ISSN: 0973-7510
E-ISSN: 2581-690X
This study aimed to investigate the effect of potential metabolite(s) produced by Paraburkholderia spp. isolated from the Rhizopogon roseolus (shouro mushroom) sporocarp on the mycelial growth of R. roseolus. For this purpose, we selected two molecularly identified bacteria: P. fungorum GIB024 and P. caledonica KN1. Direct confrontation assay at three different distances, a pour plate method that sampled bacterial spent broth either with and without agitation at 25 °C, and an indirect confrontation assay was carried out in order to assess the R. roseolus growth-promoting ability of Paraburkholderia spp. These assessments were carried out in a 1:5 diluted Melin-Norkran-modified medium with glucose (hs-dMMN) and without glucose (ls-dMMN). GIB024 promoted the growth of R. roseolus in ls-dMMN in short distance, whereas KN1 inhibited the growth of the fungus in that condition. In hs-dMMN, both bacteria have neutral or slightly promotion effect toward R. roseolus. We determined from the spent broth analysis that Paraburkholderia spp. that grew axenically under static conditions had a more pronounced mycelial growth-promoting effect on R. roseolus than under agitation conditions. We also found that high concentration of spent broth resulted in a decrease in mycelial growth-promoting ability. Volatile metabolite(s) produced by both bacteria did not promote the mycelial growth of R. roseolus. In conclusion, Paraburkholderia spp. exhibited a species- and nutrient (sugar)-dependent ability to promote the mycelial growth of R. roseolus, and the bacterial soluble metabolite(s) play a crucial role in their growth-promoting ability.
Metabolite, Ectomycorrhizal mushroom, Parabulkholderia, Rhizopogon
Bacterial-fungal interaction (BFI) is ubiquitous in nature and plays a crucial role in various environmental niches, contributing to the biochemical and physical processes in nature. Moreover, BFI has been exploited for centuries by humans in various sectors, including agriculture, food technology, health, forestry, and environmental protection. The symbiotic relationship between bacteria and fungi can be by mutualism, parasitism, or commensalism. In the mycorrhizal relationship, the fungus helps to promote plant growth through exchange as the photosynthetic products of the plant are provided to the fungus. However, it has been revealed that bacteria, known as mycorrhizal helper bacteria (MHB), are also involved in this symbiotic relationship between plants and fungi, thus making a tripartite symbiosis plant-fungus-bacterium.1,2
The fruiting body or sporocarp is the most visible part of the fungus on which spore-producing structures are formed. Sporocarp of ectomycorrhizal fungus harbors unique bacteria that might have roles in symbiotic relation between plant and the ectomycorrhizal fungus, yet they are relatively unexplored compared with bacteria in rhizospheric or “hypospheric” soil. In this research, bacteria have been isolated from the fruiting body of Rhizopogon roseolus (shouro mushroom in Japanese) and the screening for their effect on mycelial growth of the fungus have been performed.3 R. roseolus is an edible ectomycorrhizal fungus that is commonly associated with pine trees. Six out of 19 isolated bacteria had the potential to promote mycelial growth of R. roseolus according to the in vitro confrontation test. All these isolates were also molecularly identified. Among these bacteria, we found some bacteria belonging to the genus Paraburkholderia.
The genus Paraburkholderia has been found ubiquitously with high abundance as mycorrhizosphere bacteria and has fungophilic features,4,5 but the roles of these bacteria in their fungal counterparts are not well understood. Some Paraburkholderia spp. isolated from R. roseolus sporocarp were found to promote mycelial growth of the fungus using an in vitro direct confrontation assay. In the mycorrhizal association, the capacity of bacteria to enhance the growth of fungal mycelia is an indication of the role of the bacteria as MHB.6 However, the mode of action or agent responsible for the mycelial growth-promoting activity of these Paraburkholderia spp. toward the growth of R. roseolus mycelia is still unknown. Some studies have suggested that extracellular metabolites produced by the bacteria are responsible for the mycelial growth effect of their fungal counterparts in a mutualistic symbiosis.7-9 Therefore, this study aims to evaluate whether the extracellular metabolites produced by two species of Paraburkholderia isolates, namely P. fungorum and P. caledonica, have the potential to promote growth in R. roseolus mycelial growth. We will investigate the volatile and nonvolatile metabolites of the bacteria.
Microorganisms and media
The R. roseolus strain TUFC10010 was provided by the Tottori University Fungal Culture Collection, Japan. Two bacterial isolates were employed, Parabukholderia fungorum GIB024 and P. caledonica KN1, both of which were isolated from the sporocarp of R. roseolus associated with Pinus thunbergii (Japanese black pine). The bacterial isolation, as well as molecular identification, were carried out according to Pramoj Na Ayudhya et al.3 All bacterial isolates and R. roseolus isolates were maintained in potato dextrose agar (PDA) and 1:5 diluted Melin-Norkrans modified medium or 1:5 MMN (with thiamine), respectively, prior to use. In the bioassay experiment, high-sugar medium (hs-dMMN) refers to 1:5 MMN (without thiamine) in the presence of glucose and low-sugar medium (ls-dMMN) refers to 1:5 MMN (without thiamine) in the absence of glucose. The composition of the MMN-based liquid media used is summarized in Table 1.
