Emerging and re-emerging zoonoses of diverse etiologies have caused significant morbidity and mortality recently. In the past two decades, several viral zoonoses, such as Bird flu, Ebola hemorrhagic fever, Hantavirus infection, Nipah virus disease, Rift Valley fever, Swine flu, West Nile fever, SARS, MERS, COVID-19 etc., have emerged from different parts of the world. The latest to the list is the “Monkey Pox”, which has recently been renamed as “Mpox” by WHO. The ongoing 2022 multi-country outbreak of monkeypox is the largest in history to occur outside of Africa. Monkeypox is an emerging zoonotic disease that for decades has been viewed as an infectious disease with significant epidemic potential because of the increasing occurrence of human outbreaks in recent years. With increasing case numbers being reported in the current outbreak, it is important for healthcare staff everywhere to update their knowledge of this zoonotic infection, including its prevention, clinical management, prophylaxis, and basics of infection control, to understand the broader implications of the current outbreak. We provide an overview of monkeypox virus infection to serve as a primer for healthcare staff who may encounter this condition in their practice.
Monkeypox, Mpox, Emerging Diseases, Zoonosis, Multi-country Outbreak, Africa, Smallpox
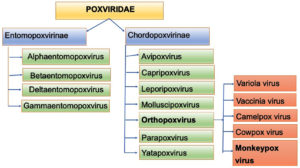
Monkeypox virus (MPXV) are large, enveloped, double-stranded DNA virus, which belongs to the Orthopoxvirus genus from Chordopoxvirinae subfamily of Poxviridae family.1
Viruses from the Poxviridae family infects various animals, such as rodents, birds, reptiles, insects and non-human primates, which when occasionally transmitted to humans, facilitate further human to human transmission.2
The Poxviridae family consists of two sub-families: Entomopoxvirinae, which infect insects and has 4 genera and 30 species; and Chorodopoxvirinae, which infect vertebrates & has 18 genera and 52 species, majority of which are of zoonotic origin (Figure 1).2,3
Various poxvirus species known to cause infections in humans includes Variola (smallpox), Vaccinia, Monkeypox, Cowpox, Camelpox, Pseudocowpox virus, Orf virus, Buffalopox, Molluscum contagiosum and Bovine papular stomatitis virus. Among these only Variola & Molluscum contagiosum virus has Human as sole host, other are zoonotic in origin.4
Monkeypox is described as 200-250 nm brick-shaped, slightly pleomorphic, enveloped virus, with dumbbell-shaped core and typical surface tubules & lateral bodies.5 Recently WHO has recommended the use of name “Mpox” as a synonym for Monkeypox.6
Various studies have shown immunological cross-reactivity & cross-protection among members of genus Orthopoxviruses.7,8 Therefore, it is seen some protection is conferred to the individuals having infection with any of the virus from genus Orthopox (OPXV) when infected with any other members of the same genus.
