Tuberculosis (TB) is a contagious disease that is a significant cause of illness worldwide and has been declared one of the top ten causes of mortality across the world. It is well known that bacteria within biofilms exhibit much higher drug resistance than individual cells. Biofilms constitute a significant threat in the clinical environment by acting as reservoirs of multidrug-resistant bacteria. Thus, the formation of biofilms has been postulated to further aid in drug insensitivity and bacterial persistence within host tissues. The rapid increase in drug resistance in Mycobacteria poses a significant challenge to TB eradication and needs to be addressed soon. In this review, we have attempted to frame a general overview of mycobacterial pathogenesis, the role of biofilm formation in enhancing its shelf life, and some natural compounds and nanoparticles as emerging novel therapeutics reported to inhibit biofilm formation in mycobacteria. Therefore, we present some recent advances which might have potential applications in new treatment regimens for Tuberculosis.
Biofilm, Mycobacteria, Therapeutics, Tuberculosis
Despite the advent of several anti-TB drugs like isoniazid, rifampicin, Linezolid, Bedaquiline, and Delamid and several strategies framed to combat this contagious disease, Tuberculosis continues to cause worldwide morbidity in around 10 million people each year.1 The causative agent, Mycobacterium tuberculosis (M.tb.), is known to form biofilms both in vitro and in vivo, and the cells enmeshed in biofilm survive high concentrations of antibiotics due to the integrity of mycobacterial cell wall structure.2,3 Infections caused by biofilm-producing bacteria are abundant and highly persistent, revealing phenotypic resistance to high concentrations of antimicrobials and controlling host immune systems.4,5
Several studies report the primary role of biofilm formation in enhancing the growth of infectious species of Mycobacteria. The first report of this hazardous Biofilm was published in the year 1978,6 and the phenomena of mycobacterial cells forming “aggregates” or “pellicles” was described in the early days of mycobacteriology.7,8 Robert Koch described the appearance as “cells which are pressed together and arranged in bundles”.9 Similar observations were made for avian bacilli,7 concluding that mycobacterial cells grow naturally in the so-called structures “biofilm”. These bacterial biofilms, composed of calcite scaffolds, limit penetration of small molecule solutes such as antibiotics and play a protective role in the complex assembly of multicellular populations.10 Several laboratory experiments were conducted to grow mycobacteria.11,12 Hence, Biofilm can be defined as a sheet of cells, full of life, which can resist several stress environments, thereby protecting bacteria and hence, promoting drug resistance among them and creating a global challenge in the eradication of tuberculosis.13
Mycobacterial Biofilms
The occurrence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains of M.tb. are a limitation in current Tuberculosis (T.B.) control strategies, thus leading to the emergence of tuberculosis epidemic to a critical condition.14 M.tb. and M. leprae are the prominent residents of the genus Mycobacterium, which are specifically human pathogens. Additionally, some environmental organisms referred to as non-tuberculous mycobacteria (NTM) are also responsible for causing certain infections in humans.15 Several mycobacterial species tend to self-assemble in highly organized, surface-attached, and matrix-encapsulated structures called biofilms and thus survive against various environmental stresses, like high pH, oxidative stress, and antibiotics.16-18 Plants are a rich source of numerous therapeutic compounds having multiple medicinal properties. Each part of the plant is the source of secondary metabolites, such as terpenes, phenolics, essential oil, alkaloids, and polypeptides. Several of this phytocompound have potent antibiofilm activity and can be used for different diseases.19 Using gold, silver, iron, zinc, copper, etc, antibacterial agents based on nanotechnology has shown a promising future to combat drug-resistant bugs.20,21 These nanoparticles can be obtained from various inorganic and organic sources, and recently, researchers have derived nanoparticles from fungal sources.22,23 These nanoparticles have also been found effective against biofilm-forming bacteria.21,24 Recently resistance to Ag, Au, or Cu nanoparticles has been observed, which creates a need to look into newer nanoparticles for prospects.25,26
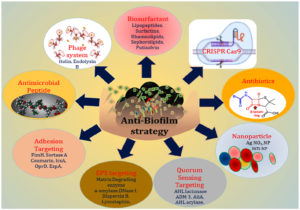
Given the role of Biofilm in drug resistance and mycobacterial persistence, there is a need to focus on newer approaches in the eradication of Tuberculosis by agents that inhibit mycobacterial biofilm production and thus aid in overcoming drug resistance (Figure 1) and thereby control the spread of this contagious disease.
Characteristics of Biofilm Formed in Mycobacterial spp.
