ISSN: 0973-7510
E-ISSN: 2581-690X
The present study aims to evaluate the efficacy of an endosymbiotic bacterial strain associated with Sesbania species and its plant growth efficacy on peanut plants. Plant samples were collected around 10 different sites in Madurai, Tamil Nadu, India. We used the conventional isolation technique to isolate the endosymbiant. The qualitative plant growth promoting characters and a quantitative growth hormone analysis were performed. The active strains were phylogenetically studied, followed by seed germination and growth promotion characters by the grow bag method. Totally 80% of the samples (n = 8) are nodulated and 8 Rhizobium sp. are isolated. Out of eight, six isolates failed to absorb congo red on YEMA medium. About 37.5% of isolates showed positive results on ammonia, phosphate, siderophore, organic acid, and HCN production. About 25% were zinc solubilizers and ACC deaminase producers. Isolates SR1 and SR5 were found to have all plant growth characters reveal the maximum production of IAA, GA and cytokine. Both strains showed more efficient seed germination on Sesbania and peanut plants (>70%) than control. Peanuts showed seed treated with SR1 and SR5 strains had a higher maximum vitality index, than control. Both Rhizobium sp. treated data on plant growth, nodulation, and LHB content were found to be statistically significant at 0.05. Phylogenetic analysis using the 16S rRNA gene and evolutionary relatedness by the neighbor-joining method reveals that the similarity of active strains was found to be Sinorhizobium meliloti SR1 and Rhizobium leguminosarum SR5. The research concludes that the Sesbania endosymbiotic Rhizobium sp. has many physiological traits, including phytohormones and enzyme production, which can help overcome soil fertility as well as other legume plant growth promotion.
Nitrogen Fixation, Phosphatase, Seed Viability, Germination Index, ACC Deaminase, Agrobacterium
Sustainable agriculture depends on using alternate products that are less harmful to the ecosystem. For usage on legumes, biological nitrogen fixation (BNF) provides an economical and efficient replacement for chemical fertilizer. By converting atmospheric nitrogen (N2) into physiologically accessible ammonium, which plants can use to generate a variety of biomolecules, BNF increases the fertility of the soil.1 Rhizobia can also be found in legume root nodules can exist in two different forms: a symbiotic form within parent legumes and a free-living form in soils2 which plays major role in nitrogen cycle. In a microaerobic condition, the root and steam nodules grow to contain nitrogen-fixing bacteria including Rhizobium, Mesorhizobium, Sinorhizobium, Bradyrhizobium, and Azorhizobium spp.3 Plant growth-promoting Rhizobium strains also promote plant development through phosphate solubilization, siderophore secretion, extracellular enzymes, phytohormones, ammonia, and organic acid production.4 Some Rhizobium spp. produce hydrogen cyanide and ACC deaminase lowering toxic ethylene levels which have a variety of plant growth stimulation.5,6 In low-fertilizer soils, siderophores producers exhibit a strong attraction for Fe3+, which they can then convert to Fe2+ for legume uptake and utilization.7 Insufficient consumption of zinc and its low bioavailability also be overcome by using Rhizobium bacteria that solubilize zinc.8 Rhizobium radiobacter, a bacterium that solubilizes zinc and Phosphate, can be a useful to increase minerals availability.9 auxins stimulate a number of growth and developmental processes, including differentiation, elongation, and cell division alsoproduced by many bacteria.10 According to Iqbal and Ashraf11 application of these biostimulants with all plant growth characters could make a major contribution to the so-called bioeconomy and production.12 The well-known medicinal herb Sesbania grandiflora Linn is found across the plains of India widely grown in the tropical zones of India is Sesbania sesban, also referred to as “Egyptian sesban.” There are 85 species of annuals, perennials, herbs, shrubs, and trees in the genus Sesbania; 40 of these species are widely distributed over tropical and subtropical regions of the world and contain nodules with different colonization.13 The finding of successful multitalent genotypes is significantly influenced by the diversity of rhizobia varies among host and its capacity to fix N2, which makes it necessary to identify appropriate rhizobia for a given cultivar. The main objective of this work is to find and determine excellent PGP Rhizobium from the root nodules of sesbania leguminous plants, and to evaluate its characteristics on growth promotion of another legume peanut field. A potentially useful resource to improve peanut nodulation and yield comprises the kind microorganism infect the root. Therefore sesbania plant nodules selected to screen the Rhizobium diversity.
Collection of Sesbania plant and root nodules
Ten sesbania plants were gathered in the 10 different local site (9.9252°N, 78.1198°E) around Madurai District, Tamil Nadu, India, during December 2022. Young, healthy plants were selected at random, and their roots and root nodules were carefully uprooted to acquire undamaged roots. The plants were collected and delivered in less than two hours to the laboratory facility. After being cut off from the roots, the plump, fresh root nodules underwent a surface sterilizing procedure to isolate the nodulating bacterial flora. Similar regions were selected for the collection of Arachis hypogaea plant.