Poxviruses are large viruses (size ranging from 140-450 nm), with linear, double-stranded DNA with a comparatively large genome size of 130-360 kbp, which codes for over 200 genes.9
Unlike other DNA viruses, which replicates inside nucleus, Poxviruses replicate inside host cytoplasm, utilising viral proteins, instead of host proteins.10 With different expression of surface glycoproteins, two distinct form of virus can initiate infection cycle inside host cell: the intracellular mature virion (IMV), that has a single membrane, and the extracellular enveloped virion (EEV), that has an additional outer membrane.11 There are four Mature virion associated proteins that facilitate MV attachment to a host cell by binding glycosaminoglycans or laminin on the cell surface. Glycosaminoglycans on the surface of mammalian cells are considered to be key receptor for binding of the virus to the host cell membrane.12 Mature virions and EEVs perform distinct duties for the virus, i.e. transmission between host animals is carried out by MVs by being very stable, whereas outer membrane of EEVs make them fragile, therefore they are responsible for existing intact cells and spreading within the host.11
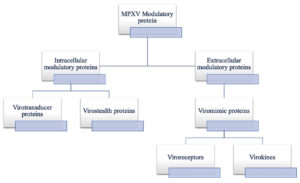
Because of such large size of Poxvirus, avoiding host defences by passing through gap junctions & rapid replication are difficult for them. Moreover, large size of Poxvirus initiate immune response in an individual relatively early, initiating an immune response very easily. Various viral proteins, coded by various virulence genes, act as modulators in host immune invasion, and these include some Intracellular proteins such as virotransducer proteins and virostealth proteins (Figure 2). Host cell response to infection is interrupted by virotransducer proteins, such as oxidative burst and apoptotic pathways. Whereas virostealth proteins play role in immune recognition molecules downregulation, including MHC-1 (major histocompatibility complex class 1) and CD+4, therefore reducing chance of virus detection by the host’s immune system. Similarly, one extracellular modulatory protein, viromimic proteins, which are present as cell surface glycoproteins, competitively inhibit host chemokines, cytokines and growth factors. As a consequence, virokines form viral mimics of above mentioned host factors, help in host responses subversion, thus helping virus survival and promote viral replication and spread.5,13 Simultaneous action of all these modulatory proteins helps in host’s immune evasion, allowing for viral replication.
History of the disease
Mpox was considered a neglected zoonotic virus as it remained confined to regions like tropical rainforest of West and Central African countries. First identified in Denmark in 1958, Mpox was isolated from crab-eating macaque monkeys kept for research purpose, by a Danish virologist Preben von Magnus.14 Later on, blood tests of different African rodents revealed evidence of monkeypox infection.
However, reportedly it took more than a decade for first human case to be reported in 1970 from the Democratic Republic of Congo (formerly Zaire).15 Majority of the human cases have been reported from west and central Africa particularly from rainforest and rural region of Congo Basin especially in DRC, since then.14,15
Sporadic outbreaks with several thousand human cases have been reported till date over the past fifty years, but have remained confined to African countries. Central and West African countries where monkeypox is endemic include Democratic Republic of the Congo (DRC), Cameroon, Cote d’Ivoire, Central African Republic, Liberia, Gabon, Sierra Leone, Republic of the Congo and Nigeria.16,17
However, there have been occasional cases or limited outbreaks in non-endemic countries which have been linked to importation of animals harboring the virus or with international travel.18
First outbreak of monkeypox outside the African continent was reported in the year 2003 in United states. 47 people in 7 states contracted the virus, who were later linked to close contact with infected prairie dogs.18,19
Cases have also been reported from United Kingdom (UK), United States (USA), Israel and Singapore, making Monkey pox as disease of global public health importance.17
Current Scenario
Current outbreak had its roots in Europe in early May 2022.20 Simultaneous cases were reported from the endemic and non-endemic countries like Europe and North America. Confirmed cases from these regions for the first time didn’t have any known epidemiological link to the endemic countries like West or Central Africa. Since then there has been steady rise in the number of cases reported and the number of countries affected, namely United Kingdom (UK), Germany, France, Italy, Portugal, Belgium, Spain, Netherlands, USA, Australia, Austria, Canada, Switzerland and Israel, and now India and sub continents, providing evidence of community spread.
WHO declared monkeypox a “public health emergency of international concern (PHEIC)” on 23rd July 2022.21
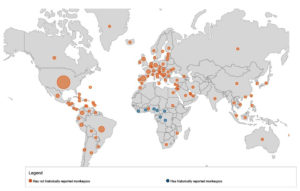
As of 4th November 2022, Centre of Disease Control & Prevention (CDC) reported 78,229 total confirmed cases of Monkeypox, and 41 deaths from total of 109 countries. Out of total 78,229 confirmed cases, 77,301 cases are reported from locations that have not historically reported monkeypox (Figure 3).22
Figure 3. CDC World map showing current distribution of confirmed MPX cases all over globe (4Th Nov 2022 data)
As per WHO data, top 10 countries with maximum number of confirmed cases are United state of America (USA), followed by Brazil, Spain, France, UK, Germany, Columbia, Peru, Mexico and Canada, accounting for 86.4% of the total reported cases all over the world.