Biofilm assembly
Biofilm assembly comprises several steps – (i) attachment to the surface on which it grows, (ii) a thin layer of biofilm formation, (iii) thickening and maturation of Biofilm, (iv) formation of the air-liquid interface, (v) dispersion (Figure 2). As the Biofilm thickens and matures, it starts to disperse further, leading to the formation of aggregates of bacterial cells. These aggregates adhere to the surface with the help of adhesins found in the cell wall of bacteria, followed by the production of extracellular polysaccharide substance (EPS) with the use of major proteins.27 The extracellular polymeric substances consist of polysaccharides, mycolic acids, and nucleic acids.28 The NTM, both clinical and laboratory strains, tend to adhere to surfaces like polyvinyl chloride (PVC), cellulose diacetate, polypropylene, etc., forming biofilm.29-31,32 In a study with Mycobacterium smegmatis as a model, it has been observed that glycopeptidolipids play a major role in the biosynthesis of the biofilm,33 while in another study, it was observed that mycolic acids, too, play a major role in the formation of biofilm structures by forming the hydrophobic extracellular matrix.34
Various functions of biofilm
The consortium of bacterial cells termed “biofilm” assist in the persistence of cells using many influences like host-pathogen relationships, cell-to-cell interactions, environmental factors, and the interchange of genetic goods. The host-pathogen interaction improves in preventing the tangible hazard to the Biofilm by helping the body to fight against it. The exopolysaccharides distributed from Biofilm are in the form of adhesins, protecting the Biofilm from external forces, penetration of antibiotics into the bacteria’s cell wall, and multi-drug resistance. Tuberculosis, several waterborne diseases, and teeth plaques-like diseases are the outcomes of biofilm formation. It has been observed that the thicker the Biofilm, the less the chances of phagocytic35 inflammatory response of the host.36
Various techniques to control Biofilm
Ultraviolet (U.V.) irradiation
Ultraviolet irradiation is used as a potent disinfectant. In comparison to the chemicals used, irradiation is safer. This technique is beneficial because of the elimination of many pathogenic bacteria, and no unsafe substances are left evaluated for the chemical therapies. U.V. radiation generates the making of oxygen-rich reactive species, which destroys the nucleic acid and punctures the cell membrane leading to an outflow of cytoplasm and cell death.37
Chlorine
Chlorine and chloramine treatment is used to purify water. It is beneficial when it is paired with a U.V. procedure. Chlorine and chloramine together act as a germicidal agent, but due to their little penetration within the extrapolysaccharide of Biofilm, they have low efficacy as anti-biofilm agents. The chlorine reacts with the protein and exopolysaccharide portions of the bacterial cell, which affects minimal hydrophobicity and adhesion. The function of chlorine grows the EPS on cell lysis, while chloramine is favourably effective in changing with microbial protein and ruining the EPS. Chloramine with chlorine comparison is found that chlorine is less effective.38
Hydrogen peroxide
Hydrogen peroxide disrupts exopolysaccharides and proteins of Biofilm. The hydroxyl ion from hydrogen peroxide treatment produced is generally accountable for disrupting macromolecular structures like constituents of nucleic acids. It has also been found that residual hydrogen peroxide benefits in controlling the adjustment of Biofilm to its environment.37
Nitric oxide
Nitric oxide possesses antimicrobial properties because of its diatomic and free radical nature. Their micromolar concentration can be efficient against Biofilm. The use of nitric oxide-based treatment shows a bactericidal consequence by detrimental bacterial DNA and inducing lipid peroxidation.39
Environmental factors responsible for biofilm formation
Many environmental factors play a major role in developing a well-defined biofilm matrix, including nutrient ions like calcium, magnesium, and iron. Specific carbon sources like glucose also enhance biofilm formation.40 Mycobacteria have a hydrophobic property, which promotes aerosolization, leading to the spread of aerosols at distant places that remain suspended for a longer tenure than droplets, enhancing the chances of distant spread and further infection. Also, it has been found in the study that due to this hydrophobic nature, where even running tap water may act as a suitable environmental growth factor for mycobacteria prone to adhering to each other, triggering biofilm formation.41,42
M. smegmatis is a non-tuberculous mycobacterium (NTM) often used as a model organism for M. tb. because of its high genotypic and phenotypic homology along with M. smegmatis being non-pathogenic and possess rapid growing ability. Under harsh environmental conditions, microorganisms amass reactive oxygen species (ROS), leading to a precarious situation called oxidative stress. The high amount of oxidative stress causes toxicity, while a low amount act as a stimulant, causing bacteria to stimulate active scavenging mechanisms.43 Various studies show a significant connection between different environments provided for growth and biofilm formation in M. smegmatis. The growth of M. smegmatis and Biofilm formation were analyzed under various environmental conditions like media deficient in iron and magnesium, carbon source-containing medium, alkaline medium, and under high oxidative stress. In the case of iron and magnesium-deficient media, the viable counts tend to decrease compared to the initial inoculum. Less mycobacterial growth and Biofilm was observed under high oxidative stress, low pH, and alkaline condition, whereas sodium acetate as a carbon source enhanced the viable counts of the isolate, thus depicting its crucial role in biofilm development.28 Mohammad Faizi et al. studied the effects of some environmental stresses on M. marinum on growth, biofilm formation, cell division, and biochemical characteristics, where it was found that biofilm formation decreased with stress conditions.44 Biofilms are recalcitrant to extreme environments and can protect microorganisms from ultraviolet (U.V.) radiation, extreme temperature, extreme pH, high salinity, high pressure, poor nutrients, antibiotics, etc., by acting as “protective clothing.”45
Clinical manifestations and epidemiology of mycobacterial biofilms
Several species of Mycobacteria have been found to cause infections affecting various organs of the human body, and biofilms have been observed not only at infection sites but also the hospital water tanks, catheters, etc. Few species, like Mycobacterium haemophilum, which belongs to the non-tuberculous group of mycobacteria tend to infect humans and animals. It affects people with weaker immune systems, including healthy children developing lymphadenitis, and also infects the dermis region with ulcers and pulmonary areas. Several published studies suggest surgical treatments in patients suffering from cervicofacial lymphadenitis due to Mycobacterium spp.46
The most crucial microbiological and clinical manifestations include the number of bacteria and their molecular strain. Patients suffering from miliary tuberculosis exhibit symptoms such as an increase in temperature during the evening time, loss of weight, and coughing for several weeks. The chest X-ray does not clearly show the changes in military Tuberculosis, so diagnosis is challenging.47,48
Lymph nodes infections
Children and infants are much more susceptible to infection as they habitually bring their hands to the mouth, leading to microbial exposure. The symptoms vary from person to person, antimicrobial treatments are ineffective, and the last cure is the surgical removal of the lymph node.49
Cutaneous infections
Non-tuberculous mycobacterial infections result in granuloma formation leading to severe infections, ulcer formation, nodules, and cell inflammations in the skin region of arms. Polluted water resources, ill fish, and surgical wounds are important sources of infections. Mycobacterium marinum causes soft tissue infections among people that maintain aquariums.50
Bone and Joints TB
Tuberculosis in the spinal constitutes several vertebral bodies, resulting in the Tuberculosis of multiple organs. In such cases, complete removal of the lesion is quite challenging.51 Antimicrobial treatment is poorly effective when not combined with surgical toilette. Chronic osteomyelitis should not be treated empirically, except in patients with clinical sepsis due to acute recurrence. Antimicrobial therapy based on culture results with susceptibility testing. Traditionally, treatment was started by the iv route. The initial iv therapy is identical in patients with acute or chronic osteomyelitis.52
Lung TB
Non-tuberculous mycobacteria (NTM) have recently become apparent as vital pathogens among cystic fibrosis (C.F.) patients worldwide. The M. abscessus establishment in lung alveoli begins with smooth strains producing glycopeptidolipids and Biofilm, while in the invasive infection, “rough” mutants are responsible for the production of trehalose mycolate and cording formation.53 In a case study on a patient suffering from chronic obstructive pulmonary Tuberculosis, scanning electron microscopy of the lung was conducted where bacilli and Biofilm were observed. Large colonies of bacteria Mycobacterium abscessus in Biofilm were reported.54 Another study showed that patients with cystic fibrosis and chronic pulmonary MABSC (Mycobacterium abscessus complex) infection have mycobacterial biofilms embedded in the alveolar walls of their end-stage lungs.55
Multi-drug resistance- Is biofilm responsible for the recurrence of tuberculosis?