Isolation of Rhizobium spp.
After 30 seconds in 50% (v/v) ethanol, root nodules were placed in a 0.1% mercury chloride solution for 60 sec. Using sterile distilled water, three rinses were carried out in an aseptic manner. Using 10 mL of 0.85% normal saline, 1 g of surface-sterilized nodules were crushed and diluted up to a 10-8 under a septic condition. One milliliter (10-7) suspension was poured on pre sterilized plates and then 25 mL of sterile Yeast extract mannitol agar was transferred over the sample and allowed to solidify. Plates were kept in an incubator for five days at 28 °C for 72 h. white mucoid colonies were selected, subcultured and then Gram’s stained.
Phosphate solubilization test14
Pikovskaya’s agar plates with 2% tri-calcium phosphate are used to assess Rhizobium spp. phosphate solubilization ability, determining the phosphate solubilization index after 3 days of incubation. The following formula is used to determine the phosphate solubilization index.
SI = zone diameter – colony diameter/ colony diameter
Zn solubilization assay15
Zinc solubility was assessed using 0.1% ZnO on yeast glucose agar(Yeast 1 g; glucose 5 g; agar 15 g; water 100 mL). Isolates inoculated on agar plates and were cultured for three days at 30 ± 2 °C. Zinc-solubilizing strains with halo zone was measured, and zinc solubilization efficiency (ZSE) was calculated using the formula
ZSE = (HZ/C) x 100,
HZ is the diameter of the solubilization halo zone, and C is the diameter of the colony.
Ammonia production test
In sterile peptone water (10 g of peptone and 5 g of NaCl per 1000 mL of distilled water), bacteria were seeded and incbated 48 h. one mL of cell free supernatant was combined with 1 mL of Nessler’s reagent (50 g potassium iodide, 35 mL saturated mercuric chloride, 25 mL distilled water, and 400 mL 40% potassium hydroxide). The development of color intensity was recorded.
Siderophores test
The qualitative CAS agar test is used for screening of siderophore production. a modified nutrient agar plates that had been aamended with CAS solution was prepared. plates are inoculated with isolated strains and were incubated at 28 °C in the dark for 48 h. The development of siderophores is confirmed by the presence of yellow to orange zones.
Hydrogen cyanide production test
Isolates were streaked onto modified agar plates after YEMA was supplemented with 2 g glycine/L. The top of the plate contained a Whatmann filter paper No. 1 soaked in a solution of 0.5% picric acid and 2% sodium carbonate. After using parafilm to encapsulate the plates, they incubated for 48-72 h at 28 ± 2 °C. HCN production was detected by the hue changing from yellow to brown
Auxin (IAA) production and estimation
Yeast extract mannitol broth supplemented with 1 g/l of L-tryptophan was prepared and inoculated with isolates. Following two days of incubation at 28 ± 2 °C , the cultures were centrifuged at 5000 rpm. The cell free supernatant and Salkowski reagent was mixed and incubated at room temperature under dark condition. IAA production was identified by the formation of a pink or red tint. Utilizing standard IAA, the concentration was estimated.
Estimation of Gibberellic acid (GA3)
In order to produce GA3, isolates were added to 50 milliliters of YEM broth, which was then cultured for two days at 28 °C and 150 rpm. cell free filtrate was mixed with ethylacetate and solvent phase was collected after 24 h shaking and concentrated by evaporation. About 2 milliliters of the extract added to 1 milliliter of DNPH then incubated for 5 minutes at 100 degrees Celsius and then cooled in a water bath. 5 milliliters of 10% potassium hydroxide were added to this, and it was left to stand until a red wine coloration appeared. Finally, the content was diluted to a ratio of 1:2 using sterile distilled water and color intensity was measured at 430 nm. Using 100% alcohol, several aliquots of standard gibberellic acid (0.2-10 mg/mL) were produced and quantified in a comparable manner for the standard curve.
Extraction and estimation of Cytokinin
In order to produce cytokinin, 100 mL of YEM supplemented with 0.01% thiamine, 2 µg of biotin per liter, and 0.2% casein hydrolysate are added to 250 mL flasks. The media was inoculated with ten milliliter of culture, and it was cultivated for two days at 160 rpm and 28 ± 2 °C. The cell free filtrate was stored in methanol at -20 °C after being extracted three times using ethyl acetate. Above 665 nm, they were measured using spectrophotometry with known standard.