Indian Scenario
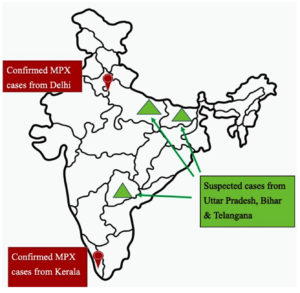
On 14th July 2022 first case of SEAR (South-East Asian region) was reported from Kerala, India in a 35 year old man arrived from Middle east.23 In subsequent weeks two more cases were reported from Kerala with international travel history. Fourth case of MPX was reported from the capital city, New Delhi, which was also the first case in India without any international travel history. Since then multiple suspected & confirmed cases were reported without any known epidemiological link which suggests the undetected circulation of Mpox virus in the community. First fatality from India was reported in a 22 year old Kerala man with a travel history from UAE. Multiple suspected cases were reported from States of Uttar Pradesh, Bihar and Telangana as well, however lab confirmed MPX cases are from Delhi and Kerala only (Figure 4).
As per CDC as well as WHO data, India has reported 17 confirmed cases and 1 death till now.22 According to ICMR study, all retrieved MPXV sequences from India belongs to A2 lineage of clade 2b.
Genetic clades
Based on the epidemiological, molecular and animal evidences, existence of two different strains of monkeypox have been suggested in different geographic regions of Africa.
The Central African (Congo Basin) clade or Clade 1, which has historically caused more severe disease and with more transmissibility, with a greater degree of morbidity and mortality and viremia; and the other, clade 2, which was isolated from West Africa is less virulent, and typically causes self-limited disease with low case fatality, and is deficient in several genes found in Central Africa strains.16
Cameroon, has been the only country where both virus clades have been found, making it a geographical division landmark between the two clades.24 Infections in the current outbreak are from Clade II, or more specifically, Clade IIb. Based on metagenomic sequencing studies, some have proposed to name the current strain as Clade 3.25
Epidemiology
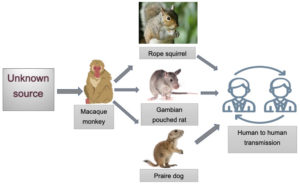
Even though Mpox was first identified in macaque monkey, but identification of the definitive animal reservoirs for the human infection remained, and continues to remain unknown. It has been isolated from different animal species which includes small mammals, such as rope squirrel, tree squirrels, dormouse, Gambian pouched rat, prairie dog, brush-tailed porcupine, and different species of monkeys as well (Figure 5).16,18
Figure 5. Diagrammatic representation of origin of MPXV and its spread to different reservoirs & humans
Early since 1980s studies have classified monkeypox case as primary case if the exposure was from an animal source, and secondary case if a human exposure have been linked.
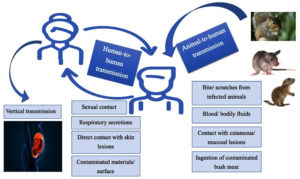
Monkeypox can be transmitted from 1). Animal to human, or 2). Human to human. Mode of transmission from animal to human are direct contact with blood, bodily fluid, mucosal or cutaneous lesions of infected animals, as well as by ingestion of inadequately cooked (bush) meat. It is mostly seen in people living near or in forest areas as they may have constant low-level or indirect exposure to infected animals (Figure 6).
It can be transmitted from one human to other by direct contact with cutaneous lesions of an infected person, or recently contaminated surfaces and objects, or by respiratory secretions. Although prolonged face-to-face contact is required for transmission by respiratory secretions, the persons in close contacts of active case, especially family members and health workers are at greater risk.
Vertical transmission (mother to fetus) via placenta have also been documented, which can lead to congenital monkeypox. Sexual transmission has also been reported in many European countries, especially in men having sex with men. However, for better understanding, more extensive studies are required.
