ISSN: 0973-7510
E-ISSN: 2581-690X
Staphylococcal foodborne illness, caused by the ingestion of contaminated foods by induced Staphylococcus aureus enterotoxins, is one of the most recurrent foodborne diseases worldwide. Food handlers working in food-service establishments who carry enterotoxigenic isolates represent a reservoir for potential contamination leading to staphylococcal food intoxication. The aim of this research was to investigate the prevalence of nasal transmission of S. aureus among food handlers in a University community. A total of 100 nasal swab specimens were obtained from the food handlers and analyzed by standard laboratory techniques for isolation and identification. Questionnaires were administered to ascertain the risk determinants associated with nasal carriage of S. aureus. Antibiotic susceptibility testing of the isolates was done by disk diffusion method. Thirty-two food handlers were found to be carriers of S. aureus. Majority were females (63%), and 30% had been certified for food safety and handling. There were however no significant association between the nasal distribution of S. aureus together with established risk factors. Among the isolates, 93.75% were found to be resistant to penicillin, 50% to gentamicin and 50% to oxacillin. These findings indicate the need for training of food handlers on food safety, as well as the knowledge of antibiotic resistance.
Staphylococcal Foodborne Illness, Prevalence, Food Handlers, Hand Swabs, University Community
Food-borne illness has been one of the major health challenges of developed and developing countries worldwide, with up to 2 million deaths estimated in the former.1,2 Microbial presence or its metabolites in food products is one of the predominant problems in the food industry with significant menace to public health.3 One of the main causes of infections is Staphylococcus aureus a pathogen of global spread and increasing resistance to antimicrobials.4 Staphylococcal gastroenteritis (Staphylococcal Food Poisoning―SFP), one of the most common food-borne ailments results from the ingestion of one or more staphylococcal enterotoxins (SEs) in foods contaminated with the bacterium. This is due to the potential of the bacterium to generate more than 22 dissimilar enterotoxins, among which five of them have been established as a paramount cause of 95% of staphylococcal food poisoning occurrences.5,6,7 Another contributing factor is the poor personal hygiene of food-handlers functioning in food-service establishments (cafeterias and restaurants). As a consequence of colonization or skin infections of food handlers, contamination of the hands or dissipation through sneezing or coryza, there is a continual contamination of foods by Staphylococcus aureus. The ability of this bacterium to produce enterotoxins, depends on the method of storage or preservation of foods which may affect its metabolic activity in the food.1 Other species of Staphylococcus can retain enterotoxigenic genes, but they are only infrequently implicated in staphylococcal food poisoning epidemics. Carriers of antibiotic resistant Staphylococcus aureus which are enterotoxigenic will enable its continued resistance to one or more antimicrobial agents and its proliferation in the community.8 Given the need for a practical understanding of Staphylococcus aureus as a high-level foodborne pathogen, a major concern is the widespread under-reporting of diseases associated with food consumption in developing communities. This study is focused on determining the prevalence, risk factors and susceptibility pattern of Staphylococcus aureus obtained from food handlers to a range of selected antibiotics.
Designated Study Areas and Population
A cross-sectional study was conducted in the food utility establishments within the Covenant University community including cafeterias (3), butteries (17) and restaurants (1). Food handlers working in the study area, who had given informed consent, were involved in the study. The study was coordinated from January to April 2018. A self-administered questionnaire was completed by food handlers to obtain data on the hygiene practice and endangering factors associated with the nasal carriage of Staphylococcus aureus. Several variables were investigated as potential risk factors of S. aureus nasal carriage. These include certain general socio-demographic variables such as age, sex and ethnicity, hygiene practices, disease history, smoking habits, daily contact with animals, sporting activities, individual hospitalization/ hospitalization of household member, incidence of chronic sinusitis, rhinitis or skin diseases, use of antibiotics in the past 6 months and frequent skin puncture.