Seed germination test16
After being injected into nutrient broth, productive rhizobial colonies that produced PGH were let to grow over night. Sesbania grandiflora seeds were surface sterilized with 70% ethanol for two minutes, after which they were treated with 1% sodium hypochlorite and repeatedly washed with sterile water. The seeds were then soaked under 1% starch solution containing rhizobial cell (1 × 108 CFU) for 30 min. Sterilized petriplates with damp filter paper containing ten seeds of each treatment were spaced equally apart and incubated at 28 °C. The seedling emergence and seed germination was calculated by:
Number of emerged seeds/number of tested seeds x 100
Seed vigor index I = Germination percentage (%) x seedling length (cm)
Plant growth promotion studies on A. hypogea (Pot trials)
The pots were 14 inches in circumference and could contain 10 kg of soil. The studies were conducted on sterile soil. Three treatments in all, with three replications of each, were used. One hundred milliliters of culture (OD 1.2, or 2.0 × 108 cfu ml-1) was applied to the soil. Ten seeds were prepared with strains of rhizobia. To maintain seed coating the seeds kept an hour in Rhizobial phosphate saline buffer (RPSB) containing the suspension of the 108 cfu. Ten seeds were planted in a 5 cm depth in each pot. After germination during 3rd and 8th day germination index were calculated.
- Germination potential(DAY 3)= seed germinated / n x 100
- Germination index (D8) seed germinated / n x 100
- Vitality index = GI X root weight
Plant growth characters
After 40 days, the crops were taken out and the following metrics were noted: Plant parameters that were assessed were height (cm), root (cm), weight (g) of root nodules, surface are of root nodule, chlorophyll content (Arnon method), were recorded. The nodules were oven dried at 60 °C for 24 hours and weight of nodule was calculated
Dry wt. of nodule = Initial weight – final weight
Leghemoglobin content in nodular tissues
Wilson and Reisenauer’s detailed description of the cyanmethaemoglobin technique served as the foundation for the assays. This approach makes use of Drabkin’s solution and is based on spectrophotometric measurement. 100 mg of frozen nodules were crushed to release leghaemoglobin under 2 mL centrifuge tube along with 0.6 mL of Drabkin’s solution. The mixture was centrifuged at 10,000 rpm and 4 °C and the supernatant was collected. The collected aqueous phase made up to 2 mL and absorption was measured at 540 nm using a UV-visible spectrophotometer. Bovine hemoglobin standard was used with regression coefficient of 0.99.
Molecular analysis17
DNA from Rhizobium cells were isolated by using HigenoMB (Himedia) kit as per user manual. amplification done by 50 µl reaction mixture contained 10 ng DNA template, 1 µl of primer, 1 U of Taq DNA polymerase (Banglore Genei, India) and 100 µM dNTPs. Universal primers 8F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1540 R (5′-AAG GAG GTG ATC CAG CC-3′), for 16S rDNA were used. The PCR products were purified and sequenced by Sangers method. All the sequences were subjected to phylogenetic analysis. The homology of partial sequences were compared with the sequences from the DNA databases and similar sequences showing above 95% were retrieved by nucleotide Basic Local Alignment Search Tool (BLAST) program at the National Center for Biotechnology Information (NCBI) BLAST server (www.ncbi.nlm.nih.gov/BLAST)
Isolation of nodule associated bacteria
Sixty-day-old Sesbania grandiflora plants were collected from ten different sites in the Madurai region (Figure 1) and are designated as SES1 to SES10. The frequency of nodulation among collected plants was 80%. The mass of the root nodule was taken, and the maximum average mass was 1.24 ± 0.02 mg in SES9 followed by 1.2 ± 0.002 in SES7 and the minimum mass of 0.67 ± 0.01 mg was recorded in SES6 sample. All other plants have 0.7 to 0.9 mg mass of root nodule. Root nodules with high mass gave maximum colony forming unit 14 × 107 and the rest gave 6-8 × 107 CFU/g. A total of eight samples were used for isolation, and 8 strains were isolated on YEMA agar plates and designated as SR1 to SR8. Out of 8 strains, 87.5% of isolated colonies developed within 48 hours, grouped into fast-growing bacteria, and isolate SR4 found to be developing colonies after 72 h considered slow growing bacteria. The data of isolation of root nodule associated Rhizobium spp. was given in Table 1. Very few studies were documented on the colonization of rhizobial isolates from root nodules of Sesbania sesban.18,19
Table (1):
Frequency of nodulation and Rhizobium isolates from Sesbania sp.
