratory, Surabaya, Indonesia. There were 29 patients who were consecutively sampled for the subjects in this research (Fig. 1). We also obtained 53 Candida isolates. The study protocol was in accordance with ethical procedure (0231/KEPK/IV/2018).
We first examined the subject’s culture9,10 that was taken from oral tissue swab. The positive Candida was grown in SDA at 37°C for 48 hours11. We used CHROMagar Candida (CHROMagar Candida, France) as the SDA medium. The growing Candida specimen were identified using cornmeal agar and tween 80 that were incubated at 42-45°C12,13. We also conducted carbohydrate test to identify Candida species14. Furthermore, we conducted resistance test using disc diffusion method on Mueller Hinton agar with 2% glucose and methylen blue. The isolate of Candida species was implanted on the agar, then a paper disk containing nystatin or fluzonazole was placed on top of it. We made a 24-48-hour-observation to look for an inhibition zone around the paper disc. The diameter of inhibition zone was measured using caliper (Rosco Diagnostica, Taastrup, Denmark). We interpreted the inhibition zone diameter using CLSI15. This study used nystatin with a dose of 100,000 UI/ml (pharma chemistry Ltd, Bekasi, Indonesia) and fluconazole at a dose of 2 mg/ml (pharma chemistry Ltd, Bekasi, Indonesia)
We assessed demographic and clinical data of patients. The collected data were then processed using IBM SPSS Statistics software version 23.0 (IBM Corp., Armonk, NY, USA). The statistical analysis used chi-square, fisher, or ANOVA with 95% CI (p <0.05).
Twenty-nine patients were divided into two groups based on their sex that consisted of 23 male subjects and 6 female subjects. The average age of male and female patients was 43.15 ± 3.67 years and 40.02 ± 10.23, respectively. They were divided into several age groups, where most subjects were found in the age range of 36 – 45 years (8 subjects; 27.59%), and followed by age group of 56 – 65 years (7 subjects; 24.13%). Most subjects had high school education background (48.27%), and unemployed (12 subjects; 41.37%) (Table 1). Most patients were Javanese (48.28%), and followed by Madurese (44.83%).
Table (1):
Demographic and Clinic Characteristics of patient Gender.
| Variable | Sex | p | |
|---|---|---|---|
| Male (n=23) | Female (n=6) | ||
| Age (mean ± SD) | 43.15 ± 3.67 | 40.02 ± 10.23 | – |
| Education (%) Not attending school Junior high school Senior high school Undergraduate/Diploma |
2 (8.70) 7 (30.44) 11 (47.83) 3 (13.04) |
2 (33.33) 1 (16.67) 3 (50.00) 0 (0.00) |
– |
| Ethnic (%) Java Madura Other |
10 (43.48) 11 (47.83) 2 (8.70) |
4 (66.67) 2 (33.33) 0 (0.00) |
– |
| Oral candidiasis (%) Recurrent First infection |
15 (65.22) 8 (34.78) |
5 (83.33) 1 (16.67) |
0.079 |
| Treatment history (%) Systemic antifungal Topical antifungal |
4 (17.39) 8 (34.78) |
1 (16.67) 3 (50.00) |
0.002* |
| ARV treatment (%) | 20 (86.96) | 3 (50.00) | 0.518 |
SD=standard deviation; ARV=antiretroviral; *significant 0.05
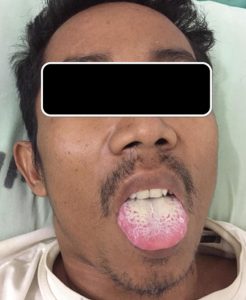
The subject’s clinical condition was as follows: 27 subjects (93.10%) had lesions on the tongue, 1 subject (3.45%) in the mucous membrane, and 1 subject (3.45%) in the corner of the lips (Figure 2). Most subjects were recurrent oral candidiasis patients (male = 65.22% and female = 83.33%) (p = 0.079). Some subjects had a history of systematic antifungal treatment (male = 17.39% and female = 16.67%) and topical antifungal (male = 34.78% male and female = 50.00%), with p = 0.002. Most subjects used antiretroviral (ARV) as much as 86.96% in men and 10.34% in women (p = 0.518; Table 1).SD=standard deviation; ARV=antiretroviral; *significant 0.05
Culture comparison based on the sex found that most subjects were infected with non-Candida albicans bacteria as much as 55.00% in male subjects, while most female subjects were infected with Candida albicans bacteria as much as 53.85% (p = 0.035). Most subjects were resistant to fluconazole as much as 77.50% in men and 61.54% in women (p = 0.823). The majority of subjects were sensitive to nystatin as much as 92.50% in men and 92.31% in women (p = 0.167; Table 2).