Table (1):
Chemical and quantity composition of Melin-Norkrans modified medium (MMN)-based liquid media for culture maintaining and bioassay in this study.
Chemical composition |
1:5 MMN |
hs-dMMN |
ls-dMMN |
|---|---|---|---|
Malt extract |
1 g |
1 g |
1 g |
Glucose |
2 g |
2 g |
– |
(NH4)2HPO4 |
0.05 g |
0.05 g |
0.05 g |
KH2PO4 |
0.1 g |
0.1 g |
0.1 g |
MgSO4.7H2O |
0.03 g |
0.03 g |
0.03 g |
NaCl |
5 mg |
5 mg |
5 mg |
CaCl2 |
10 mg |
10 mg |
10 mg |
Thiamine hydrochloride |
20 µg |
– |
– |
FeCl3.6H2O 1% (w/v in deionized water) |
0.24 mL |
0.24 mL |
0.24 mL |
Deionized water |
999 mL |
999 mL |
999 mL |
Dual culture (direct confrontation) between the bacterial isolate and R. roseolus at various distances
Preparation of R. roseolus for dual culture assay
Sterilized hs-dMMN and ls-dMMN with 2% agar (w/v) were used as media for the dual culture assay. One plug (0.6 cm in diameter) of the shouro mycelium (grown on 1:5 diluted MMN agar plate with 2% agar) from the active part was placed on the edge of the agar medium in the Petri dish. The R. roseolus was incubated in a dark incubator at 25°C for one week prior to the dual culture assay.
Preparation of the bacterial isolate
One colony was picked from a bacterial plate (7-day-old in PDA) and inoculated in 50 mL of hs-dMMN liquid for 3 days in a dark incubator at 25°C without agitation. The bacterial cells were then separated from the supernatant by centrifugation at 2200 × g for 20 min. The supernatant was discarded, and NaCl 0.9% (w/v) solution was added and mixed with the bacterial cells until the OD600 reached approximately 0.2.
Dual culture assay
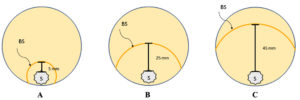
One loop of the bacterial suspension was placed at three different distances (5, 25, and 45 mm) from the active edge of one week old R. roseolus (Fig. 1). A 0.9% (w/v) NaCl solution was used as a control. The plates were then incubated in a dark incubator at 25°C, and mycelial elongation growth was observed weekly.
Fig. 1. Schematic figures of the design experiment of dual culture assay between R. roseolus and bacterial isolate in a petri dish (ø 8.5 cm). One loop of bacterial suspension (BS) with OD600 ≈ 0.2 was cycled around the edge of actively growing one-week R. roseolus mycelial colony (S) in three different distances: 5 (A), 25 (B), and 45 mm (C).
Investigation of the volatile metabolites of bacterial isolates toward growth of Rhizopogon roseolus
Preparation of R. roseolus plate
The hs-dMMN and ls-dMMN with 2% agar were used as media for growing R. roseolus. One plug (0.6 cm in diameter) of R. roseolus mycelia (grown on hs-dMMN agar plate) from the active part was placed at the center of the agar medium in a nine-centimeter Petri dish. R. roseolus was incubated in a dark incubator at 25°C for one week prior to the dual culture assay.
Preparation of the bacterial isolate plates
Similar to the R. roseolus plate, the hs-dMMN and ls-dMMN with 2% agar were used as media to inoculate the bacterial isolate. One colony was picked from the bacterial plate (in PDA) and inoculated in 50 mL of hs-dMMN (without thiamine) liquid medium in the presence of glucose for 3 days in a dark incubator at 25°C without agitation. Further, 30 µL of each bacterial suspension broth was inoculated into the agar plate (pH before wet sterilization was maintained at 6 ± 0.1). For the control, uninoculated liquid medium was spread onto the agar medium. The bacterial isolates were maintained in a dark chamber at 25°C for three days.
Indirect contact between the R. roseolus and bacteria
Indirect contact was applied to investigate the effect of volatile metabolites produced by bacteria on the mycelial growth of R. roseolus. The lids of both the bacterial and R. roseolus plates were removed, and the R. roseolus plate was inverted and placed on top of the bacterial plate (Fig. 6C). R. roseolus grown in hs-dMMN was placed on top of a bacterial isolate grown in hs-dMMN; R. roseolus grown in ls-dMMN was placed on top of the bacterial isolate grown in ls-dMMN. The control of the bacterial plate was also placed beneath the R. roseolus plate. The two plate bases were then sealed with a double layer of Parafilm®. The plates were maintained at 25°C in a dark chamber. A sterile agar plate without bacterial inoculation was used as a control. The diameter of R. roseolus was measured every five days after bacterial inoculation for 35 days of incubation.