Clinical Picture
Monkeypox presents with similar signs and symptoms as that of smallpox, but generally much milder. Clinical sign & symptoms are fever, headache, body aches, swollen, a general feeling of discomfort, and exhaustion. Lymphadenopathy is a prominent feature of monkeypox, which is not seen with other diseases that may initially appear similar, such as chickenpox, smallpox or measles. Incubation period range from 5-21 days.
Within 1 to 3 days after onset of fever, multiple vesiculo-pustular rash appears, usually beginning from face, sometimes on other body parts as well. Initially papular rash progress to vesiculation, then pustulation, and finally crusting, found in different stages simultaneously in different body parts. Lesions tend to be more intensive on face & extremities, rather than trunk. Lesions may also appear in oral mucosa, genitalia as well as conjunctiva.
The case is considered infectious starting from the appearance of symptoms till scabbing and re-epithelialization of cutaneous lesions.16 In rare instances, Complications can develop, including pneumonitis, encephalitis, keratitis, corneal scarring (which can lead to blindness) and secondary bacterial infections.26 These severe outcomes are reported largely from HIV positive persons or other immunocompromised persons and young children.27
Monkeypox is generally self-limited disease with the symptoms lasting from 2 to 4 weeks. In the general population the case fatality of monkeypox has referred from 0 to 11%. But in young children it is reported to be higher. In the recent outbreak the case fatality ratio is somewhat less ranging around 3-6%.
Case definition & identification
Ministry of Health & Family welfare (MoHFW), Government of India, has defined:
(a) Suspected case as “a person of any age having history of travel to affected countries within last 21 days presenting with an unexplained acute rash AND one or more of the following signs or symptoms: Swollen lymph nodes, Fever, Headache, Body aches, profound weakness”.
(b) Probable case as “a person meeting the case definition for a suspected case, clinically compatible illness and has an epidemiological link (face-to-face exposure, including health care workers without appropriate PPE; direct physical contact with skin or skin lesions, including sexual contact; or contact with contaminated materials such as clothing, bedding or utensils is suggestive of a strong epidemiological link)”
(c) Confirmed case as “a case which is laboratory confirmed for monkeypox virus (by detection of unique sequences of viral DNA either by polymerase chain reaction (PCR) and/or sequencing”.28
Diagnosis
For the diagnosis of monkeypox infection, clinician must have high index of suspicion and must be aware of typical as well as atypical presentation of the disease. A thorough history should be taken, including travel & sexual history, as well as any history of close contact with any person with similar looking rashes or suspected or confirmed monkeypox infection.
Other similar looking rash illness which should be included in the differential diagnosis includes measles, chickenpox, bacterial skin infection, syphilis, scabies, and medication-associated allergies. Distinguishing feature of monkeypox from chickenpox or smallpox is the presence of lymphadenopathy during the prodromal stage.
In case of a suspected monkeypox case, an appropriate sample should be collected, with proper infection prevention practices, and transported safely to the designated laboratory. Samples with optimal diagnostic results are skin lesions, the roof or fluid from vesicles and pustules, lesion base scrapings, and dry crusts. Wherever feasible, biopsy can be taken. Packing and shipping of the specimens should be strictly in accordance to the hospital and institution guidelines.26 As per MoHFW guidelines, blood sample (one in EDTA vial, and one sample in plain vial) and urine sample should also be collected.28 Samples from the skin lesions should be stored in a dry sterile tube with no viral transport media and transported maintaining cold chain.
Laboratory test of choice is Polymerase chain reaction (PCR) owing to its high sensitivity and specificity. As per MoHFW guidelines, initially PCR for Orthopoxvirus genus [Monkeypox, Cowpox, Camelpox, Buffalopox] are done on the specimen. If it come positive for Orthopoxvirus, then only confirmation test for Monkeypox DNA either by conventional PCR or real time PCR are done. Moreover, characterization of the positive clinical specimens can be performed by virus isolation and the Next Generation Sequencing of clinical samples (Miniseq and Nextseq).28
Orthopoxviruses are serologically cross-reactive, therefore serological tests based on antigen and antibody reactions as well as antigen detection methods are not recommended for diagnosis as they do not provide monkeypox-specific confirmation.