There has been some progress in testing, detection, and treatment of MDR/XDR-TB between 2018 and 2019.1 With the rapidly increasing emergence of multiple drug resistance, treating Tuberculosis has become a significant challenge. Critical factors in biofilm formation identified as drug targets represent a novel and promising platform for developing better antibiotics.56 The drug resistance process produces in three steps. Firstly, the microbes procure resistance genes, followed by the expression of those resistant genes, followed by natural selection for microbes expressing those resistance genes. The bacteria acquire resistance to single and multiple drugs through horizontal gene transfer by transformation, conjugation, and transduction. Microbes can also receive resistance genes by spontaneous mutation of existing genes. Considerable drug resistance is developed when an additional gene is set in the bacteria that already harbors genes resistant to drugs.57-59 Chronic infections and disease recurrence result from antibiotic resistance of bacteria, especially in the microbiome of Biofilm.
Resistance mechanisms of biofilm communities are not similar to the planktonic ones, such as target site mutations, lower cell permeability, efflux pumps, drug modifying enzymes, and drug neutralizing proteins. Biofilm has a property of tolerance to different environmental stress conditions in which antibiotic resistance is also included; this extra property in Biofilm promotes the multiplication of mycobacterial cells.48,60,61 It is also found that mycobacterial biofilms, apart from environmental aggressions, are also resistant to disinfectants, unlike planktonic cells. In the study, it has been found that Biofilm formed by Mycobacterial strains plays a role in the necrosis and cavity formation in the alveolar tissues, resulting in becoming the crucial factor for antimicrobial resistance leading to the failure of the treatment, which, if not eradicated, can cause recurrence of Tuberculosis. A recent study has shown that the M. tuberculosis cyclophilin peptidyl-prolyl isomerase (PpiB), an essential gene, is responsible for biofilm formation and has resistance to anti-tuberculosis drugs. A detailed understanding of Biofilm is hence necessary for the appropriate management of T.B. patients and many NTM diseases.15
Therapeutics to combat biofilm forming mycobacteria, recalcitrant to antibiotics
Natural compounds
Natural compounds exhibiting antibiofilm activity, viz. phenolics, essential oils, terpenoids, lectins, alkaloids, polypeptides, and polyacetylenes that, have high anti-biofilm properties (Table 1). Phenolics class of compounds consists of seven sub-classes as quinones, phenolic acids, flavonoids, flavones, tannins, and ceramics, out of which tannins (especially condensed tannins) possess anti-biofilm activity.19,62,63 The primary mechanism of anti-biofilm action of these phytocompounds is to cause bacterial membrane disruption, inhibit quorum sensing, inhibit the cell-to-matrix adhesion, interact with eukaryotic DNA, and block viral fusion.64,65 Some of the phytocompounds play significant roles in the downregulation of cell adhesion-related genes (Figure 3) which are reported to play an important role in cell adhesion.66
Table (1):
Natural compounds effective against biofilm formation in Mycobacterium
Secondary metabolites OR extracts |
Source |
Mode of Action |
Reference |
|---|---|---|---|
Quinazole |
2-amino quinazoline |
Non-Toxic mechanism |
67 |
Leaf extracts |
P. curatellifolia, (Parinari curatellifolia) |
The presence of saponins, steroids, alkaloids, tannins, flavonoids, and cardiac glycosides did biofilm inhibition. |
69 |
Azadirachta indica |
From Dried leaves |
Disruption of cellular membranes |
70 |
Hippophae rhamnoides |
From Dried berries |
Forms complexes with cell walls and inhibits enzyme activity. They also bind to adhesins and inhibit matrix formation |
70 |
Juglans regia
|
From Dried bark |
Cellular disruption |
70 |
Ophiobolin K |
Emericella variecolor
(Marine fungus) |
Cellular disruption |
71 |
Meridianin D |
Aplidium meridian |
Cause multiple disorders and block mycobacterial cell wall biosynthesis |
67 |
(E)-2-(methyl (phenyl) amino) ethyl 2-(2-hydroxyundecanamido)-7 |
Arisaemasinii Krause |
Disperse the preformed Biofilm |
74 |
Figure 3. Novel approaches in the eradication of the tuberculosis using biofilm inhibition strategies