No |
Sample Code |
Sampling site |
Average Nodule weight (mg) |
CFU X107 |
Strain code |
Growth pattern |
|---|---|---|---|---|---|---|
1. |
SES1 |
Kallikudi |
0.77 ± 0.17 |
8 |
SR1 |
Fast |
2. |
SES2 |
Velaneri |
0.8 ± 0.28 |
6 |
SR2 |
Fast |
3. |
SES3 |
Chinnakannur |
0.88 ± 0.002 |
7 |
SR3 |
Fast |
4. |
SES4 |
Soorikulam |
0 |
– |
– |
– |
5. |
SES5 |
S. Vellakulam |
1.2 ± 0.002 |
7 |
SR4 |
slow |
6. |
SES6 |
Chatrapatti |
0.67 ± 0.01 |
4 |
SR5 |
Fast |
7. |
SES7 |
Kodangipatti |
0 |
– |
– |
– |
8. |
SES8 |
Palamedu |
0.76 ± 0.02 |
7 |
SR6 |
Fast |
9. |
SES9 |
Alanganallur |
1.24 ± 0.02 |
14 |
SR7 |
Fast |
10. |
SES10 |
Kumaram |
0.9 ± 0.002 |
8 |
SR8 |
Fast |
Qualitative Plant growth hormone screening
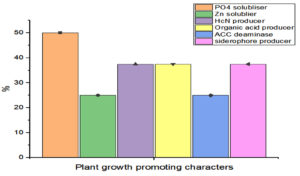
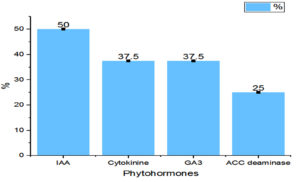
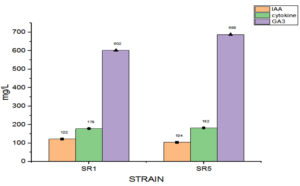
The potential of the Rhizobial strains on promoting plant growth promoting (PGP) characters data is given in Table 2. Among the isolated bacteria, different frequency of PGPR characteristics were discovered. The percentage of Rhizobium spp. with tested PGP properties is shown in Figure 2. Out of eight isolates, SR1, SR5, SR6 and SR7 were found to be phosphate solubilizers, whereas SR1 and SR5 were positive for zinc solubilization. While the isolates SR1, SR5 and SR7 showed production of siderophore, HCN and organic acid. The percentage of frequency was 37.5% of ammonia, siderophore, organic acid, and HCN producers, 50% phosphate solubilizer, and 25% zinc solubilizer. The ability of growth hormone production among isolates was identified by the qualitative method, and the data is given in Table 3. Figure 3 represents the percentage frequency of IAA (50%), Ctikinine and Gibberlic (37.5%) acid, and ACC deaminase (25%) production. Based on the data isolates, SR1 and SR5 strains were found to be effective, which showed the production of phytohormones, deaminase, NH3, siderophore, HCN, solubilization of phosphate and zinc, etc. The quantitative analysis of growth hormones produced by Rhizobium sp. is given in Figure 4. The auxin production was 122 and 104 mg/L among SR1 and SR5 isolates. The concentration of cytokines was 178 and 182 mg/L, and GA3 was calculated as 602 and 688 mg/L, respectively, by SR1 and SR5. The biocontrol of several soil-borne plant diseases is mostly dependent on Rhizobium spp. by the production of ammonia, siderophore, and HCN.20 The outcomes are consistent with previous research that found several species of Rhizobium and Mesorhizobium to be capable of solubilizing phosphate.21 According to Khanghahi et al.22 Rhizobium can dissolve an intractable source of zinc. Similarly, phosphate solubilizing Rhizobium sp. previously isolated from Sesbania sesban stem nodules was reported by Sridevi and Mallaiah.23 Production of siderophore, oranic acid, pophatase, and deaminase by different Rhizobium spp. is in line with the previous findings of various researchers.24,25
Table (2):
Plant growth traits of Rhizobium sp. from Sesbania sp.
