ISSN: 0973-7510
E-ISSN: 2581-690X
Cholecystectomy, a common surgical procedure to remove the gallbladder, generally has a low complication rate. However, the emergence of MDR pathogens poses significant challenges in the post-operative management of infections. Multidrug-resistant Klebsiella pneumoniae (MDR-KP) is one of the most emerging issues in bacterial resistance. A 57 year-old woman came to the emergency unit at Dr. Soetomo General Academic Hospital with a chief complaint of pain in the upper and lower abdomen for four days. The patient had a history of cholecystectomy surgery in another hospital two weeks before admission. A cito laparotomy was performed. During surgery, turbid peritoneal fluid mixed with pus and abdominal wall fasciitis necroticans were found. From microbiology examination, MDR-KP was detected in pus. The patient was treated with Meropenem 1 g/8 hours and Amikacin 250 mg/8 hours. Unfortunately, the patient died due to sepsis and multi-organ dysfunctions. This case underscores the importance of vigilant post-operative monitoring and the need for effective infection control measures to manage and prevent MDR-KP infections in surgical patients. Enhanced surveillance and antibiotic stewardship are crucial to mitigating the risks associated with MDR organisms in healthcare settings.
Klebsiella pneumoniae, Multidrug-resistant, MDR-KP, Infection, Post Cholecystectomy
Cholecystectomy, the surgical removal of the gallbladder, is a standard procedure to treat various gallbladder diseases, most notably cholelithiasis and cholecystitis. While elective cholecystectomy is generally a straightforward and low-risk surgery, the procedure becomes more complex in the context of post-infection scenarios, particularly following biliary tract infections or systemic infections that involve the gallbladder, including abscesses.1
Post-infection cholecystectomy is most commonly due to pathogens such as Escherichia coli and Klebsiella pneumoniae for Gram-negative bacteria and Enterococcus species for Gram-positive bacteria.2 Infections can arise from surgical site infection, complications of gallstone disease, obstructive jaundice, or secondary to systemic infections such as sepsis. Surgical site infections result from invading pathogens into the surgical wound, typically occurring within 30 days of surgery.3
The emergence of MDR pathogens poses significant challenges in the post-operative management of infections. Klebsiella pneumoniae, a Gram-negative bacterium, is a common cause of healthcare-associated infections, including pneumonia, bloodstream infections, and urinary tract infections.4,5 It was found that up to 10% of nosocomial infections were caused by Klebsiella pneumoniae.6 Multidrug-resistant Klebsiella pneumoniae (MDR-KP) is one of the most emerging issues in bacterial resistance. One of the systematic review studies calculated that the combined prevalence of nosocomial MDR-KP was 32.8% worldwide.6 One of the significant post-infection cholecystectomy is surgical site infections (SSIs), particularly in cases where MDR-KP is involved. SSIs not only prolong hospital stays and increase healthcare costs but also contribute to higher morbidity and mortality rates.3 In the context of MDR-KP, SSIs pose even more significant challenges due to the pathogen’s resistance to multiple antibiotic classes and its ability to evade conventional infection control measures. Hereby, this paper presented a case of MDR-KP infection in a post-cholecystectomy patient.
Case Presentation
A 57 year-old woman came to the emergency unit at Dr. Soetomo General Academic Hospital with a chief complaint of worsened pain in the upper and lower abdomen for four days. The patient had a history of cholecystectomy surgery in another hospital two weeks before admission. The patient has not been able to mobilize since the operation, only bed rest. The yellowish fluid comes out of the drain in the stomach, averaging 300-600 ml per day. The patient could eat and drink, bowel movements were normal, and the last one this afternoon was soft brownish in consistency. The patient did not have nausea and vomiting. The patient said that since the operation, yellowish fluid has come out, and it has been seeping from the drain site. The surgical wound was opened at the edge. There was no previous history of jaundice. From the physical examination, the patient had a blood pressure of 109/57 mmHg, heart rate of 116 beats/minute, respiration rate of 22 times/minute, temperature of 37.2 degrees Celsius, and a defens regio epigastric. From the laboratory examination, the Leukocyte count: 19.24×103, Neutrophils: 79%, Platelets: 734×103, C-Reactive Protein: 18.03, BUN: 33.9, Creatinine serum: 18.03, total bilirubin: 1.7, direct bilirubin: 1.2, and albumin 3.07.