Table (2):
Comparison of culture results on male and female subjects .
| Variable | Sex | p | |
|---|---|---|---|
| Male (n=40) | Female (n=13) | ||
| Bacterium (%) Candida albicans Non-Candida albicans |
18 (45.00) 22 (55.00) |
7 (53.85) 6 (46.15) |
0.035* |
| Fluconazole (%) Sensitive Resistance |
9 (22.50) 31 (77.50) |
5 (38.46) 8 (61.54) |
0.823 |
| Nystatin (%) Sensitive Resistance |
37 (92.50) 3 (7.50) |
12 (92.31) 1 (7.69) |
0.167 |
*significant 0.05
Most candida albicans bacteria were resistant to fluconazole (68.00%), and most non-Candida albicans bacteria were also resistant to fluconazole (78.57%) (p = 0.048). Most Candida albicans bacteria were sensitive to nystatin drugs (92.00%), while non-candida albicans bacteria were mostly sensitive to nystatin drugs as much as 92.86% (p = 0.791; Table 3).
Table (3):
Comparison of fluconazole and nystatin sensitivity tests in the candida albicans and non-candida albicans groups.
| Variable | Bacterium | p | |
|---|---|---|---|
| CA (n=25) | NCA (n=28) | ||
| Fluconazole (%) Sensitive Resistance |
8 (32.00) 17 (68.00) |
6 (21.43) 22 (78.57) |
0.048* |
| Nystatin (%) Sensitive Resistance |
23 (92.00) 2 (8.00) |
26 (92.86) 2 (7.14) |
0.791 |
CA=Candida albicans; NCA=Non-candida albicans; *significant 0.05
The demographic data of this research included age, educational level and occupation. The highest age range was found in the adult group (63%). According to the data of Directorate General PPM & PL of the Ministry of Health in 2016, there were more than 50% HIV/AIDS patients were young adults and productive age groups with age group of 25-49 years old16. The finding of this study supported that adults included in productive and sexually active groups were more likely to engage in unprotected sexual behavior that was prone to HIV transmission17.
HIV/AIDS infection is a disease that has a huge social impact. Ninety percent of HIV/AIDS patients are likely to have oral cavity diseases that will have impact on the life quality, including occupational sector. This study found that 45% of the subjects were unemployed, and 9 subjects (45%) were high school graduates. HIV/AIDS patients with a low educational and socio-economic background have a bad oral health that makes them prone to oral cavity diseases18.
This study found that most patients were infected HIV due to heterosexual behavior (65%). This finding reflected the general condition of HIV/AIDS in East Java, in which the virus is mostly transmitted through heterosexual behavior (69.6%), followed by narcotics (21.9%)19. All patients in this study had white patches in their oral cavity, and being diagnosed with pseudomembranous oral candidiasis. This finding was consistent with a study conducted in India in 2013, in which pseudomembranous candidiasis was found in 72% of the subjects20. The ARV administration to HIV/AIDS patients could significantly reduce oral candidiasis. Some patients in this study previously had ARV therapy, while the new HIV/AIDS patients had not received the therapy yet21,22. Fungal infection was still found in most patients with antifungal therapy history, both systemic and topical23.
A disc diffusion method was conducted to measure sensitivity of all Candida species to nystatin. The results of sensitivity test were in the form of inhibition zone diameter. The criteria of susceptibility and resistance to antifungal agents were determined according to the interpretation of inhibition zone diameter for fungi by Rosco Diagnostica Company24. This study found that neither Candida albicans nor Candida non-albicans species that resisted to nystatin. Nystatin currently becomes the primary therapy for oral candidiasis in patients with HIV/AIDS.
The resistance against nystatin was divided into two groups, namely intrinsic and extrinsic sensitivity. The extrinsic sensitivity shows a change in sensitivity pattern of Candida species, from sensitive to resistance against antifungal therapy. On the other hand, intrinsic sensitivity has occurred early on antifungal therapy. This study found some intrinsic sensitivities in a form of infection caused by Candida krusei, which occurred in 8% of subjects25.
Some literatures stated that nystatin resistance is very minimum, but the therapy has side effects and toxicity. Nystatin generally works by distracting fungal cytoplasmic membrane and interacting with ergosterol. Ergosterol is important to maintain integrity and function of the enzymes of fungal membrane. Nystatin produces holes in cell membrane that becomes a way out for potassium ion and magnesium cellular components. This causes damages in proton gradient of cell membrane that leads to fungal cell death. Nystatin has a high binding capacity to ergosterol and low binding capacity to 3 hydroxy or oxysterol, such as fecosterol and episterol that becomes an important reason for nystatin resistance26.