Quinazole derivatives have been found to inhibit biofilm formation through non-toxic mechanisms. The study resulted in the identification of 2-AQ products with biofilm inhibition activity against M. smegmatis.67 Potentiation of antibiotic action by plant metabolites has been done by inhibiting biofilm formation via quorum sensing, thereby aiding in fighting drug resistance.68
Leaf extract of P. curatellifolia has been found to act as a potential source of phytochemicals that inhibit the growth and biofilm formation of M. smegmatis. Biofilm formation in M. smegmatis was seen to be effectively inhibited by ethanol extract, dichloromethane extract and water extract. The screening of the extract for inhibition of Biofilm was done by micro-broth dilution.69 The efficient reduction and removal of M. smegmatis biofilm has been accomplished using fresh leaves of Azadirachta indica.70 Biofilm disinfection and removal was measured by a modified quantitative spectrophotometric method which allows a rapid detection of concentration-dependent anti-biofilm activity of various agents.71
Ophiobolin K, 6-epi-ophiobolin K, and 6-epi-ophiobolin G isolated from a culture of marine-derived fungus of Emericella varied color inhibit biofilm of M. smegmatis, and it has been reported that Ophiobolin K was also effective against the biofilm formation of M. bovis BCG and was able to restore the antimicrobial activity of isoniazid against M. smegmatis by inhibiting biofilm formation (Table 2). The inhibition of M. smegmatis biofilm has been done physically by using the M63 complete medium containing 0.05 % Tween 80, and the CFU of M. smegmatis was seen to decrease in a time-dependent fashion.72 Meridian D, a marine natural product of marine invertebrate Aplidium, meridian has been reported to inhibit Biofilm formed by M. smegmatis by an unknown mechanism.67 In a study conducted by Paushali et al., it was observed that Biofilm of species of mycobacteria when washed and treated with certain enzymes like cellulase, DNase1, lipase, á amylase, proteinase K had antibiofilm activities in which it was found that cellulase and proteinase K had disrupted mycobacterial biofilms. In contrast, DNase1 had little effect and a-amylase did not affect the mycobacterial Biofilm at all.73
Table (2):
Antibiofilm and antimycobacterial activity of certain compounds
S.N. |
Name of Compound |
Source of Compound |
Action |
References |
|---|---|---|---|---|
1 |
3-O-Methyl-butyl gallate |
Isolated from Loranthus micranthus |
The synergistic approach in the killing of mycobacteria, |
90 |
2 |
Cerulenin |
Isolated from Cephalosporium caerulens |
Inhibited the growth of a variety of mycobacteria species, including multi-drug resistant strains of M. tuberculosis. |
91 |
3 |
Teixobactin |
Isolated from Eleftheria terrae |
Teixobactin inhibited the M. tuberculosis H37Rv (MIC: 0.1 mM) to the cell wall synthesis by binding to a highly-conserved lipids II and III motif. |
92 |
4 |
Platensimycin |
Isolated from S. platensis |
Inhibited mycobacterial cell wall biosynthesis by targeting M.tb. KasA and KasB. |
93 |
5 |
Thiolactomycin |
Isolated from Nocardia species |
Inhibited cell wall biosynthesis by targeting M.tb. KasA and KasB. |
94 |
6 |
Pyridomycin |
Isolated from Streptomyces species |
Pyridomycin competitively inhibited NADH and blocked both the NADH cofactor-and lipid substrate-binding pockets of InhA |
95 |
7 |
Ophiobolin K |
Isolated from Emericella variecolor |
Showed anti-biofilm formation activity against M. smegmatis and M. bovis BCG with MIC values of 4.1 and 8.2 mM. |
96 |
(E)-2-(methyl (phenyl) amino) ethyl 2-(2-hydroxyundecanamido)-7 compound isolated from Arisaemasinii krause (Araceae) is a perennial herb which was identified as the active anti-biofilm component. Biofilm formed by M. smegmatis could be disrupted and inhibited by this component; it could also disperse the performed biofilm.74 Extraction of Sphedamnocarpus pruriens and S. Africana-lutea showed potential biofilm formation inhibition.75 The possible antibiofilm activity of the plant extracts could be due to their chemical constituents. S. Africana-lutea has been reported to have phenolic compounds, which can be complex with bacterial cell walls and disrupt microbial membranes by inhibiting matrix formation.76 Both extracts showed the presence of flavonoids, which have been reported to bind to and inhibit matrix formation.70 Since S. pruriens is from the same family as H. benghalensis, there might be similarities in their phytochemical composition.