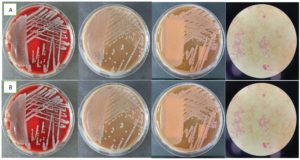
A cito laparotomy was performed. During surgery, turbid peritoneal fluid mixed with pus and abdominal wall fasciitis necroticans were found. The aspirate pus and the blood from both sides were sent to the clinical microbiology laboratory for culture examination. The blood specimens were cultured in BACTEC (BD, USA) and showed positive results in less than 24 hours. Then, the blood specimens from BACTEC were subcultured into the Blood Agar, Chocolate Agar, and Mac-Conkey Agar. The Blood Agar and Chocolate Agar showed opaque, raised, and mucoid colonies; the Mac-Conkey Agar showed pink, large mucoid colonies, and the bacterial Gram staining showed negative rods (Figure). BD PhoenixTM M50 (BD, USA) was used for microorganism isolation and antibiotic susceptibility tests. From microbiology examination, Multidrug-resistant Klebsiella pneumoniae was detected in pus and blood. The patient was treated with Meropenem 1 g/8 hours and Amikacin 250 mg/8 hours. Unfortunately, the patient died due to sepsis and multiorgan dysfunction.
Surgical site infections result from invading pathogens into the surgical wound, typically occurring within 30 days of surgery.3 The management of post-infection cholecystectomy in cases caused by MDR-KP presents significant clinical challenges due to the pathogen’s virulence, including a protective capsule, fimbriae for adherence, biofilm formation, and production of enzymes that degrade antibiotics (e.g., extended-spectrum beta-lactamases (ESBLs) and carbapenemases). These virulence factors enable Klebsiella pneumoniae to colonize surgical wounds effectively, resist host immune responses, and persist in the wound environment.7
MDR-KP can enter the surgical site through various mechanisms. The bacteria may originate from the patient’s biliary system or gastrointestinal tract, especially in patients with prior biliary infections or sepsis. Residual bacteria may infect the wound if the infection is not completely eradicated before surgery. MDR-KP can form biofilms on surgical materials, such as drains or sutures, making it difficult to eliminate the infection. Biofilms protect the immune system and antibiotic treatments, contributing to chronic, difficult-to-treat infections.8
SSIs caused by MDR-KP present similarly to SSIs caused by other pathogens but tend to be more severe and difficult to treat. Common clinical features include inflammation around the surgical wound, purulent discharge from the wound, post-operative fever, localized pain or tenderness at the incision site, and, in severe cases, the wound may open due to infection and tissue breakdown.9
MDR-KP is characterized by its resistance to multiple classes of antibiotics, including beta-lactams, carbapenems, aminoglycosides, and fluoroquinolones, limiting the available treatment options and leading to higher morbidity and mortality rates. Several mechanisms, including the acquisition of resistance genes through horizontal gene transfer, mutations in target sites, and the overexpression of efflux pumps, drive the development of MDR-KP.7 Plasmids are crucial in disseminating resistance genes among Klebsiella pneumoniae strains and across different bacterial species. Notably, the production of extended-spectrum beta-lactamases (ESBLs) and carbapenemases, such as KPC (Klebsiella pneumoniae carbapenemase), OXA-48, and NDM-1 (New Delhi metallo-beta-lactamase), has significantly contributed to the resistance profile of MDR-KP.10
Many factors, including the overuse and misuse of antibiotics in healthcare settings, inadequate infection control practices, and the global movement of people and goods, facilitate the spread of MDR-KP. Hospitals, particularly intensive care units, are hotspots for the transmission of MDR-KP due to the high usage of invasive devices, prolonged hospital stays, and the presence of immunocompromised patients.11
Addressing the challenge of MDR-KP requires a multi-faceted approach. Surveillance systems are essential for monitoring the prevalence and spread of MDR-KP and for identifying outbreaks early. Antibiotic stewardship programs are pivotal in managing infections caused by MDR organisms, including Klebsiella pneumoniae. In the context of post-infection cholecystectomy, judicious use of broad-spectrum antibiotics is necessary to limit the emergence of further resistance while ensuring that patients receive adequate coverage for the causative pathogen. The rise of Carbapenem-resistant Klebsiella pneumoniae (CRKP) underscores the need for clinicians to be aware of regional resistance patterns and to utilize local antibiograms when selecting empirical therapy.12
Isolating patients infected with MDR pathogens is critical in preventing nosocomial transmission. The prevalence of MDR-KP in hospital settings, particularly in intensive care units, highlights the importance of rigorous adherence to hygiene protocols, including hand hygiene, personal protective equipment, and environmental cleaning. Additionally, the use of antimicrobial-coated surgical instruments or bile drainage catheters may offer some protection against post-operative infections.13
In cases of targeted therapy, newly available drugs like Ceftazidime/Avibactam can be utilized as monotherapy. However, it is crucial to monitor the development of drug resistance to new medications; hence, molecular microbiological surveillance needs to be put in place. The recent report found the combination of Ceftazidime/Avibactam and Aztreonam was effective in combating MBL-producing K. pneumoniae.14 Zidebactam and Nacubactam, two novel inhibitors, demonstrated encouraging outcomes in early research, demonstrating a wide range of efficacy against MBL producers as well.15 Furthermore, research and development of new antibiotics and alternative therapeutic strategies, such as bacteriophage therapy, immunotherapies, and anti-virulence agents, are urgently needed to combat MDR-KP. Rapid diagnostic tools can also aid in the timely identification and appropriate treatment of MDR-KP infections.