Although there is an increased in nystatin resistance, this remains a rare occurrence in fungal pathogenic isolates since nystatin could not be used for systemic fungal infection. Therefore, the indications are not as much as the azole group. The incident of nystatin-resistant strains may be largely not considered. Most fungal species are considered susceptible to nystatin. However, some of which intrinsically less susceptible to this antifungal agent, such as Candida glabrata, Scedosporium prolificans and Aspergillus terreus. Some species are also more susceptible to nystatin resistance, including Candida lusitaniae, Candida guilliermondii and Candida krusei27,25.
Factors such as recurrent oral candidiasis and history of antifungal usage are considered to cause differences of Candida species. Those factors are thought to be a predisposing factor that changes the type of Candida into Candida non-albicans. This may occur in patients with recurrent oral candidiasis as they are also exposed to antifungal medication thus supports the previous hypothesis. The characteristics of an antifungal drug are also factors that play a role in a difficult-to-treat infection. Fungistatic drugs will further encourage the formation of resistance compared to fungicidal drugs. Absorption, distribution and metabolism of a drug also contribute to the overall effectiveness of treatment based on the location of an infection. Antifungal drug dosages, including quantity, frequency, administration schedule and cumulative doses, can also play a role in treating a fungal infection. The administration of antifungal medication along with other prescription can also change the effectiveness of antifungal drugs. In addition, in the course of advanced HIV/AIDS, extensive fungal colonization was also found27.
This study found a change in the spectrum causing oral candidiasis, since Candida albicans species were mostly found, while the number of Candida non-albicans was increasing.
In the invitro test, no Candida isolate was found to be resistant to nystatin. Therefore, oral nystatin is still effectively used as a standard therapy for HIV/AIDS patients with oral candidiasis.
ACKNOWLEDGEMENTS
Special thanks to Hastika Saraswati, MD who has assisted in our paper translate and Bernadya Yogatri Anjuwita Saputri, MD who helped us in the research. In addition, we would like to thank the Surabaya Health Laboratory, Indonesia, for helping us to carry out fluconazole resistance tests.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
DM conceived the study. R contributed in study design. DM and YL collected data. CSM participated in data analysis and interpretation. DM drafted the manuscript. R and YL revised the manuscript. All authors read and approved the manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The dataset used and/or analyzed during the current study are available from corresponding author on reasonable request.
ETHICS STATEMENT
The study protocol was in accordance with ethical procedure (0231/KEPK/IV/2018). All subject was received consent forms.
- Patil S, Rao RS, Majumdar B, Anil S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Frontiers in Microbiology, 2015; 6: 1391-1391.
Crossref - Goulart LS, Souza WWRd, Vieira CA, Lima JSd, Olinda RAd, Araujo Cd. Oral colonization by Candida species in HIV-positive patients: association and antifungal susceptibility study. Einstein (Sao Paulo, Brazil), 2018; 16(3): eAO4224-eAO4224.
Crossref - Anwar KP, Malik A, Subhan KH, Profile of candidiasis in HIV infected patients. Iranian journal of microbiology, 2012; 4(4): 204-209.
- Ribeiro Ribeiro AL, de Alencar Menezes TO, de Melo Alves-Junior S, de Menezes SAF, Marques-da-Silva SH, Rosario Vallinoto AC. Oral carriage of Candida species in HIV-infected patients during highly active antiretroviral therapy (HAART) in Belem, Brazil. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 2015; 120(1): 29-33.
Crossref - Lyu X, Zhao C, Yan Z-M, Hua H, Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Design, Development and Therapy, 2016; 10: 1161-1171.
Crossref - Patil S, Majumdar B, Sarode SC, Sarode GS, Awan KH, Oropharyngeal Candidosis in HIV-Infected Patients-An Update. Frontiers in Microbiology, 2018; 9: 980-980.
Crossref - Mungrue K, Sahadool S, Evans R, Boochay S, Ragoobar F, Maharaj K, Green S, Pennerman T, Tayopa O, Assessing the HIV rapid test in the fight against the HIV/AIDS epidemic in Trinidad. HIV/AIDS (Auckland, NZ), 2013; 5: 191-198.
Crossref - Huang X, Liu X, Chen J, Bao Y, Hou J, Lu X, Xia W, Xia H, Song A, Liu Z, Su B, Chen H, Chen Y, Wu H, Evaluation of Blood-Based Antibody Rapid Testing for HIV Early Therapy: A Meta-Analysis of the Evidence. Frontiers in immunology, 2018; 9: 1458-1458.
Crossref - Byadarahally Raju S, Rajappa S, Isolation and identification of Candida from the oral cavity. ISRN dentistry, 2011; 2011: 487921-487921.
Crossref - Coronado-Castellote L, Jiminez-Soriano Y, Clinical and microbiological diagnosis of oral candidiasis. Journal of Clinical and Experimental Dentistry, 2013; 5(5):e279-e286.