Sample Collection and Identification of Isolates
A total of 100 nasal swab samplings were procured from food handlers in the study areas and cultured on mannitol salt agar. Characteristic colonies were confirmed by Gram staining, catalase test, coagulase test and culture on blood agar for characteristic beta hemolysis of S. aureus according to Emeakaroha et al.9
Antimicrobial Susceptibility Testing (AST)
S. aureus isolates were analysed for antibiotic sensitivity by applying the Kirby-Bauer disk diffusion method in line with CLSI (Clinical and Laboratory Standards Institute) guidelines.10 The antimicrobial drugs tested include oxacillin (5µg), gentamicin (30µg), ciprofloxacin (5µg), sulphamethoxazole/trimethoprim (25µg), penicillin (10µg), erythromycin (15µg) and tetracycline (30µg).
Data Analysis
Data obtained were evaluated using IBM SPSS (Statistical Package for the Social Sciences) version 20.0 Software and chi-square (X2). A P value of ≤ 0.05 was considered statistically significant.
A total of 39 staphylococcal isolates were identified from the 100 samples collected, out of which 32 (32%) were Staphylococcus aureus and 7 were coagulase negative staphylococci. Based on gender difference, female food handlers (63%) were more compared to the males (37%). Young adults within the age of 18-19 (59%), workers with service experience of 1-10 years (59%) and high school leavers (44%) dominated the population of food handlers. The pedagogic levels, age category, sex, marital status and service years in relation to isolated identified are presented in Table 1. Based on hand washing practices, 91% of food handlers routinely cleaned hands with soap and water, 9% used only water for handwashing after using the toilet, 91% washed hands before preparing/serving food and 80% reported a habit of hand washing after touching nose in the middle of handling food items. Only 50% of the food handlers had received training in food safety, preparation and handling (Table 2). There was no significant association between potential risk factors investigated in connection to nasal carriage of S. aureus as shown in Table 3.
Table (1):
Socio-demographic characteristics of food handlers.
| Staphylococci | ||||||
|---|---|---|---|---|---|---|
| Absent | Present | Total | ||||
| Count | % | Count | % | Count | ||
| Gender | Male | 21 | 56.76% | 16 | 43.24% | 37 |
| N=100 | Female | 40 | 63.49% | 23 | 36.51% | 63 |
| Age Group | 18-29 | 31 | 52.54% | 28 | 47.46% | 59 |
| N=100 | 30-50 | 9 | 75.00% | 3 | 25.00% | 12 |
| 50-70 | 21 | 72.41% | 8 | 27.59% | 29 | |
| Education | Elementary | 6 | 75.00% | 2 | 25.00% | 8 |
| N=98 | High school | 29 | 65.91% | 15 | 34.09% | 44 |
| Tertiary | 26 | 56.52% | 20 | 43.48% | 46 | |
| Marital Status | Single | 36 | 55.38% | 29 | 44.62% | 65 |
| N=100 | Married | 25 | 71.43% | 10 | 28.57% | 35 |
| Years of Practice | <1 | 15 | 51.72% | 14 | 48.28% | 29 |
| N=95 | 1-10 | 41 | 69.49% | 18 | 30.51% | 59 |
| 11-15 | 2 | 66.67% | 1 | 33.33% | 3 | |
| >15 | 3 | 75.00% | 1 | 25.00% | 4 | |
Table (2):
Hygiene practices of food handlers.