Investigation of the axenic soluble metabolites of bacterial isolates toward growth of R. roseolus
Preparation of bacterial spent broth
The pour plate method was used to investigate the effect of extracellular metabolites produced by bacteria under axenic conditions on the mycelial growth of R. roseolus. Initially, one colony was picked from a bacterial plate (7-day-old in PDA) and inoculated in 50 mL of hs-dMMN for 3 days in a dark incubator at 25°C without agitation. Further, 10 µL of bacterial suspensions of GIB024 and KN1 were inoculated in two different liquid media, hs-dMMN and ls-dMMN (pH before wet sterilization was maintained at 6 ± 0.1). The bacterial isolates were maintained in a dark chamber at 25°C without agitation (S) and with agitation at 80 rpm (R) for 3 days. Detailed information on the spent broth conditions is presented in Table 2. The bacterial cells were then separated from the liquid broth by centrifugation at 2200 × g for 40 min. The supernatant was then filtered aseptically through Minisart® 0.45 µm filter paper, followed by Acrodisc® 0.1 µm filter paper and collected in sterile falcon tubes as bacterial spent broth for further assays.
Table (2):
Conditions for investigating the ability of crude bacterial spent broth toward growth of R. roseolus via pour plate method. The bacterial spent broth was added to the warm agar medium for R. roseolus in three concentrations: 10%, 20%, and 30% (v/v).
Condition |
Agitation |
Bacterial spent broth medium |
R. roseolus medium |
|---|---|---|---|
S ls-dMMN |
Static |
ls-dMMN |
ls-dMMN |
S hs-dMMN |
80 RPM |
hs-dMMN |
hs-dMMN |
R ls-dMMN |
Static |
ls-dMMN |
ls-dMMN |
R hs-dMMN |
80 RPM |
hs-dMMN |
hs-dMMN |
Pour plate method
As with bacteria, we used two different agar media for R. roseolus, hs-dMMN and ls-dMMN (pH before wet sterilization was maintained at 6 ± 0.1). Approximately 10%, 20%, and 30% (v/v) of the bacterial spent broth was added to warm hs-dMMN or ls-dMMN agar medium in a 9 cm Petri dish until a total volume of 15 mL was reached and then mixed slowly. The bacterial spent broth from the hs-dMMN liquid medium was added to warm hs-dMMN agar medium, while the bacterial spent broth from bacteria grown in ls-dMMN liquid medium was added to warm ls-dMMN agar medium. For control, instead of the bacterial spent broth, uninoculated hs-dMMN or ls-dMMN were added to warm hs-dMMN and ls-dMMN agar media, respectively. The amount of agar in the Petri dish for each concentration of spent broth was maintained at 2% (w/v). After the agar media solidified, one plug (0.6 cm in diameter) of R. roseolus mycelia (grown on hs-dMMN agar plate) from the actively growing edge was placed on the center of the agar medium in the Petri dish and sealed with Parafilm®. The Petri dish was incubated in a dark chamber at 25°C, and the mycelial diameter was observed after 14 days. The percentage of mycelial growth was calculated as [(diameter of R. roseolus with spent broth – diameter of R. roseolus in control)/ diameter of R. roseolus in control] × 100%.
Statistical analysis
All the procedures for the dual culture assay, pour plate assay, and indirect confrontation assay were repeated in triplicate. Statistical analysis of dual culture data was performed through Dunnett’s multiple comparisons test (one-way ANOVA) between control and bacterial test. Statistical analysis of effect of bacterial spent broth (pour plate assay) was performed through unpaired t test between GIB024 and KN1. Statistical analysis of indirect confrontation data was performed through Tukey’s multiple comparisons test (two-way ANOVA). For figure that contained statistical analysis data, the asterisk sign, indicates the summary of the comparison test, is stated in each figure legend. Without asterisk sign or ns means not significantly different (P > 0.05). All data and illustrations were processed using GraphPad Prism software version 9.2.0.
Paraburkholderia–R. roseolus in dual culture interaction
The interaction between R. roseolus and the bacterial isolates in the direct confrontation assay depended on the distance between the tip of R. roseolus mycelium and the bacterial line in both hs-dMMN and ls-dMMN agar media (Fig. 2; the appearance of the dual culture plate is shown in Fig. 3). We extended the period of incubation and post-bacterial inoculation observation until 35 days because the fungal plug was initially put on the edge of the Petri dish, thus allowing enough space and time for fungal mycelia to reach the farthest edge of the Petri dish (diameter of petri dish is 9.0 cm). Based on our observation, after 35 days of bacterial inoculation, the fungal mycelia were able to elongate maximally 4.5 cm from the initial point of mycelial active edge (day 0 post-bacterial inoculation). We also observed that each strain of bacteria promoted R. roseolus mycelial growth differently. GIB024 promoted the growth of R. roseolus at shorter distances, particularly in low sugar medium, whereas KN1 inhibited the growth of the fungus in that medium. Instead, KN1 increased the mycelial growth of R. roseolus at long distances in a high sugar medium.