Case Management
Till date, no safe and proven cure exists for monkeypox.22 Case management for monkeypox should be fully optimized toward patient isolation, rehydration therapy and nutritional support, protection of compromised skin and mucous membranes, symptom alleviation, and monitoring and treatment of complications. Secondary bacterial infections which is a common complication of monkeypox, should be treated according to the protocol.21,27
Although, at present, there is no are no US FDA (Food and Drug Administration) approved treatments for Mpox specifically, some of antivirals, namely cidofovir, brincidofovir (a lipid-conjugate prodrug of cidofovir), and tecovirimat, having activity against smallpox, are reserved and used for the treatment of severe cases of monkeypox or in immunosuppressed individuals.29,30
Apart from antiviral agents, FDA had previously approved VIGIV intravenous (vaccinia immune globulin) for the management of complications due to vaccinia immunisation, which includes progressive vaccinia and severe generalized vaccinia.31 VIGIV provides passive immunity through specific antibodies against OPXV, which are retrieved from pooled human plasma who are vaccinated against smallpox.
Presently, all these (VIGIV, cidofovir, and tecovirimat) are available options for OPXV infection treatment in an outbreak scenario, under EA-IND (Expanded Access Investigational New Drug) protocols held by the CDC (Centers for Disease Control and Prevention).22
Preventive Measures
Infection Control measures to be followed during contact with infected person & its surroundings, as per CDC Interim Infection Control and Exposure Management Guidelines are:
- Performing hand hygiene and use of PPE, including gloves and gowns as contact precautions.
- Use of NIOSH-certified N95 or filter disposable respirator for droplet or aerosol precaution.
- Use of goggles or face shield for eye protection in cases where splash or spray of bodily fluids is likely to occur.
- Contaminated wastes generated during patient care, such as patient dressings, should be segregated & disposed strictly in accordance to facility based or local government guidelines. Patient-care equipment should be handled carefully to avoid any contamination of skin or clothings. Environmental surfaces in immediate vicinity of the active case should be cleaned & disinfected properly according to established guidelines.32
Apart from these, unprotected contact with known reservoirs should be avoided, specifically sick or dead animals. Even the contact to the body parts, meat and blood is dangerous. Therefore food including those should be thoroughly cooked before consumption.26
Success of containment of an outbreak particularly resides on active Surveillance and rapid identification of new cases so that chain of transmission can be broken.
Chief prevention strategies includes Educational intervention and raising awareness among general population about risk factors of the disease.
Immunization
Several past studies have concluded that vaccination against smallpox may provide approximately 85% protection against monkeypox and reduce disease severity.33,34 Because of genetic similarity between monkeypox and smallpox viruses, smallpox vaccines may be used to prevent monkeypox infections.
Presently smallpox vaccination is recommended by CDC for protection against monkeypox, but only for a limited group of individuals who are at a greater risk of exposure to the virus, namely public health workers and animal control workers involved in animal or human monkeypox case investigations, healthcare providers responsible for mpox patient care, or who have been in close contact with such patients, veterinarians and veterinary technicians, and laboratory personals. However, in immunocompromised individuals (HIV positive persons, transplant recipients, patients on cancer treatment, or on immunosuppressive medication, patient with primary immune deficiency disorders or severe autoimmune disorders) vaccines should be considered with cautions.22,35
Currently, there are two smallpox vaccines available for monkeypox prevention in US: JYNNEOS and ACAM2000. ACAM2000, a live replication-competent Vaccinia virus (a member of the OPXV genus), have a risk for serious adverse events, such as progressive vaccinia,36 eczema vaccinatum,37 and myopericarditis.38 However, Jynneos is a nonreplicating modified Vaccinia Ankara virus vaccine, which was licensed for both prevention of monkeypox and smallpox in the United States in 2019. (Table)
Table :
Comparison between two vaccines approved for monkeypox.