Nanoparticles
In the current era of multi-drug resistance, nanomedicine has exhibited significant potential for the global tuberculosis eradication program.77 Nanotechnology-based drug delivery systems can enhance the bioactive potential of therapeutic agents. Promising nanoparticles, such as liposomes, microemulsions, cyclodextrins, solid lipid nanoparticles, polymeric nanoparticles, and metallic nanoparticles, have effectively inhibited microbial biofilms by targeted drug delivery. Using nanoparticles as efflux pump inhibitors assists in reviving the bactericidal effect and biofilm-forming ability of conventional antibiotics.78 Drug delivery by lipid or polymer nanoparticles is deemed a favorable strategy for defeating biofilm resistance.79,80 Day-to-day inventions and research in the field of engineering and science has led to the development in the nanotechnology field. This field of nanotechnology has given several chances to design new biomaterials and surfaces with anti-infective, antifouling, bactericidal, and anti-biofilm properties. Previously Pati et al. conducted a study by using Zinc oxide nanoparticles (ZnO-NPs) along with rifampicin treatment, revealing that it induces intracellular bacterial killing by generating reactive oxygen species and co-localization with BCG and Mycobacterium smegmatis in macrophages respectively.81 Acarbose and cyclosporine-A show a bacteriostatic effect, while gallium nanoparticle tends to show a bactericidal effect along with the disruption of M.tb. H 37Rv biofilm formation and decrease in dosage of anti-tubercular drugs- isoniazid and ethambutol.82 Silver nanoparticles have been found to possess a high negative surface charge, showing colloidal stability under low pH, leading to cell membrane and biofilm disruption.83 In another study conducted by Saifullah et al., graphene oxide was used as a nanocarrier formulation, was loaded with the anti-TB antibiotic ethambutol, and showed antibiofilm and antimycobacterial activity.84
Metal nanomaterials
Silver and gold display bactericidal activity against numerous pathogenic microorganisms. Silver and gold have been used since ancient times because of their medicinal properties. Mostly antimicrobic cream and other gels possess silver and gold to inhibit microbic contamination of injuries.85 Other metal nanoparticles made from zinc, copper, cerium, titanium and their metal oxides show similar clinical benefits. However, their toxic effects on humans and the environment prevail over their benefits and uses. Additionally, current advances in biotechnology, chemistry, and nanotechnology allow the synthesis of AgNPs and AuNPs with cheaper, greener, and simpler methods, as well as their surface modification and fine-tuning for better and synergistic action.86,87
Non-metallic inorganic nanomaterials
The nano-sized non-metallic inorganic material like silicon, proposed to have antiadhesive facilities and fast delivery of antimicrobials to the required location within the oral cavity, have obtained attraction. The innovative ideas established for silicon controls oral biofilms. The nitric oxide (NO)-releasing silica nanoparticles are used to kill biofilm-based microbial cells.88
The alarming situation demands new strategies to inhibit biofilm formation
In brief, the microbial inhabitants explain organized approaches, including biofilms, to resist antibiotics. The antibiotic resistance in Biofilm is because of a specific amalgamation of mechanisms like poor penetration, efflux pump, conjugation of drugs with extra polysaccharides, persisters, and demonstration of genes in reaction to certain environmental stress conditions. The stubborn aspect of biofilms and their antibiotic tolerance enforce a potent challenge to applying older techniques. Most antibacterial agents have obstacles in entering the matrix of the Biofilm. So, the best way to solve the problem of multi-drug resistance is to inhibit biofilm generation using certain other compounds other than antibiotics, which fail to do so. The advantages of using nanosystems for biofilm inhibition applications include high drug loading effectiveness, constant or extended release of drugs, amplified drug steadiness, and better-quality bioavailability. Specific nanoparticles, including metallic-oxide nanoparticles, polymeric, liposomes, and lipid-based nanoparticles, are used as drug delivery and anti-biofilm agents.
Furthermore, nanoparticles can be proposed to be linked with outer incentives to generate magnetic, photothermal, or photodynamic accomplishes to disrupt the extrapolysaccharide matrix of the biofilm.88,89 The use of metallic nanoparticles in treating lung infections may assist in treating Tuberculosis as the metallic nanoparticles penetrate through the macrophages and phagolysosomes, which are loaded with mycobacteria because of their small size and fast movement. Because of the increasing problem of multi-drug resistance in mycobacterium species, the eradication program of Tuberculosis has become more challenging. Due to this resistance, certain novel therapeutics have come into the limelight that have exhibited efficacy in inhibiting Mycobacterium growth. Cost-effective compounds extracted from natural means have shown promising results along with specific nanoparticles. Many mycobacterial species mentioned above, including non-tuberculous mycobacteria and Mycobacterium tuberculosis, induce a chronic course of disease in humans and require a long remission. Tuberculosis infection is associated with biofilm formation in-vivo. The prolonged treatment and toxicity of drugs and the emergence of multidrug-resistant (MDR) strains hampers the development of effective disease control and add to a country’s disease burden, thus increasing its economic burden. Even after the introduction of various modalities of treatment for Tuberculosis, there is still a great void in compliance with anti-tuberculosis drugs and hence a dire need for an alternative method of treatment that not only decreases the mycobacterial-burden but also negatively affects the biofilm-forming ability of Mycobacterium spp. Several studies have exhibited that biofilms, which are formed by the mycolic acids, glycol peptide lipids, cellulose, and extracellular DNA, play an essential role in the development of the resistance by becoming the physical barrier to the effectiveness of first-line as well as second-line TB. drugs and other ions. Hence, some phytochemical or nano-therapeutic intervention(s) are needed to address the challenge posed by biofilm formation and consequent multi-drug resistance emerging in mycobacterial strains. Another primary requirement for research against TB. is a better understanding of the mechanisms adopted by mycobacteria to develop chronic infection wherein Mycobacteria eludes both the host immune response and antibiotic treatment.
ACKNOWLEDGMENTS
The authors would like to thank Integral University, Lucknow, India, for providing the manuscript communication number IU/R&D/2022-MCN0001449.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RS conceptualized the study and performed supervision. NP applied methodology. Performed investigation, data interpretation and formal analysis. NP wrote the manuscript. KS, FA; PP and RS reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Global tuberculosis report. 2020. https://www.who.int/publications-detail-redirect/9789240013131. Accessed November 22, 2020.
- Chen S, Teng T, Wen S, Zhang T, Huang H. The aceE involves in mycolic acid synthesis and biofilm formation in Mycobacterium smegmatis. BMC Microbiol. 2020;20(1):259.
Crossref - Wang C, Zhang Q, Tang X, et al. Effects of CwlM on autolysis and biofilm formation in Mycobacterium tuberculosis and Mycobacterium smegmatis. Int J Med Microbiol. 2019;309(1):73-83.
Crossref - Pang JM, Layre E, Sweet L, et al. The Polyketide Pks1 Contributes to Biofilm Formation in Mycobacterium tuberculosis. J Bacteriol. 2012;194(3):715-721.