Crossref - Saigal S, Bhargava A, Mehra SK, Dakwala F, Identification of Candida albicans by using different culture medias and its association in potentially malignant and malignant lesions. Contemporary Clinical Dentistry, 2011; 2(3): 188-193.
Crossref - Nadeem SG, Hakim ST, Kazmi SU, Use of CHROMagar Candida for the presumptive identification of Candida species directly from clinical specimens in resource-limited settings. The Libyan Journal of Medicine, 2010; 5: 10.3402/ljm.v3405i3400.2144.
Crossref - Baradkar V, Mathur M, Kumar S , Hichrom candida agar for identification of candida species. Indian Journal of Pathology and Microbiology, 2010; 53(1): 93-95.
Crossref - Kali A, Srirangaraj S, Charles P, A cost-effective carbohydrate fermentation test for yeast using microtitre plate. Indian Journal of Medical Microbiology, 2015; 33(2): 293.
Crossref - Sahu C, Jain V, Mishra P, Prasad KN, Clinical and laboratory standards institute versus European committee for antimicrobial susceptibility testing guidelines for interpretation of carbapenem antimicrobial susceptibility results for Escherichia coli in urinary tract infection (UTI). Journal of Laboratory Physicians, 2018; 10(3):289-293.
Crossref - Lattif AA, Banerjee U, Prasad R, Biswas A, Wig N, Sharma N, Haque A, Gupta N, Baquer NZ, Mukhopadhyay G, Susceptibility pattern and molecular type of species-specific Candida in oropharyngeal lesions of Indian human immunodeficiency virus-positive patients. J. Cli. Microb., 2004; 42(3): 1260-1262.
Crossref - Ishii H, Matano T, Development of an AIDS vaccine using Sendai virus vectors. Vaccine, 2015; 33(45): 6061-6065.
Crossref - Kambu Y, Waluyo A, Kuntarti K, Umur orang dengan HIV AIDS (ODHA) berhubungan dengan tindakan pencegahan penularan HIV. Jurnal Keperawatan Indonesia, 2016; 19(3): 200-207.
Crossref - Bajomo AS, Ayo-Yusuf OA, Rudolph MJ, Tsotsi NM, Impact of oral lesions among South African adults with HIV/AIDS on oral health-related quality of life. Journal of Dental Sciences, 2013; 8(4): 412-417.
Crossref - Oktafiani D, Megasari NLA, Fitriana E, Nasronudin, Lusida MI, Soetjipto, Detection of Human Herpesvirus-8 Antigen In Hiv-Infected Patients In East Java, Indonesia. African Journal of Infectious Diseases, 2018; 12(2): 43-46.
Crossref - Fauk NK, Kustanti CY, Wulandari R, Damayani AD, Mwanri L, Societal determinants of HIV vulnerability among clients of female commercial sex workers in Indonesia. PloS one, 2018; 13(11): e0207647-e0207647.
Crossref - Prabawanti C, Dijkstra A, Riono P, Hartana G, A survey on HIV-related health-seeking behaviors among transgender individuals in Jakarta, based on the theory of planned behavior. BMC Public Health, 2015; 15: 1138-1138.
Crossref - Altman K, Vanness E, Westergaard RP, Cutaneous manifestations of human immunodeficiency virus: a clinical update. Current Infectious Disease Reports, 2015; 17(3): 464-464.
Crossref - Patel PK, Erlandsen JE, Kirkpatrick WR, Berg DK, Westbrook SD, Louden C, Cornell JE, Thompson GR, Vallor AC, Wickes BL, Wiederhold NP, Redding SW, Patterson TF, The Changing Epidemiology of Oropharyngeal Candidiasis in Patients with HIV/AIDS in the Era of Antiretroviral Therapy. AIDS Research and Treatment, 2012; 2012: 262471-262471.
Crossref - Sariguzel F, Berk E, Koc A, Sav H, Aydemir G, Evaluation of CHROMagar Candida, VITEK2 YST and VITEK® MS for identification of Candida strains isolated from blood cultures. Infez. Med., 2015; 23: 318-322
- Mohamadi J, Motaghi M, Panahi J, Havasian MR, Delpisheh A, Azizian M, Pakzad I, Anti-fungal resistance in candida isolated from oral and diaper rash candidiasis in neonates. Bioinformation, 2014; 10(11): 667-670.
Crossref - Moges B, Bitew A, Shewaamare A, Spectrum and the In vitro Antifungal Susceptibility Pattern of Yeast Isolates in Ethiopian HIV Patients with Oropharyngeal Candidiasis. International Journal of Microbiology, 2016; 2016: 8.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.