S.code |
NH3 |
Posphate |
Siderophore |
Organic acid |
HCN |
Zinc solublization |
|---|---|---|---|---|---|---|
SR1 |
+ |
+ |
+ |
+ |
+ |
+ |
SR2 |
– |
– |
– |
– |
– |
– |
SR3 |
+ |
– |
– |
– |
– |
– |
SR4 |
– |
– |
– |
– |
– |
– |
SR5 |
+ |
+ |
+ |
+ |
+ |
+ |
SR6 |
– |
+ |
– |
– |
– |
– |
SR7 |
– |
+ |
+ |
+ |
+ |
– |
SR8 |
– |
– |
– |
– |
– |
– |
Table (3):
Plant growth hormone producing isolated Rhizobial colonies
S. code |
IAA |
Cytokine |
GA |
ACC deaminase |
|---|---|---|---|---|
SR1 |
+ |
+ |
+ |
+ |
SR2 |
– |
– |
– |
– |
SR3 |
– |
– |
– |
– |
SR4 |
– |
– |
– |
– |
SR5 |
+ |
+ |
+ |
+ |
SR6 |
+ |
– |
– |
– |
SR7 |
+ |
+ |
+ |
– |
SR8 |
– |
– |
– |
– |
Plant growth studies
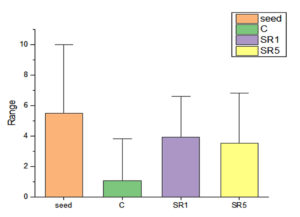
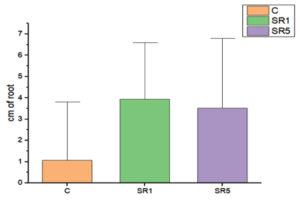
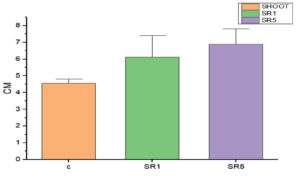
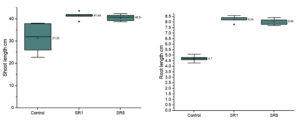
Seed germination capability of Rhizobium spp. among Sesbania seeds was tested and represented on Figure 5. The number of seed germinated among treatments was compared with control (Figure 6). The growth was recorded on 7th day and summarized in Table 4. The germination rate of the untreated group was 40% with a vigor index (VI) of 88.8. SR1 treated group showed maximum seed germination of 80%, 11.01*80 value is 880 and SR5 had a 70% germination rate along with VI of 873. Except for the control, the rhizobium-treated root showed the presence of IAA detected by Salwoski reagent treatment. Seedling length of a 7-day-old plant among control, SR1 and SR5 treatments was 7.22, 11.01 and 12.48 cm. Figure 7 represents the root length (RL) of control and treated groups, indicating that Rhizobium SR1 treated plants roots were 4.91 ± 1.14 cm and SR5 showed 5.68 ± 1.46 cm long. Likewise, the shoot length (SL) was calculated as 6.10 ± 0.99 and 6.80 ± 0.90 cm among the treated samples. The root and shoot growth among the control group was 2.67 ± 1.004573 and 4.55 ± 0.25 cm. Growth patterns among shoots and roots were compared. The two-tailed P-value of shoot between control and SR1 equals 0.0126 and SR5 was 0.0008 is found to be significant. Between the control and SR1 root lengths, the two-tailed probability is equal to 0.0158. The variation meets the standard requirements for statistical significance. According to conventional requirements, the statistical P-value of SR5 and control equals 0.0379, meaning that the difference is deemed statistically significant (Figure 8). Seed quality and seed coating are attributable to seed vigour.26 However, seed microbial loading, plant inheritance, external factors, and soil variables all affect seed germination.27 According to Chanda et al.28 increased seed germination depends on size of seed and growth hormone availability. Results on the effect of inoculation strains on sesbania seed germination, length of shoot, and root were highly significant compared to control.29
Table (4):
Seed germination and vigor index of Sesbania
Properties |
Control |
SR1 |
SR5 |
|---|---|---|---|
Percentage of seed germination |
40 |
80 |
70 |
Root length cm |
2.67 ± 1.004573 |
4.91 ± 1.14 |
5.68 ± 1.46 |
Shoot height cm |
4.55 ± 0.25 |
6.10 ± 0.99 |
6.80 ± 0.90 |
Seedling length |
7.22 |
11.01 |
12.48 |
Seedling Vigor index |
288.8 |
880 |
873.6 |
Root IAA Qualitative |
– |
+ |
+ |
Impact of Rhizobium on peanut growth
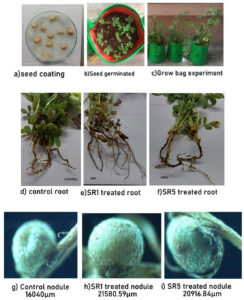
The potential of Rhizobium spp. on peanuts was studied under a grow bag experiment (Figure 9a-c). Both isolates showed 80% germination index. The germination potential of SR1 was 70% and SR5 was 60%. The vitality index of SR1 treated plant showed 154 with a root weight of 1.9 g and the SR-5-treated plant VI-144 with a root weight of 1.8 g. Comparing to the control, both the Rhizobium sp. treated bags showed high germination and vitality index. The rate of seed germination potential in control was 40%, and GI was 60% with a 90% vitality index (Table 5).