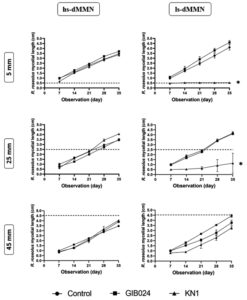
Fig. 2. Weekly observation (x-axis in day) of R. roseolus mycelial length (y-axis in cm) in dual culture plate with bacterial isolates (GIB024 in square, KN1 in up-pointing triangle, NaCl 0.9% (w/v) solution as control in circle) at various distance (5, 25, and 45 mm) from the edge of one-week actively growing R. roseolus mycelium. The assay was carried out in two agar plate types, 1:5 MMN with glucose (hs-dMMN) and without glucose (ls-dMMN). Dotted line (—) marks the distance between bacterial line and the initial apical tip of one-week R. roseolus mycelia (see BS in Fig. 1). Statistical analysis of the data was performed through Dunnett’s multiple comparisons test (one-way ANOVA) between control and bacterial test, the asterisk sign indicates the summary of the comparison test, * (P < 0.05). Without asterisk sign means not significantly different (P > 0.05).
The shortest distance (5 mm) facilitated immediate contact between the bacterial colony and the actively growing mycelial tip of R. roseolus. Under these conditions, distinct interactions were observed. In ls-dMMN, GIB024 promoted fungal growth at a distance of 5 mm. However, the promoting effect decreased at a further distance between the bacterium and the fungus (at 25 mm), or even showed an inhibitory effect (at 45 mm). On the other hand, in ls-dMMN, KN1 inhibited the growth of the fungus at the shortest distance (5 mm), thus making the mycelia of R. roseolus unable to cross the bacterial colony line (Fig. 2). However, the antifungal effect decreased with increasing distance between the bacterium and fungus. These findings suggest that metabolites produced by R. roseolus itself might trigger the KN1 of toxic metabolite(s) that inhibit fungal growth in such low sugar environments. In hs-dMMN however, KN1 slightly promoted R. roseolus growth at the shortest distance (5 mm), while GIB024 had a neutral effect on fungal growth. At a distance of 25 mm, the promoting effect of KN1 increased, but it decreased at a distance of 45 mm. In hs-dMMN, GIB024 slightly promoted the growth of R. roseolus at a distance of 25 mm, but this effect decreased with increasing distance between the bacterium and the fungus.
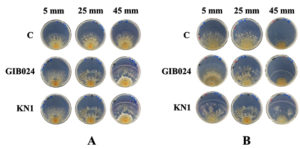
Fig. 3. Thirty-five-day-old post-bacterial inoculation of dual culture plates of R. roseolus mycelium with two bacterial isolates (GIB024 = P. fungorum, KN1 = P. caledonica, C = Monoculture of R. roseolus) at various distances, 5, 25, and 45 mm from the edge of one-week R. roseolus mycelia. The assay was done in two agar plate types, (A) 1:5 MMN with glucose (hs-dMMN) and (B) without glucose (ls-dMMN).
Mycelial growth promoting ability of the bacterial spent broth
Extracellular metabolites produced by the bacteria that accumulate in the bacterial spent broth potently promote the mycelial growth of R. roseolus. The spent broth mixture with the growth medium increased the diameter of colonies of the fungus mycelia. The spent broth was added to the medium at three different concentrations: 10%, 20%, and 30% (Fig. 4A-D). However, various fermentation conditions, such as the presence of additional glucose (sugar) in the fermentation media and agitation during the fermentation period affected the bacteria to produce spent broth with various positive effects toward the growth of R. roseolus. Spent broth of GIB024 grown in low sugar medium had a greater ability to promote the growth of R. roseolus than that grown in high sugar medium (Fig. 4A). On the other hand, the other bacterium, KN1, preferred high sugar medium to produce growth factors for R. roseolus mycelial growth (Fig. 4B). This tendency agrees with the dual culture assay (Fig. 1), where GIB024 promoted R. roseolus growth in ls-dMMN and KN1 in hs-dMMN. Agitation conditions during bacterial fermentation also influence the ability of bacteria to produce mycelial growth factors. Bacteria grown under static conditions in both types of media were more effective in producing mycelial growth-promoting metabolites than with agitation.
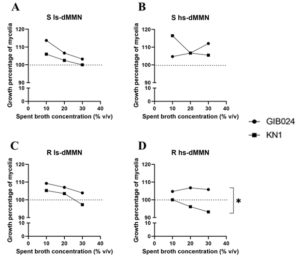
Fig. 4. Effect of two bacterial spent broths of Paraburkholderia species (GIB024 in circle and KN1 in square) toward the growth of R. roseolus through pour plate method. The spent broth was added to the R. roseolus medium in three concentrations: 10%, 20%, and 30%. The spent broth was obtained by growing bacteria in various fermentation conditions by manipulating the sugar concentration (1:5 MMN with glucose [hs-dMMN] and without glucose [ls-dMMN]) and agitation condition (S= static condition, R= with agitation 80 RPM). The influence of bacterial spent broth concentration of GIB024 and KN1 toward growth percentage of R. roseolus mycelia through pour plate assay is plotted in (A) bacteria grown in S ls-dMMN condition; (B) bacteria grown in S hs-dMMN condition; (C) bacteria grown in R ls-dMMN condition; (D) bacteria grown in R hs-dMMN condition. The dotted line (—) marks the growth percentage of the control (100%), growth percentage of R. roseolus below the line indicates inhibition effect of the bacterial spent broth. Statistical analysis of the data was performed through unpaired t test between GIB024 and KN1, the asterisk sign indicates the summary of the comparison test, * (P < 0.05). Without asterisk sign means not significantly different (P > 0.05).