| Staphylococci | OR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Absent | Present | Total Count | |||||
| Count | % | Count | % | ||||
| Received | Yes | 19 | 63.33% | 11 | 36.67% | 30 | 1.152 |
| Certification | No | 42 | 60.00% | 28 | 40.00% | 70 | (0.476-2.785) |
| Method of hand | Using water only | 4 | 44.44% | 5 | 55.56% | 9 | 2.096 |
| washing after | Using soap & | 57 | 62.64% | 34 | 37.36% | 91 | (0.526-8.344) |
| using the toilet | water (ref) | ||||||
| Wash hands after | Yes (ref) | 51 | 63.75% | 29 | 36.25% | 80 | 1.583 |
| touching nose | No | 10 | 52.63% | 9 | 47.37% | 19 | (0.577-4.343) |
| Wash hands before | Yes | 58 | 63.74% | 33 | 36.26% | 91 | 2.929 |
| preparing food | No(ref) | 3 | 37.50% | 5 | 62.50% | 8 | (0.658-13.047) |
Table (3):
Prevalence of S. aureus in relation to established risk factors.
| Staphylococci | OR 95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Absent | Present | Total Count | |||||
| Count | % | Count | % | ||||
| History of Sinusitis | Yes | 8 | 80.00% | 2 | 20.00% | 10 | 0.368 |
| No | 53 | 59.55% | 36 | 40.45% | 89 | (0.074-1.834) | |
| History of Rhinitis | Yes | 12 | 70.59% | 5 | 29.41% | 17 | 1.616 |
| No | 49 | 59.76% | 33 | 40.24% | 82 | (0.521-5.018) | |
| Skin Disease | Yes | 3 | 60.00% | 2 | 40.00% | 5 | NC |
| No | 58 | 61.05% | 37 | 38.95% | 95 | ||
| History of hospitalization | Yes | 3 | 42.86% | 4 | 57.14% | 7 | NC |
| in the past 6 months | No | 58 | 62.37% | 35 | 37.63% | 93 | |
| Used antibiotics in the | Yes | 25 | 64.10% | 14 | 35.90% | 39 | 1.240 |
| past 6 months | No | 36 | 59.02% | 25 | 40.98% | 61 | (0.541-2.843) |
| Frequent skin puncture | Yes | 1 | 100.00% | 0 | 0.00% | 1 | NC |
| No | 60 | 60.61% | 39 | 39.39% | 99 | ||
| Contact with animals | Yes | 19 | 65.52% | 10 | 34.48% | 29 | 1.312 |
| No | 42 | 59.15% | 29 | 40.85% | 71 | (0.533-3.227) | |
| Household member | Yes | 13 | 59.09% | 9 | 40.91% | 22 | 0.873 |
| who has contact with animals | No | 48 | 62.34% | 29 | 37.66% | 77 | (0.332-2.295) |
| Household member who | Yes | 20 | 64.52% | 11 | 35.48% | 31 | 1.242 |
| is a healthcare worker | No | 41 | 59.42% | 28 | 40.58% | 69 | (0.516-2.990) |
| Household member | Yes | 6 | 66.67% | 3 | 33.33% | 9 | NC |
| admitted to the hospital | No | 54 | 60.00% | 36 | 40.00% | 90 | |
| in the past year | |||||||
| Participate in close | Yes | 30 | 63.83% | 17 | 36.17% | 47 | 1.195 |
| contact sport | No | 31 | 59.62% | 21 | 40.38% | 52 | (0.530-2.695) |
NC- Not computed because one category contains too few respondents.
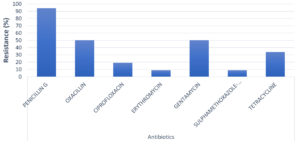
The results of the antimicrobial testing showed that 93.75% of S. aureus were resistant to Penicillin, 50% to Gentamycin and 50% to Oxacillin (Table 4). However, 84.38% of the isolates were susceptible to Erythromycin and Trimethoprim-Sulphamethoazole (Figure).