JYNNEOS |
ACAM2000 |
|
|---|---|---|
Developed from |
Nonreplicating modified Vaccinia Ankara virus |
Live replication-competent Vaccinia virus |
Produced by |
Bavarian Nordic |
Emergent Biosolutions |
Marketed as |
Jynneos (in USA), ImvanexTM (in EU), ImvamuneTM (in Canada) |
ACAM2000 (USA) |
Administration |
Subcutaneously in 2 doses, 28 days apart |
Percutaneously by multiple puncture technique in single dose |
Serious adverse effects |
fewer risk |
Risk for serious adverse events, such as progressive vaccinia, eczema vaccinatum, and myopericarditis (because of its replication-competent property) |
Safety for use in immunocompromised individuals |
Safer, as it does not produce live virus in vaccinated individuals |
Not-safe |
Aventis Pasteur Small Pox Vaccine is a third vaccine candidate similar to ACAM2000, and is an experimental smallpox vaccine made from replication-competent Vaccinia virus. It is authorized for use in the US under IND protocol (Investigational New Drug) or via ‘emergency use authorization’ in situations where the above mentioned two vaccines are not available. Unfortunately, no vaccines are available in India till now.
When the world is still recovering from global pandemic caused by a zoonotic virus, SARS CoV-2, re-emergence of another zoonotic virus, Monkeypox virus, become a global concern because of ongoing multi-country outbreak with chains of transmissions reported outside of endemic African countries. However, COVID-19 pandemic have taught us that awareness and preparedness are the most important ways to tackle these dire situations. Defining the attributes of current outbreak will be the best help in deciding action plans with available resources to contain the outbreak. As most common modes of transmission of MPXV from one human to another are respiratory droplets or direct contact with skin lesions, social distancing & contact tracing is crucial. Implementation of adequate screening methods in healthcare settings & education of all healthcare providers, including infectious disease specialists, and keeping high level of suspicion of typical as well as atypical presentation, with the help of well-defined clinical case definitions, are crucial steps in identifying cases. Prevention of new infections and breaking chain of transmission by timely isolation of suspected and confirmed cases and close monitoring of contacts are of essence. New vaccine development and ongoing research are imperative for emerging & re-emerging diseases.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Joklik WK. The poxviruses. Bacteriol Rev. 1966;30(1):33-66.
Crossref - Haller SL, Peng C, McFadden G, Rothenburg S. Poxviruses and the evolution of host range and virulence. Infect Genet Evol. 2014;21:15-40.
Crossref - Hughes AL, Irausquin S, Friedman R. The evolutionary biology of poxviruses. Infect Genet Evol. 2010;10(1):50-59.
Crossref - Oliveira GP, Rodrigues RAL, Lima MT, Drumond BP, Abrahao JS. Poxvirus Host Range Genes and Virus-Host Spectrum: A Critical Review. Viruses. 2017;9(11):331.
Crossref - Okyay RA, Bayrak E, Kaya E, et al. Another Epidemic in the Shadow of Covid 19 Pandemic: A Review of Monkeypox. Eurasian J Med Oncol. 2022;6(2):95-99.
Crossref - WHO recommends new name for monkeypox disease. 2022. https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease. Accessed November 30, 2022.
- Shchelkunov SN, Marennikova SS, Moyer RW. Orthopoxviruses Pathogenic for Humans. Springer US. 2005.
Crossref - Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID, Organization WH. Smallpox and Its Eradication. World Health Organization; 1988;6:1371-1409. https://apps.who.int/iris/handle/10665/39485. Accessed September 24, 2022.
- Lefkowitz EJ, Wang C, Upton C. Poxviruses: past, present and future. Virus Res. 2006;117(1):105-118.