Table (5):
Seed germination impact of Rhizobium spp. on peanut
Properties |
Control |
SR1 treated |
SR5 treated |
|---|---|---|---|
Germination potential |
40 |
70 |
60 |
Germination Index |
60 |
80 |
80 |
root weight |
1.5 |
1.9 |
1.8 |
Vitality index |
90 |
152 |
144 |
The 40th day growth of bags showed the formation of a root nodule, and its total surface was microscopically measured (Figure 9d-f). The root nodule of the control was 16040 µm (Figure 9g), and the weight of the nodule was 124 mg/plant for 54 ± 5.43 number. SR1 root nodule surface area was 21580 µm (Figure 9h), average number of nodules is 111.8 ± 21.98 with 212 mg weight/plant. SR5 treated plants have 130.8 ± 25.81 root nodules with a total weight of 248 mg and a surface area of 20916 µm (Figure 9i). Figure 10a represents the shoot length, and Figure 10b represents the difference in root length between treatment and control. The average shoot length was recorded as 31.32 ± 6.85, 41.44 ± 1.73, and 40.54 ± 1.56 cm, and the root length was 4.7 ± 0.29, 8.24 ± 0.29, and 8.06 ± 0.29 cm, respectively, on control, SR1, and SR5. The number of nodules, root, and shoot length was given in Table 6.
Table (6):
Plant growth promotion of Rhizobium spp. on peanut
Group |
Root length cm |
Soot length cm |
Average nodule number |
Weight mg/plant |
IAA µg/g |
|---|---|---|---|---|---|
Control |
4.7 ± 0.29 |
31.32 ± 6.85 |
54 ± 5.43 |
124 |
72 |
SR1 |
8.24 ± 0.29 |
41.44 ± 1.73 |
111.8 ± 21.98 |
212 |
148 |
SR5 |
8.06 ± 0.29 |
40.54 ± 1.56 |
130.8 ± 25.81 |
248 |
152 |
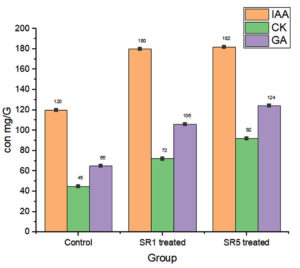
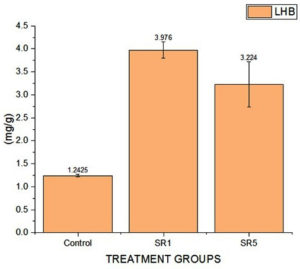
Further, the root sample growth hormone analysis reveals that the contents of IAA were 120, 180, and 182 mg/g of samples. Cytokine were 45, 72, and 92. The estimated GA content was 65, 106, and 124 mg/g (Figure 11). The length of the shoots and roots was more influenced by the Rhizobium sp. inoculation. In comparison to untreated pots, the length of the shoots and roots had risen by almost 50% and 23%, respectively. Rhizobium treated root nodules with isolates have a significantly positive effect (p-value < 0.0001, Table 7) on the leghaemoglobin content of pea nodules. A rise in the amount of hemoglobin was noted in SR5 followed by SR7. The comparative data among control and treated groups on LHB quantification was given in Figure 12 represent that SR1 root nodules have a maximum LHB estimated as 3.976 ± 0.17855 mg g-1. Isolate SR5 also shoed LHB concentration 3.224 ± 0.49445 mg g-1. Both treated plant nodules showed twofold higher LHB compared to untreated plant nodules (1.242 ± 0.03194 mg g-1).
Table (7):
Estimated LHB among treated and untreated plants
| Group | Mean (mg/g) | Standard Deviation | SE of Mean | P value |
|---|---|---|---|---|
| Control | 1.242 | 0.03194 | 0.01428 | <0.0001 |
| SR1 | 3.976 | 0.17855 | 0.07985 | |
| SR5 | 3.224 | 0.49445 | 0.22112 |
The current study’s findings demonstrate Rhizobium’s capacity for root colonization, which is further supported by an increase in root branches. Senthilkumar and Krishnamoorthi30 reported higher groundnut yields, plant growth, and germination percentages due to Rhizobium treatment. Biopriming of seed inoculation by PGB has an advantage that may enable more uniform germination, fast plant growth, and improved vitality index.31 In chickpea, it has been discovered that the treatment of Rhizobium sp. that produces IAA increases root length/biomass, shoot growth, and seedling germination.32
Phylogenetic analysis
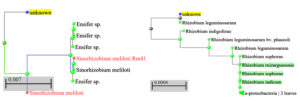
Two isolates’ 16S rRNA sequences were examined to find the taxa in the NCBI database. After aligning the sequences, a dendrogram was created. There were two different strains identified in the 16S rRNA sequencing. The SR1 have 1359 bp sequence showed relatedness to Sinorhizobium meliloti SEMIA gene, and the similarity index was 100% and 94.24% with Ensifer sp. RhPF3. the amount of genetic change with Ensifer was 0.05 and Sinorhizobium meliloti was 0.02 branch length (Figure 13a) and the genbank accession number is PP907784. The megablast of Isolate SR5 reveals that the isolate was 100% identity to Rhizobium leguminosarum and 99.71% similarity with R. indicum, Rhizobium acidisoli, Rhizobium anhuiense, Rhizobium sophorae, Rhizobium hidalgonense, and Rhizobium laguerreae. The branch length between SR5 and R. leguminosarum was 0.00083, and 0.0012 with Rhizobium acidisoli and 0.159 among Rhizobium hidalgonense, Rhizobium laguerreae (Figure 13b). The sequence is submitted to NCBI and the accession number is PP907783. Colonization relatedness of Sesbania sp. associated strains frequently related to the genera of Rhizobium sp., Mesorhizobium sp., Agrobacterium radiobacter and Sinorhizobium sp. was reported by Cummings et al.33