Crossref - Chakraborty P, Kumar A. The extracellular matrix of mycobacterial biofilms: could we shorten the treatment of mycobacterial infections? Microb Cell. 2019;6(2):105-122.
Crossref - Costerton JW, Geesey GG, Cheng KJ. How bcteria stick. Sci Am. 1978;238(1):86-95.
Crossref - Löwenstein E. Vorlesungen Über Bakteriologie, Immunität, Spezifische Diagnostik Und Therapie Der Tuberkulose. Gustav Fischer Verlag; 1920. Accessed September 19, 2023. https://agris.fao.org/agris-search/ search.do?recordID=US201300338650.
- Calmette A. L’infection Bacillaire et La Tuberculose Chez l’homme et Chez Animaux; Processus d’infection et de Defense; Étude Biologique et Expérimentale. Masson; 1920. Accessed September 19, 2023. https://agris.fao.org/agris-search/search. do?recordID=US201300661759
- Koch R. The Etiology of Tuberculosis. Rev Infect Dis. 1982;4(6):1270-1274.
Crossref - Keren-Paz A, Kolodkin-Gal I. A brick in the wall: Discovering a novel mineral component of the biofilm extracellular matrix. N Biotechnol. 2020;56:9-15.
Crossref - Dubos RJ, Davis BD. Factors affecting the growth of tubercle bacilli in liquid media. J Exp Med. 1946;83(5):409-423.
Crossref - Cynthia P, Dubos RJ, Middlebrook G. Infection of Mice with Tubercle Bacilli Grown in Tween-Albumin Liquid Medium. Proc Soc Exp Biol Med. 1947;64(2):173-174.
Crossref - Solokhina A, Bruckner D, Bonkat G, Braissant O. Metabolic activity of mature biofilms of Mycobacterium tuberculosis and other non-tuberculous mycobacteria. Sci Rep. 2017;7(1):9225.
Crossref - Esteban J, Garcia-Coca M. Mycobacterium Biofilms. Front Microbiol. 2018;8:2651.
Crossref - Basaraba RJ, Ojha AK. Mycobacterial Biofilms: Revisiting Tuberculosis Bacilli in Extracellular Necrotizing Lesions. Tuberculosis and the Tubercle Bacillus. 2017:533-539.
Crossref - Hall-Stoodley L, Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005;13(1):7-10.
Crossref - Costerton JW, Stewart PS, Greenberg EP. Bacterial Biofilms: A Common Cause of Persistent Infections. Science. 1999;284(5418):1318-1322.
Crossref - Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9(6):588-594.
Crossref - Yong YY, Dykes GA, Choo WS. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit Rev Microbiol. 2019;45(2):201-222.
Crossref - Fatima F, Siddiqui S, Khan WA. Nanoparticles as Novel Emerging Therapeutic Antibacterial Agents in the Antibiotics Resistant Era. Biol Trace Elem Res. 2021;199(7):2552-2564.
Crossref - Lee NY, Ko WC, Hsueh PR. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front Pharmacol. 2019;10:1153.
Crossref - Waseem M, Nisar MA. Fungal-Derived Nanoparticles as Novel Antimicrobial and Anticancer Agents. IntechOpen. 2016.
Crossref - Beyth N, Houri-Haddad Y, Domb A, Khan W, Hazan R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid Based Complement Alternat Med. 2015;e246012.
Crossref - Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227-1249.
Crossref - Zhao Y, Jiang X. Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale. 2013;5(18):8340-8350.
Crossref - Zazo H, Colino CI, Lanao JM. Current applications of nanoparticles in infectious diseases. J Control Release. 2016;224:86-102.
Crossref - Menozzi FD, Rouse JH, Alavi M, et al. Identification of a heparin-binding hemagglutinin present in mycobacteria. J Exp Med. 1996;184(3):993-1001.
Crossref - Sousa S, Bandeira M, Carvalho PA, Duarte A, Jordao L. Nontuberculous mycobacteria pathogenesis and biofilm assembly. Int J Mycobacteriol. 2015;4(1):36-43.
Crossref - Vess RW, Anderson RL, Carr JH, Bond WW, Favero MS. The colonization of solid PVC surfaces and the acquisition of resistance to germicides by water micro-organisms. J Appl Bacteriol. 1993;74(2):215-221.
Crossref - Ridgway HF, Rigby MG, Argo DG. Adhesion of a Mycobacterium sp. to cellulose diacetate membranes used in reverse osmosis. Appl Environ Microbiol. 1984;47(1):61-67.
Crossref - Zamora N, Esteban J, Kinnari TJ, Celdrán A, Granizo JJ, Zafra C. In-vitro evaluation of the adhesion to polypropylene sutures of non-pigmented, rapidly growing mycobacteria. Clin Microbiol Infect. 2007;13(9):902-907.
Crossref - Martin-de-Hijas NZ, Garcia-Almeida D, Ayala G, et al. Biofilm development by clinical strains of non-pigmented rapidly growing mycobacteria. Clin Microbiol Infect. 2009;15(10):931-936.
Crossref - Recht J, Kolter R. Glycopeptidolipid Acetylation Affects Sliding Motility and Biofilm Formation in Mycobacterium smegmatis. J Bacteriol. 2001;183(19):5718-5724.
Crossref - Ojha A, Anand M, Bhatt A, Kremer L, Jacobs WR, Hatfull GF. GroEL1: A Dedicated Chaperone Involved in Mycolic Acid Biosynthesis during Biofilm Formation in Mycobacteria. Cell. 2005;123(5):861-873.
Crossref - Ciofu O, Moser C, Jensen Po, Hoiby N. Tolerance and resistance of microbial biofilms. Nat Rev Microbiol. 2022;20(10):621-635.
Crossref - Alhede M, Alhede M, Qvortrup K, et al. The origin of extracellular DNA in bacterial biofilm infections in vivo. Pathogens and Disease. 2020;78(2):ftaa018.