Crossref - Moss B. Poxvirus DNA Replication. Cold Spring Harb Perspect Biol. 2013;5(9):a010199.
Crossref - Moss B. Poxvirus Cell Entry: How Many Proteins Does it Take? Viruses. 2012;4(5):688-707.
Crossref - McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005;3(3):201-213.
Crossref - Petersen E, Kantele A, Koopmans M, et al. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect Dis Clin North Am. 2019;33(4):1027-1043.
Crossref - Cohen J. Is an old virus up to new tricks? Science. 1997;277(5324):312-313.
Crossref - Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593-597.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15-25.
Crossref - Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLOS Negl Trop Dis. 2022;16(2):e0010141.
Crossref - Reed KD, Melski JW, Graham MB, et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N Eng J Med. 2004;350(4):342-350.
Crossref - Multistate Outbreak of Monkeypox – Illinois, Indiana, and Wisconsin, 2003. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5223a1.htm. Accessed September 24, 2022.
- Monkeypox cases reported in UK and Portugal. European Centre for Disease Prevention and Control. 2022. https://www.ecdc.europa.eu/en/news-events/monkeypox-cases-reported-uk-and-portugal. Accessed September 24, 2022.
- United Nations. Monkeypox Declared a Global Health Emergency by the World Health Organization. Https://News.Un.Org/En/Story/2022/07/1123152. Accessed 23 July 2022.
- CDC. Monkeypox in the U.S. Centers for Disease Control and Prevention. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html. Accessed September 27, 2022.
- India confirms first case of monkeypox in WHO South-East Asia Region. https://www.who.int/southeastasia/news/detail/15-07-2022-india-confirms-first-case-of-monkeypox-in-who-south-east-asia-region. Accessed November 8, 2022.
- Nakazawa Y, Mauldin MR, Emerson GL, et al. A Phylogeographic Investigation of African Monkeypox. Viruses. 2015;7(4):2168-2184.
Crossref - Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28(8):1569-1572.
Crossref - Monkeypox. https://www.who.int/news-room/fact-sheets/detail/monkeypox. Accessed September 24, 2022.
- Ogoina D, Iroezindu M, James HI, et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210-e214.
Crossref - Guidelines for Management of Monkeypox Disease. Ministry of Health and Family Welfare. GOI. https://main.mohfw.gov.in/diseasealerts-0. Accessed September 24, 2022.
- Adalja A, Inglesby T. A Novel International Monkeypox Outbreak. Ann Intern Med. 2022;175(8):1175-1176.
Crossref - Grosenbach DW, Honeychurch K, Rose EA, et al. Oral Tecovirimat for the Treatment of Smallpox. N Engl J Med. 2018;379(1):44-53.
Crossref - Wittek R. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int J Infect Dis. 2006;10(3):193-201.
Crossref - Centers for Disease Control and Prevention. Updated interim infection control and exposure management guidance in the health-case and com- munity setting for patients with possible monkeypox virus infection. 2003. http://www.cdc.gov/ncidod/monkeypox/infectioncontrol.htm.
- Hutin YJF, Williams RJ, Malfait P, et al. Outbreak of Human Monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7(3).
Crossref - Wenger J. Effectiveness of safer smallpox vaccine demonstrated against monkeypox. NIH News. 2004. http://www.nih.gov/news/pr/mar2004/niaid-10.htm.
- Centers for Disease Control and Prevention. Smallpox Vaccine and Monkeypox. Http://Www.Cdc.Gov/Ncidod/Monkeypox/Smallpoxvac- Cine_mpox.Htm.
- Bray M, Wright ME. Progressive Vaccinia. Clin Infect Dis. 2003;36(6):766-774.
Crossref - Reed JL, Scott DE, Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54(6):832-840.
Crossref - Halsell JS, Riddle JR, Atwood JE, et al. Myopericarditis Following Smallpox Vaccination Among Vaccinia-Naive US Military Personnel. JAMA. 2003;289(24):3283-3289.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.