A lower concentration of the spent broth was more effective in increasing the growth of R. roseolus mycelia. According to the bioassay, supplementation of 10% (v/v) of KN1 bacterial spent broth grown in high sugar medium under static conditions provided the highest R. roseolus-promoting ability among other bacterial spent broth conditions (Fig. 4B, the appearance of the colony is shown in Fig. 5). However, the addition of KN1 to R. roseolus medium resulted in a decrease in R. roseolus growth (Fig. 4C and Fig. 4D). For most fermentation conditions, particularly in low sugar media, for each bacterium, the spent broth concentration added to the plate was negatively correlated with the growth promoting ability of R. roseolus mycelium (R2 > 0.8; negative 1/ slope; see Table 3). An exception to this is GIB024, which when grown in high sugar medium both in static and agitated conditions, showed positive correlation and no correlation, respectively, toward R. roseolus mycelium growth.
Table (3):
The parameter of the best fit values as well as the goodness of fit as the effect of two bacterial spent broths of Paraburkholderia species (GIB024 and KN1) toward the growth of R. roseolus through pour plate method.
| Parameter | S ls-dMMN | S hs-dMMN | R ls-dMMN | R hs-dMMN | ||||
|---|---|---|---|---|---|---|---|---|
| GIB024 | KN1 | GIB024 | KN1 | GIB024 | KN1 | GIB024 | KN1 | |
| 1/ slope | -0.5215 | -0.2990 | 0.3690 | -0.5485 | -0.2690 | -0.3980 | 0.0545 | -0.3400 |
| R2 | 0.9595 | 0.9904 | 0.9317 | 0.8290 | 0.9895 | 0.9022 | 0.2906 | 0.9939 |
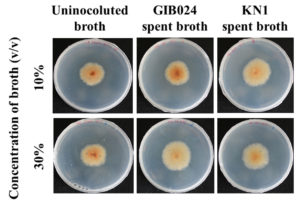
Fig. 5. The appearance of 14-day-old mycelial colonies of R. roseolus grown in hs-dMMN (1:5 MMN with glucose) with addition of 10% and 30% v/v bacterial spent broth that grown in S ls-dMMN (1:5 MMN with glucose and without agitation condition) for 3 days, in dark chamber at 25°C. The control plate contained hs-dMMN only (uninoculated broth), without the addition of bacterial spent broth. The fungal colony size of control plates is smaller than plates with the bacterial spent broth, indicated the presence of mycelial-growth metabolite(s) in the bacterial spent broth of GIB024 as well as KN1.
Indirect confrontation assay between Rhizopogon roseolus and bacterial isolates
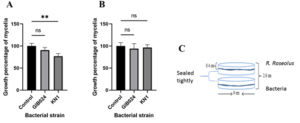
Volatile compounds produced by the bacteria through indirect confrontation had neutral and inhibitory effects on the growth of R. roseolus, depending on the bacterial nutrient medium condition. In high sugar media, the volatile compounds produced by the bacteria had a neutral effect on the growth of R. roseolus (Fig. 6A). On the other hand, gas metabolites released by the bacteria that grew in low sugar media showed variability in the growth of R. roseolus (Fig. 6B). GIB024 inoculated in low sugar medium had a neutral effect, whereas KNI in the same medium inhibited the growth of R. roseolus. These findings agreed with the direct confrontation assay (Fig. 1), in which KN1 inoculated in low sugar media at various distances from the mycelia apical tip inhibited the growth of R. roseolus, including the farthest distance of 45 mm, which resembled the indirect confrontation assay condition.
Fig. 6. Indirect confrontation of bacterial isolates, GIB024 and KN1, toward the growth of R. roseolus mycelia in a closed system 10 days post-confrontation. The nutrient scenario variation was applied on the assay. (A) both R. roseolus and bacteria were grown in hs-dMMN. (B) both R. roseolus and bacteria were grown in ls-dMMN. (C) a representative Figure to describe the design of plate petri dish for the indirect confrontation assay, showing the separated position of R. roseolus mycelial colony in agar medium (above part) as well as the bacterial colony in agar medium (below part). Statistical analysis of the data was performed through Tukey’s multiple comparisons test (two-way ANOVA), the asterisk sign indicates the summary of the comparison test, ** (P < 0.005), ns = not significant.