Crossref - Luo C, Liu H, Xie F, et al. Single nucleus multi-omics identifies human cortical cell regulatory genome diversity. Cell Genomics. 2022;2(3):100107.
Crossref - Chen RE, Zhang X, Case JB, et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med. 2021;27(4):717-726.
Crossref - Sivaloganathan DM, Brynildsen MP. Quantitative Modeling Extends the Antibacterial Activity of Nitric Oxide. Front Physiol. 2020;11:330.
Crossref - Carter G, Wu M, Drummond DC, Bermudez LE. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J Med Microbiol. 2003;52(9):747-752.
Crossref - Iii JOF. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol. 2009;107(2):356-367.
Crossref - Esteban J, Martin-de-Hijas NZ, Kinnari TJ, Ayala G, Fernandez-Roblas R, Gadea I. Biofilm development by potentially pathogenic non-pigmented rapidly growing mycobacteria. BMC Microbiol. 2008;8:184.
Crossref - Gambino M, Cappitelli F. Mini-review: Biofilm responses to oxidative stress. Biofouling. 2016;32(2):167-178.
Crossref - Faezi Ghasemi M, Moslem M, Mirpour M. Effect of Environmental Stresses on Growth Pattern, Biofilm Formation and Biochemical Characteristics of Mycobacterium marinum CCUG20998. Zahedan Journal of Research in Medical Sciences. 2016;18(9):e2664.
Crossref - Yin W, Wang Y, Liu L, He J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int J Mol Sci. 2019;20(14):3423.
Crossref - Lindeboom JA, Coppenraet LESB van, Soolingen D van, Prins JM, Kuijper EJ. Clinical Manifestations, Diagnosis, and Treatment of Mycobacterium haemophilum Infections. Clin Microbiol Rev. 2011;24(4):701-717.
Crossref - Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res. 2012;135(5):703-730.
- Sharma D, Misba L, Khan AU. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control. 2019;8(1):76.
Crossref - Lindeboom JA, Kuijper EJ, Bruijnesteijn van Coppenraet ES, Lindeboom R, Prins JM. Surgical Excision versus Antibiotic Treatment for Nontuberculous Mycobacterial Cervicofacial Lymphadenitis in Children: A Multicenter, Randomized, Controlled Trial. Clin Infect Dis. 2007;44(8):1057-1064.
Crossref - Blackwell V. Mycobacterium marinum infections. Curr Opin Infect Dis. 1999;12(3):181-184.
Crossref - Wang B, Kong L, Zhu Z, et al. Recurrent complex spinal tuberculosis accompanied by sinus tract formation: causes of recurrence and clinical treatments. Sci Rep. 2018;8(1):6933.
Crossref - Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125(4):353-364.
Crossref - Degiacomi G, Sammartino JC, Chiarelli LR, Riabova O, Makarov V, Pasca MR. Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients. Int J Mol Sci. 2019;20(23):5868.
Crossref - Fennelly KP, Ojano-Dirain C, Yang Q, et al. Biofilm Formation by Mycobacterium abscessus in a Lung Cavity. Am J Respir Crit Care Med. 2016;193(6):692-693.
Crossref - Qvist T, Eickhardt S, Kragh KN, et al. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur Respir J. 2015;46(6):1823-1826.
Crossref - Xiang X, Deng W, Liu M, Xie J. Mycobacterium biofilms: factors involved in development, dispersal, and therapeutic strategies against biofilm-relevant pathogens. Crit Rev Eukaryot Gene Expr. 2014;24(3):269-279.
Crossref - Hajipour MJ, Fromm KM, Akbar Ashkarran A, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499-511.
Crossref - Jayaraman R. Antibiotic resistance: an overview of mechanisms and a paradigm shift. Curr Sci. 2009;96(11):1475-1484.
- Ganjian H, Nikokar I, Tieshayar A, Mostafaei A, Amirmozafari N, Kiani S. Effects of Salt Stress on the Antimicrobial Drug Resistance and Protein Profile of Staphylococcus aureus. Jundishapur J Microbiol. 2012;5(1):328-331.
Crossref - Richards JP, Cai W, Zill NA, Zhang W, Ojha AK. Adaptation of Mycobacterium tuberculosis to Biofilm Growth Is Genetically Linked to Drug Tolerance. Antimicrob Agents Chemother. 2019;63(11):e01213-19.
Crossref - Mabhula A, Singh V. Drug-resistance in Mycobacterium tuberculosis: where we stand. Med Chem Commun. 2019;10(8):1342-1360.
Crossref - Mishra R, Panda AK, De Mandal S, Shakeel M, Bisht SS, Khan J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front Microbiol. 2020;11:56625.
Crossref - Trentin D da S, Giordani RB, Zimmer KR, et al. Potential of medicinal plants from the Brazilian semi-arid region (Caatinga) against Staphylococcus epidermidis planktonic and biofilm lifestyles. J Ethnopharmacol. 2011;137(1):327-335.
Crossref - Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564-582.
Crossref - Lu L, Hu W, Tian Z, et al. Developing natural products as potential anti-biofilm agents. Chinese Medicine. 2019;14(1):11.
Crossref - Adnan M, Patel M, Deshpande S, et al. Effect of Adiantum philippense Extract on Biofilm Formation, Adhesion With Its Antibacterial Activities Against Foodborne Pathogens, and Characterization of Bioactive Metabolites: An in vitro-in silico Approach. Front Microbiol. 2020;11:823.
Crossref - M. Brackett S, E. Cox K, L. Barlock S, et al. Meridianin D analogues possess antibiofilm activity against Mycobacterium smegmatis. RSC Med Chem. 2020;11(1):92-97.