Table (4):
Antibiotic Resistance Pattern of S. aureus Isolates.
| Antibiotics | Staphylococcus aureus (n=32) | ||
|---|---|---|---|
| Resistance n (%) | Intermediate n (%) | Sensitive n (%) | |
| Penicillin G | 30 (93.75) | 0 (0) | 2 (6.25) |
| Oxacillin | 16 (50) | 2 (6.25) | 14 (43.75) |
| Ciprofloxacin | 6 (18.75) | 2 (6.25) | 24 (75.00) |
| Erythromycin | 3 (9.38) | 2 (6.25) | 27 (84.38) |
| Gentamycin | 16 (50) | 0 (0) | 16 (50) |
| Sulphamethoxazole- | |||
| Trimethoprim | 3 (9.38) | 2 (6.25) | 27 (84.38) |
| Tetracycline | 11 (34.38) | 3 (9.38) | 18 (56.25) |
The overall prevalence rate of 82% S. aureus isolates of this study is in line with previous report of 37.14% of S. aureus isolated among food handlers in Obafemi Awolowo University,11 but in contrast to the 20.5% of isolates among food handlers in Gondar University 1 and 21.2% of nasal carriage according to Simsek et al12 In this study, no relation was found between the nasal carriage of S. aureus and sex, age, education, marital status, present work location and service years (P > 0.05). This corresponds to Simsek et al.12, Dagnew et al.1 and Getenet et al.13 where the frequency of the nasal transfer of S. aureus was independent of the sociodemographic variables of the food handlers. Hygiene practices that include method of hand wash after using the toilet, after touching the nose, before preparing/serving food and frequency of hand wash had no association to the carriage of S. aureus.
With only 36.67% of the food handlers who were carriers of S. aureus having been trained with a certification in food safety preparation and handling, there is the need for the training of all individuals in food services. In the findings of Nasrolahei et al,14 the rate of bacterial contamination of foods by food handlers was high despite the practice of annual check–up thus portraying poor personal and environmental hygiene as well as ignorance of health promotion practices. There was negative significant association between the nasal carriage of S. aureus and the risk factors investigated in this study. Additionally, there was no significant association between the isolates and sample collection areas. There is nonetheless the need for the enforcement of basic hygiene practices among food handlers, as they serve as reservoirs for staphylococci, which could result in food intoxication.
Among the isolates obtained, there was a 93.75% resistance of S. aureus to Penicillin, in line with report that 90% of staphylococcal isolates and specifically 91% from the human nasal passage are resistant to penicillin.15 The 50% resistance of S. aureus to Gentamicin found in this study is in contrast to the 25.58% resistance reported by Alsamarai et al16 and complete susceptibility as reported by Achek et al.17 The 18.75% resistance observed for Ciprofloxacin by S. aureus is in contrast to the 0%, 6.97% and 9.8% reported in the studies of Wolde et al.18, Alsamarai et al16 and Dagnew et al.1 respectively. Similar report by Akinduti et al.19 revealed the resistance of S. aureus to some antibiotics including Penicillin, Ciprofloaxin among others.
The findings of this study revealed that food handlers with antibiotic-resistant S. aureus may constitute significant risk to the end users, enabling the spread of drug resistant S. aureus infections. Therefore, implementation of regular food handlers’ training on food safety, regular medical examination and consistent observation of personal hygiene of food handlers is of optimum necessity.
ACKNOWLEDGMENTS
All the authors wish to acknowledge the financial support provided by Covenant University, Nigeria for the publication of this research work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
OOA conceived and designed the experimental study. ANW collected the samples, isolated and characterised the bacteria. OOA and ANW analysed and presented the results in figure and tables. OOA, ANW, MIO and BOO prepared and edited the manuscript. All the authors read and approved the final manuscript for publication.
FUNDING
This work was supported by the Covenant University Centre for Research Innovation and Discovery (CUCRID), Nigeria.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Covenant Research Ethics Committee (CHREC), Covenant University, Ota, Ogun State, Nigeria (CU/BIOSCREC/BIO/2016/016).
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Dagnew M, Tiruneh M, Moges F, Tekeste Z. Survey of nasal carriage of Staphylococcus aureus and intestinal parasites among food handlers working at Gondar University, Northwest Ethiopia. BMC Public Health. 2012;12(1):837.