This case report underscores the importance of vigilant post-operative monitoring and the need for effective infection control measures to manage and prevent MDR-KP infections in surgical patients. Enhanced surveillance and antibiotic stewardship are crucial to mitigating the risks associated with MDR organisms in healthcare settings.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Written informed consent was obtained from the participant before enrolling in the study.
- Dobradin A, Jugmohan S, Dabul L. Gallstone-Related Abdominal Abscess 8 Years After Laparoscopic Cholecystectomy. J Soc Laparoendosc Surg. 2013;17(1):139-142.
Crossref - Yun SP, Seo H-I. Clinical aspects of bile culture in patients undergoing laparoscopic cholecystectomy. Medicine. 2018;97(26):e11234.
Crossref - Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017;152(8):784-791.
Crossref - Levani Y, Wasito EB. Streptococcus mitis and Klebsiella pneumoniae Mixed Infection in Severe Burn Injury Patient. J Pure Appl Microbiol. 2024;18(2):867-872.
Crossref - Martin RM, Bachman MA. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol. 2018;8:1-15.
Crossref - Mohd Asri NA, Ahmad S, Mohamud R, et al. Global Prevalence of Nosocomial Multidrug-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis. Antibiotics. 2021;10(12):1508.
Crossref - Wang G, Zhao G, Chao X, Xie L, Wang H. The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int J Environ Res Public Health. 2020;17(17):1-17.
Crossref - Gonzalez-Ferrer S, Penaloza HF, Budnick JA, et al. Finding Order in the Chaos: Outstanding Questions in Klebsiella pneumoniae Pathogenesis. Ottemann KM, ed. Infect Immun. 2021;89(4):e00693.
Crossref - Seidelman J, Anderson DJ. Surgical Site Infections. Infect Dis Clin North Am. 2021;35(4):901-929.
Crossref - Wyres KL, Wick RR, Judd LM, et al. Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae. PLOS Genet. 2019;15(4):e1008114.
Crossref - Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80(3):629-661.
Crossref - Bassetti M, Righi E, Carnelutti A, Graziano E, Russo A. Multidrug-resistant Klebsiella pneumoniae : challenges for treatment, prevention and infection control. Expert Rev Anti Infect Ther. 2018;16(10):749-761.
Crossref - Geremia N, Zuglian G, Salvador A, et al. Impact of infection control measures and antibiotic stewardship programs on multidrug-resistant Klebsiella pneumoniae prevalence in Intensive Care Unit. infect dis trop med. 2024;10: e1413.
- Khattab S, Askar AM, Abdellatif HAA, Othman AAA, Rayan AH, Azab H. Synergistic combination of ceftazidime and avibactam with Aztreonam against MDR Klebsiella pneumoniae in ICU patients. Sci Rep. 2025;15(1):5102.
Crossref - Russo A, Fusco P, Morrone HL, Trecarichi EM, Torti C. New advances in management and treatment of multidrug-resistant Klebsiella pneumoniae. Expert Rev Anti Infect Ther. 2023;21(1):41-55.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.