The growth of R. roseolus, as the impact of confrontation with Paraburkholderia spp., depended on the media (presence of excess carbon source, in this case glucose), bacterial strain, and distance between R. roseolus and the bacteria. Nutrient availability has been acknowledged as one of the main factors in BFI determining the inhibiting or promoting effect on the fungal growth.10 Among the various macro- and micro-nutrients, carbon and nitrogen sources are the two major sources that play a major role in the effect of nutrient status on the BFI.11 The quality and quantity of carbon sources have an impact on the interaction between fungi and bacteria, either mutualistically or parasitically. The type of carbon source has been found to determine the selectivity of bacteria toward fungal partners. One example is oxalotrophic bacteria in the soil ecosystem that uses oxalic acid released by certain soil fungi as chemoattractants and as a carbon source, thus shaping the microbial community around the mycorrhizosphere.12 The amount of sugar in the zone of the bacterial-fungal interface can determine whether a positive or negative interaction will occur. Our findings suggest that this phenomenon was also observed during the interaction between Paraburkholderia spp. and R. roseolus, although it is also strain dependent. For BFIs that prefer low sugar conditions, the stress gradient hypothesis (SGH) condition has been raised, particularly in the marginal soil condition with limited major nutrients and an abundance of recalcitrant compounds.13 Furthermore, antimicrobial agents are sometimes observed to be released by microbes involved in SGH conditions, when carbon sources are abundant.
The interaction between R. roseolus and Paraburkholderia is also influenced by physical factors, such as the distance between bacteria and mycelial apex of the fungus. In our study, a short distance, that allowed immediate contact between fungal mycelia and bacterial colonies on the surface of the agar, was more beneficial for GIB024 than KN1, suggesting the role of bacterial biofilm or endobacteria feature mechanism of this bacterium toward R. roseolus mycelia. The distance between bacteria and the apical tip of the fungal mycelia also influences the distribution of either soluble metabolites released by the bacteria through the agar pores or the volatile compounds emitted in the interface beyond the agar surface. Since the movement of the non-volatile metabolites through the agar relies mostly on the passive diffusion mechanism, which depends on the molecular weight of the metabolites produced by the bacteria, high-molecular-weight biomolecules, for example, will reach the mycelial tip for a longer time than a low molecular weight compound. In addition, the agar pore size can be a significant barrier to the movement of high molecular weight compounds.
The interaction between bacteria and fungi is species-dependent. Although both of our bacterial isolates belong to the same genus, Paraburkholderia, their characteristics through interaction with R. roseolus and utilization of nutrients in the environment media are varied. A taxonomic and biochemical characteristic comparison of these two bacteria, formerly both belonging to the clade of Burkholderia, showed that P. caledonica has higher sugar-utilization ability than P. fungorum, but lacks enzymatic activity compared to P. fungorum.14 Consistent with the direct confrontation and pour plate assays, P. caledonica KN1 was shown to increase the growth of R. roseolus mycelium in high sugar conditions, and inhibited fungal growth in low sugar medium through the production of toxic soluble and volatile metabolites. The phenomenon of species- or strain-dependent interactions with fungi has been widely reported in various environmental niches, as well as for various functional interactions.15,16 In the mushroom field, several strains of Pseudomonas putida isolated from soil samples from some mushroom farms showed variability in the mushroom growth-promoting ability toward Agaricus bisporus.17 The intraspecies variation tendency of not only bacteria but also other kingdoms are caused by genetic and epigenetic factors. From an ecological and evolutionary perspective, this variation strategy is used as a mechanism for maintaining survivability under several environmental conditions that are sometimes unfavorable18
Bacterial extracellular metabolites, particularly non-volatile metabolites, contribute to the growth of R. roseolus. Although the fermentation conditions varied, bacterial spent broth of both bacterial strains, GIB024 and KN1, showed a positive mycelial growth-promoting effect. The bioassay of spent broth of these bacteria was correlated with the direct confrontation assay on agar plates under various scenarios. The mechanism by which bacteria promote the growth of fungal counterparts in a mutualistic symbiotic relationship has been thoroughly studied. Some proposed mechanisms, include direct mechanisms, such as releasing specific or general growth factors and facilitating important and crucial nutrients to the fungi. An indirect mechanism through the inhibition of fungal enemies has also been suggested. Several metabolites produced by bacteria that positively impact the growth of saprophytic as well as mycorrhizal fungi have been identified and characterized. A MHB, Streptomyces strain Ach 505, was reported to produce the soluble metabolite auxofuran, which specifically increased the growth of the ectomycorrhizal fungus Amanita muscaria.7 In some studies, several bacteria have been reported to release non-specific compounds such as thiamin19 or induce the mycosynthesis of growth hormone indole-3-acetic acid (IAA)20 during interactions with their fungal counterparts.