Out of ten plant samples processed, eight showed nodules and two sesbania (SES4 and SES7) collected at Soorikulam and Kodangipatti region did not show nodulation. According to Jensen et al.34 populations of rhizobia are negatively impacted by high soil N levels as well as the protracted shortage of legume host plants, which implies that a legume crop may have less applause. Nodule samples plated on YEMA showed the formation of whitish yellow gummy, which is found to be circular, semi-translucent, and mucilaginous. All the isolates were Gram-negative catalase positive, oxidase negative rods. The species in this genus have been divided into two groups, such as fast and slow-growers. Our findings concur with those of Taurian et al.35 who discovered N2-fixing bacteria that grow both quickly and slowly in nodule-forming plants.
Among the samples, SES5 plant isolate designated as SR4 found to be slow growing. The phosphate and zinc solubilization, as well as their ability to produce ACC deaminase, ammonia, siderophores, HCN, and organic acids among isolates, were recorded. SR1 and SR5 were promising substitutes for P and Zn supplementation that solubilize P and Zn. The vital plant nutrients phosphate (P) and zinc (Zn) are needed for nodulation, nitrogen fixation, growth of plants, and productivity.36
The addition of Nesler’s reagent to the culture tube showed three isolates had positive results on ammonia liberation. Four isolates showed a clear zone around the colony on Pikovskaya’s agar, and two strains showed clearance of zinc on agar plates. Among the eight Rhizobium isolates, three isolates were found to produce HCN, siderophore, and organic acid. Totally 4 isolates were IAA positive, 3 are cytokine and gibberelic acid producers, and two are positive ACC deaminase producers. The number of positive isolates was 3<4<3<3<3<2, respectively, on NH3, phosphate, siderophore, organic acid, HCN, and zinc solubilization. Likewise, the growth hormones IAA, GA, and cytokinin were maximum produced among SR1 an SR5. In order to promote plant growth, many Rhizobium that inhabit plant roots can produce gibberellin and indole acetic acid.37
The efficiency of two strains, SR1 and SR5 over the seed germination reveals that both are potent growth stimulators. Percentage of seed germination, root and shoot length, seedling length Seedling vigour index among Rhizobium inoculated sees was greater than control. Certain improved seed germination indices, are promoted and enhanced by the PGPR trait of SR1 and SR5 also reported by Kumar et al.38 The groundnut germination and nodulation efficacy of SR1 and SR5 indicates that the seeds loaded with those Rhizobium strains greatly improved the nodule LHB, size, and surface volume, as well as the seedling emergence and vigor. The early treatment of seedlings subsequently promotes the growth and development by rhizobial strains to enhance seed emergence and seedling characteristics. More vigour index among SR5 and SR1 treated seeds establishes the health of the seedling, germination, and nodulation. A partial 16S rRNA gene sequence of the 2 isolates acquired in this investigation was clearly determined to assign taxonomic classification down to the species level through the application of Sanger sequencing. Sequenced isolates were taxonomically identified by aligning their sequences to the NCBI 16S BLAST database using the BLAST algorithm. The taxonomy of the highest scoring sequence was chosen after the results of the current investigation were evaluated according to e-value. Sequence data revealed Sinorhizobium and Rhizobium as the effective symbionts of Sesbania nodule. The result is contrast to the report of Nusrat et al.39 who state that the colonization of Rhizobium closely related to Mesorhizobium, Agrobacterium, and Bradyrhizobium in Sesbania plants.
The current study emphasized the Sinorhizobium meliloti SR1 and Rhizobium leguminosarum SR5 isolated from the Sesbania root nodule capable of performing growth hormone production, mineral solubilization, and disease-protecting compound HCN and deaminase. Dominant plant growth characteristics recorded from Sinorhizobim sp. and R. leguminosorum were found to enhance maximum seed germination, vigor index, and the growth of legume plants. Thus, this strain can be applied to improve soils’ agricultural quality and aid in the development of crops.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Microbiology, Bharathidasan University for providing all necessary support to carry out the research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RKV designed the study. NS carried out the experiments. NS wrote the manuscript. RKV reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Zhu YG, Peng J, Chen C, et al. Harnessing biological nitrogen fixation in plant leaves. Trends Plant Sci. 2023;28(12):1391-1405.