Crossref - Abebe E, Gugsa G, Ahme M. Review on major food-borne zoonotic bacterial pathogens. J Trop Med. 2020;2020:4674235.
Crossref - Nwinyi OC, Obehi E, Adesina T, Oniha MI, Olopade B. Antibiotic susceptibility patterns of bacteria species isolated from ice-cream vended in Ota and Lagos Metropolis. Res J Microbiol. 2017;12(1):50-57.
Crossref - Akinnola OO, Ogunleye BO, Odewunmi RT, Ajayi AS. Prevalence of Staphylococcus aureus on the hands of health-care workers and the environment of a Nigerian University Health Centre. Trop J Nat Prod Res. 2020;4(11):884-886
- Jay JM, Loessner MJ, Golden DA. Staphylococcal gastroenteritis. Food Microbiol. 2005;545-566
- Argudin MA, Mendoza MC, Rodicio MR. Food poisoning and Staphylococcus aureus enterotoxins Toxins. 2010;2(7):1751-1773.
Crossref - Argudin MA, Mendoza MC, Gonzalez-Hevia MA, Bances M, Guerra B, Rodicio MR. Genotypes, exotoxin gene content, and antimicrobial resistance of Staphylococcus aureus strains recovered from foods and food handlers. Appl Environ Microbiol. 2012;78(8):2930-2935.
Crossref - Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol. 2020;10:107.
Crossref - Emeakaroha MC, Nkwocha IG, Adieze NC, Adieze IE. Antimicrobial susceptibility pattern of Staphylococcus aureus, and their nasal and throat carriage among food handlers at the Federal University of Technology, Owerri Nigeria. International Journal of Biomolecules and Biomedicine. 2017;6(3):1-7.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests. 12th Edition, Clinical and Laboratory Standards Institute, Wayne, PA. 2017
- Omololu-Aso J, Omololu-Aso O O and Otusanya O O 2017 Nasal Colonization of Methicillin Resistance Staphylococcus aureus among Food Handlers in the Eateries Obafemi Awolowo University Ile Ife, Nigeria. J Clin Nutr Diet. 2017;3:1-4.
Crossref - Simsek Z, Koruk I, Copur AC, Gurses G. Prevalence of Staphylococcus aureus and intestinal parasites among food handlers in Sanliurfa, Southeastern Anatolia JPHMP. 2009;15(6):518-523.
Crossref - Nasrolahei M, Mirshafiee S, Kholdia S, Salehiana M, Nasrolaheia M. Bacterial assessment of food handlers in Sari City, Mazandaran Province, north of Iran. J Infect Public Health. 2017;10(2):171-176.
Crossref - Getenet B, Girma M, Tesfaye K, Getnet T, Seid TM. Nasal and hand carriage rate of Staphylococcus aureus among food handlers working in Jimma Town, Southwest Ethiopia. Ethiop J Health Sci. 2019;29(5):605-612.
Crossref - Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clinc Invest. 2003;111(9):1265-1273.
Crossref - Alsamarai AM, Abbas HM, Atia QM. Nasal carriage of methicillin resistant S. aureus in food provider in restaurant at Samara city. World Journal of Pharmacy and Pharmaceutical Sciences. 2015;4(6):50-55.
- Achek R, Hotzel H, Cantekin Z, et al. Emerging of antimicrobial resistance in staphylococci isolated from clinical and food samples in Algeria. BMC Res Notes. 2018;11(1):663.
Crossref - Wolde T, Abate M, Mehari L. Prevalence and Antibiotics Resistance Pattern of Staphylococcus aureus among Food Handlers of Jigjiga University Student’s Cafeteria. Age. 2016;19(30):10.
- Akinduti AP, Osiyemi JA, Banjo TT, et al. Clonal diversity and spatial dissemination of multiantibiotics resistant Staphylococcus aureus pathotypes in Southwest Nigeria. PLoS ONE. 2021;16(2):e0247013.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.