The volatile compounds (VCs) produced by our Paraburkholderia isolates did not increase the growth of R. roseolus. Furthermore, the nutrient conditions, particularly sugar availability, influenced the VCs produced by the bacteria, thus affecting their interaction with the fungal counterpart, and causing a possible shift from neutral or positive interactions to negative interactions. Bacterial VCs have been recognized as one of the modes of action of BFI in various environmental and ecological niches, including soil biogeochemistry. In some studies, the interaction between BFI and VCs provides interkingdom communication without physical contact, but effectively influences the physiological and morphological conditions of both bacteria and fungi depending on the nature of the interaction (parasitic or mutual).21-24 Consistent with our results, in the sporocarp or mycorrhizosphere microenvironment, the role of soluble metabolite exchange in close contact between residing bacterial and fungal mycelia is more pronounced than that of long-distance gas signals. Under these conditions, the endophytic bacteria found in the sporocarp have been selected from the corresponding hyposphere or mycorrhizosphere, suggesting physical contact through the fungal highway mechanism, thus providing a high possibility of interaction between bacteria and fungi via extracellular soluble metabolites during the interface between bacterial cells and fungal mycelium.25,26 During mutualistic interactions between the mold Aspergillus nidulans and the soil bacterium Bacillus subtilis, for example, there was an observed migration of the bacterium along hyphae of A. nidulans, followed by the secretion of thiamine as a growth-inducing factor in the apical zone of the fungal hyphae.9
The quantity of bacterial spent broth affected the growth of R. roseolus. The efficacy of the growth-promoting effect of cell-free spent broth of bacteria on the growth of fungal counterparts in a mutualistic symbiosis relationship tends to be low, thus increasing the spent broth concentration, resulting in a decrease in the promoting effect on fungal growth.27 Inversely, for the parasitic interaction between bacteria and fungi, the antifungal activity of bacterial spent broth increased as the concentration of the spent broth increased. Effectivity of spent broth to promote mycelial growth was observed at lower concentrations, indicating that the possible extracellular metabolites contained in the spent broth have roles in molecular signaling and might be extended to interkingdom quorum sensing between bacteria and fungi.
The production of growth promoting extracellular metabolites is influenced by a variety of environmental conditions, such as nutrient status in the growth medium and agitation during the fermentation period. Glucose or sugar availability obviously affected the ability of our bacterial isolates to secrete mycelial growth-promoting compounds. Generally, in conditions with excess major nutrients such as sugar, nitrogen, or soluble phosphorus, bacteria suppress the production of their secondary metabolism products.28,29 However, in some cases, the abundance of certain nutrients triggers the formation and secretion of secondary metabolites.30 Agitation during the fermentation period influences the physiology of bacteria and dissolved oxygen, and thus extracellular metabolites produced by the bacteria.31
Soluble extracellular metabolites, and not non-volatile metabolites, produced by Paraburkholderia fungorum and P. caledonica can promote the growth of Rhizopogon roseolus mycelia. However, the production of these potential metabolites is dependent on various fermentation conditions, such as nutrients (particularly sugar concentration) and agitation during fermentation. Paraburkholderia spp. exhibit a species- and nutrient (glucose)-dependent ability to promote the mycelial growth of R. roseolus, and the bacterial soluble metabolite(s) play a crucial role in their growth-promoting ability.
ACKNOWLEDGMENTS
The authors would like to express heartfelt thanks to Professor I Made Sudiana of Research Organization for Environmental and Life Sciences, National Research and Innovation Agency (BRIN) Indonesia for providing information regarding interaction between plant and bacteria.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
TPN, SPNA, TA, NS performed the experiments. TPN statistically analyzed the data and made illustrations. NS and TPN interpreted the results and drafted the manuscript. All authors read, critically revised and approved the final manuscript for publication.
FUNDING
This work was supported by a Grant-in-aid for Scientific Research No. 21K05710 from the Japan Society for the Promotion of Science.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Frey-Klett P, Garbaye J, Tarkka M. The mycorrhiza helper bacteria revisited. New Phytol. 2007;176(1):22-36.
Crossref - Deveau A, Bonito G, Uehling J, et al. Bacterial-fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol Rev. 2018;42(3):335-352.
Crossref - Pramoj Na Ayudhya S, Riffiani R, et al. Isolation of bacteria from fruiting bodies of Rhizopogon roseolus and their effect on mycelial growth of host mushroom. Mushroom Sci Biotechnol. 2019;27(4):134-139.
Crossref - Shirakawa M, Uehara I, Tanaka M. Mycorrhizosphere bacterial communities and their sensitivity to antibacterial activity of ectomycorrhizal fungi. Microbes Environ. 2019;34(2):191-198.
Crossref - Pratama AA, Jimenez DJ, Chen Q, et al. Delineation of a subgroup of the genus Paraburkholderia, including P. terrae DSM 17804T, P. hospita DSM 17164T, and four soil-isolated fungiphiles, reveals remarkable genomic and ecological features-proposal for the definition of a P. hospita species clus. Genome Biol Evol. 2020;12(4):325-344.
Crossref - Bending GD. What are the mechanisms and specificity of mycorrhization helper bacteria? New Phytol. 2007;174(4):707-710.
Crossref - Riedlinger J, Schrey SD, Tarkka MT, Hampp R, Kapur M, Fiedler H-P. Auxofuran, a novel metabolite that stimulates the growth of fly agaric, is produced by the mycorrhiza helper bacterium Streptomyces strain AcH 505. Appl Environ Microbiol. 2006;72(5):3550-3557.
Crossref - Uehling JK, Entler MR, Meredith HR, et al. Microfluidics and metabolomics reveal symbiotic bacterial–fungal interactions between Mortierella elongata and Burkholderia include metabolite exchange. Front Microbiol. 2019;10:2163.