Crossref - Singh B, Jain M, Singh S, et al. Plants as Future Source of Anti-Mycobacterial Molecules and Armour for Fighting Drug Resistance. Asian J Anim Vet Adv. 2015;10(9):443-460.
Crossref - Bhunu B, Mautsa R, Mukanganyama S. Inhibition of biofilm formation in Mycobacterium smegmatis by Parinari curatellifolia leaf extracts. BMC Complement Altern Med. 2017;17(1):285.
Crossref - Pitts B, Hamilton MA, Zelver N, Stewart PS. A microtiter-plate screening method for biofilm disinfection and removal. J Microbiol Methods. 2003;54(2):269-276.
Crossref - Abidi SH, Ahmed K, Sherwani SK, Bibi N, Kazmi SU. Detection of Mycobacterium Smegmatis Biofilm and its Control by Natural Agents. Int J Curr Microbiol App Sci. 2014;3(4):801-812.
- Arai M, Niikawa H, Kobayashi M. Marine-derived fungal sesterterpenes, ophiobolins, inhibit biofilm formation of Mycobacterium species. J Nat Med. 2013;67(2):271-275.
Crossref - Chakraborty P, Bajeli S, Kaushal D, Radotra BD, Kumar A. Biofilm formation in the lung contributes to virulence and drug tolerance of Mycobacterium tuberculosis. Nat Commun. 2021;12(1):1606.
Crossref - Jiang CH, Gan ML, An TT, Yang ZC. Bioassay-guided isolation of a Mycobacterium tuberculosis bioflim inhibitor from Arisaema sinii Krause. Microbial Pathogenesis. 2019;126:351-356.
Crossref - Oosthuizen CB, Gasa N, Hamilton CJ, Lall N. Inhibition of mycothione disulphide reductase and mycobacterial biofilm by selected South African plants. S Afr J Bot. 2019;120:291-297.
Crossref - Kamatou GPP, Zy RL van, Vuuren SF van, et al. Biological activities of South African Salvia species and isolated compounds. Planta Med. 2007;73(9):149.
Crossref - Minakshi P, Ghosh M, Brar B, et al. Nano-antimicrobials: A New Paradigm for Combating Mycobacterial Resistance. Curr Pharm Des. 2019;25(13):1554-1579.
Crossref - Gupta D, Singh A, Khan AU. Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res Lett. 2017;12(1):454.
Crossref - Dos Santos Ramos MA, Da Silva PB, Sposito L, et al. Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. Int J Nanomed. 2018;13:1179-1213.
Crossref - Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607-623.
Crossref - Pati R, Mehta RK, Mohanty S, et al. Topical application of zinc oxide nanoparticles reduces bacterial skin infection in mice and exhibits antibacterial activity by inducing oxidative stress response and cell membrane disintegration in macrophages. Nanomed: Nanotechnol Biol Med. 2014;10(6):1195-1208.
Crossref - Kumar A, Alam A, Grover S, et al. Peptidyl-prolyl isomerase-B is involved in Mycobacterium tuberculosis biofilm formation and a generic target for drug repurposing-based intervention. NPJ Biofilms and Microbiomes. 2019;5(1):1-11.
Crossref - Estevez MB, Raffaelli S, Mitchell SG, Faccio R, Alborés S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules. 2020;25(9):2023.
Crossref - Saifullah B, Chrzastek A, Maitra A, et al. Novel Anti-Tuberculosis Nanodelivery Formulation of Ethambutol with Graphene Oxide. Molecules. 2017;22(10):1560.
Crossref - Mohanta YK, Biswas K, Jena SK, Hashem A, Abd_Allah EF, Mohanta TK. Anti-biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front Microbiol. 2020;11:1143.
Crossref - Barabadi H, Mojab F, Vahidi H, et al. Green Synthesis, Characterization, Antibacterial and Biofilm Inhibitory Activity of Silver Nanoparticles Compared to Commercial Silver Nanoparticles. Inorg Chem Commun. 2021;129:108647.
Crossref - Chatterjee A, Mridha D, Banerjee J, et al. Green synthesis of iron oxide nanoparticles and their ameliorative effect on arsenic stress relief in Oryza sativa seedlings. Biocatal Agric Biotechnol. 2021;38:102207.
Crossref - Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-Biofilm Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. Biomaterials. 2009;30(14):2782-2789.
Crossref - Lin YK, Yang SC, Hsu CY, Sung JT, Fang JY. The Antibiofilm Nanosystems for Improved Infection Inhibition of Microbes in Skin. Molecules. 2021;26(21):6392.
Crossref - Mishra SK, Tripathi G, Kishore N, Singh RK, Singh A, Tiwari VK. Drug development against tuberculosis: Impact of alkaloids. Eur J Med Chem. 2017;137:504-544.
Crossref - Mahboub BH, Vats MG. Tuberculosis – Current Issues in Diagnosis and Management. 2013.
Crossref - Ling LL, Schneider T, Peoples AJ, et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517(7535):455-459.
Crossref - Brown AK, Taylor RC, Bhatt A, Fütterer K, Besra GS. Platensimycin Activity against Mycobacterial â-Ketoacyl-ACP Synthases. Plos ONE. 2009;4(7):e6306.
Crossref - Rieder RJ, Zhao Z, Zavizion B. New Approach for Drug Susceptibility Testing: Monitoring the Stress Response of Mycobacteria. Antimicrob Agents Chemother. 2009;53(11):4598-4603.
Crossref - Hartkoorn RC, Pojer F, Read JA, et al. Pyridomycin bridges the NADH- and substrate-binding pockets of the enoyl reductase InhA. Nat Chem Biol. 2014;10(2):96-98.
Crossref - Wellington S, Hung DT. The Expanding Diversity of Mycobacterium tuberculosis Drug Targets. ACS Infect Dis. 2018;4(5):696-714.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.