Crossref - Abeysinghe G, Kuchira M, Kudo G, et al. Fungal mycelia and bacterial thiamine establish a mutualistic growth mechanism. Life Sci Alliance. 2020;3(12):e202000878.
Crossref - Velez P, Espinosa-Asuar L, Figueroa M, et al. Nutrient dependent cross-kingdom interactions: Fungi and bacteria from an oligotrophic desert oasis. Front Microbiol. 2018;9:1755.
Crossref - Lohberger A, Spangenberg JE, Ventura Y, et al. Effect of organic carbon and nitrogen on the interactions of Morchella spp. and bacteria dispersing on their mycelium. Front Microbiol. 2019;10:124.
Crossref - Sun Q, Li J, Finlay RD, Lian B. Oxalotrophic bacterial assemblages in the ectomycorrhizosphere of forest trees and their effects on oxalate degradation and carbon fixation potential. Chem Geol. 2019;514:54-64.
Crossref - Hammarlund SP, Harcombe WR. Refining the stress gradient hypothesis in a microbial community. Proc Natl Acad Sci. 2019;116(32):15760-15762.
Crossref - Coenye T, Laevens S, Willems A, et al. Burkholderia fungorum sp. nov. and Burkholderia caledonica sp. nov., two new species isolated from the environment, animals and human clinical samples. Int J Syst Evol Microbiol. 2001;51(3):1099-1107.
Crossref - Carlson E. Effect of strain of Staphylococcus aureus on synergism with Candida albicans resulting in mouse mortality and morbidity. Infect Immun. 1983;42(1):285-292.
Crossref - Jabra-Rizk MA, Falkler Jr WA, Merz WG, Kelley JI, Baqui A, Meiller TF. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. J Clin Microbiol. 1999;37(5):1464-1468.
Crossref - Zarenejad F, Yakhchali B, Rasooli I. Evaluation of indigenous potent mushroom growth promoting bacteria (MGPB) on Agaricus bisporus production. World J Microbiol Biotechnol. 2012;28(1):99-104.
Crossref - Godhe A, Rynearson T. The role of intraspecific variation in the ecological and evolutionary success of diatoms in changing environments. Philos Trans R Soc B Biol Sci. 2017;372(1728):20160399.
Crossref - Deveau A, Brule C, Palin B, et al. Role of fungal trehalose and bacterial thiamine in the improved survival and growth of the ectomycorrhizal fungus Laccaria bicolor S238N and the helper bacterium Pseudomonas fluorescens BBc6R8. Environ Microbiol Rep. 2010;2(4):560-568.
Crossref - Hoffman MT, Gunatilaka MK, Wijeratne K, Gunatilaka L, Arnold AE. Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS One. 2013;8(9):e73132.
Crossref - Kai M, Effmert U, Berg G, Piechulla B. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiol. 2007;187(5):351-360.
Crossref - Schmidt R, Etalo DW, de Jager V, et al. Microbial small talk: volatiles in fungal–bacterial interactions. Front Microbiol. 2016;6:1495.
Crossref - Schmidt R, de Jager V, Zuhlke D, et al. Fungal volatile compounds induce production of the secondary metabolite Sodorifen in Serratia plymuthica PRI-2C. Sci Rep. 2017;7(1):1-14.
Crossref - Weisskopf L, Schulz S, Garbeva P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat Rev Microbiol. 2021;19(6):391-404.
Crossref - Liu Y, Sun Q, Li J, Lian B. Bacterial diversity among the fruit bodies of ectomycorrhizal and saprophytic fungi and their corresponding hyphosphere soils. Sci Rep. 2018;8(1):1-10.
Crossref - Steffan BN, Venkatesh N, Keller NP. Let’s Get Physical: Bacterial-Fungal Interactions and Their Consequences in Agriculture and Health. J Fungi. 2020;6(4):243.
Crossref - Aspray TJ, Jones EE, Davies MW, Shipman M, Bending GD. Increased hyphal branching and growth of ectomycorrhizal fungus Lactarius rufus by the helper bacterium Paenibacillus sp. Mycorrhiza. 2013;23(5):403-410.
Crossref - Martin JF. Phosphate control of the biosynthesis of antibiotics and other secondary metabolites is mediated by the PhoR-PhoP system: an unfinished story. J Bacteriol. 2004;186(16):5197-201.
Crossref - Ruiz B, Chavez A, Forero A, et al. Production of microbial secondary metabolites: regulation by the carbon source. Crit Rev Microbiol. 2010;36(2):146-167.
Crossref - Gotoh T, Koyama T, Ogura K. Farnesy1 Diphosphate Synthase and Solanesyl Diphosphate Synthase Reactions of Diphosphate-Modified Allylic Analogs: The Significance of the Diphosphate Linkage Involved in the Allylic Substrates for Prenyltransferase. J Biochem. 1992;112(1):20-27.
Crossref - Somerville GA, Proctor RA. Cultivation conditions and the diffusion of oxygen into culture media: the rationale for the flask-to-medium ratio in microbiology. BMC Microbiol. 2013;13(1):1-2.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.