Crossref - Fujihara S. Biogenic amines in rhizobia and legume root nodules. Microbes Environ. 2009;24(1):1-13.
Crossref - Suzuki S, Aono T, Lee KB, et al. Rhizobial factors required for stem nodule maturation and maintenance in Sesbania rostrata-Azorhizobium caulinodans ORS571 symbiosis. Appl Environ Microbiol. 2007;73(20):6650-9.
Crossref - Hou BC, Wang ET, Li Y, et al. Rhizobial resource associated with epidemic legumes in Tibet. Microb Ecol. 2009;57(1):69-81.
Crossref - Dakora FD, Phillips DA. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil. 2002;245(1):35-47.
Crossref - Berg G. Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol. 2009;84(1):11-18.
Crossref - Lebrazi S, Fikri-Benbrahim K. Rhizobium-legume symbioses: heavy metal effects and principal approaches for bioremediation of contaminated soil. Legumes for Soil Health and Sustainable Management, Springer:539: 2018.
Crossref - Backer R, Rokem JS, Ilangumaran G, et al. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci. 2018;9:1473.
Crossref - Verma M, Singh A, Dwivedi DH, Arora NK. Zinc and phosphate solubilizing Rhizobium radiobacter (LB2) for enhancing quality and yield of loose leaf lettuce in saline soil. Environ Sustain. 2020;3(6):209-218.
Crossref - Asgher M, Khan MIR, Anjum NA, Khan NA. Minimising toxicity of cadmium in plants-role of plant growth regulators. Protoplasma. 2015;252(2):399-413.
Crossref - Iqbal M, Ashraf M. Gibberellic acid-mediated induction of salt tolerance in wheat plants: growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis Environ Exp Bot. 2013;86:76-85.
Crossref - Rengalakshmi R, Manjula M, Prabavathy VR, Jegan S, Selvamukilan B. Building bioeconomy in agriculture: Harnessing soil microbes for sustaining ecosystem services. Towards a sustainable bioeconomy: Principles, challenges and perspectives. World sustainability series, Springer. 2018.
Crossref - Farruggia FT, Lavin M, Wojciechowski MF. Phylogenetic systematics and biogeography of the pantropical genus Sesbania Adanson (Leguminosae). Systematic Botany. 2018;43(2):414-429.
Crossref - Chen Q, Liu S, Bai Y, et al. Screening and identification of phosphate-solubilizing bacteria from reclaimed soil in Shanxi mining area. Plant Nutr Fertilizer Sci. 2014;20(6):1505-1516.
Crossref - Yasmin R, Hussain S, Rasool MH, Siddique MH, Muzammil S. Isolation, Characterization of Zn Solubilizing Bacterium (Pseudomonas protegens RY2) and its Contribution in Growth of Chickpea (Cicer arietinum L) as Deciphered by Improved Growth Parameters and Zn Content. Dose Response. 2021;19(3):15593258211036791.
Crossref - Sistani NR, Kaul HP, Desalegn G, Wienkoop S. Rhizobium Impacts on Seed Productivity, Quality, and Protection of Pisum sativum upon Disease Stress Caused by Didymella pinodes: Phenotypic, Proteomic, and Metabolomic Traits. Front Plant Sci. 2017;8:1961.
Crossref - Ilangumaran G, Subramanian S, Smith DL. Complete genome sequences of Rhizobium sp. strain SL42 and Hydrogenophaga sp. strain SL48, microsymbionts of Amphicarpaea bracteata. Front Microbiomes. 2024;3:1309947.
Crossref - Singh K, Gera R, Kumar R. Isolation and characterization of siderophore producing rhizobia from Sesbania sesban using different types of Indian soils. Int J Chem Stud. 2018;6(3):797-80.
- Dhull S, Singh K, Gera R. Intrinsic antibiotic resistance (IAR) of different rhizobial strains isolated from root nodules of [Cyamopsis tetragonoloba (L.) Taub.]. Chem Sci Rev Lett. 2018;7(26):692-697.
- Rasool A, Mir MI, Zulfajri M, Hanafiah MM, Unnisa SA, Mahboob M. Plant growth promoting and antifungal asset of indigenous rhizobacteria secluded from saffron (Crocus sativus L.) rhizosphere. Microb Pathog. 2021;150:104734.
Crossref - Wolde-Meskel E, van Heerwaarden J, Abdulkadir B,et al. Additive yield response of chickpea (Cicer arietinum L.) to Rhizobium inoculation and phosphorus fertilizer across smallholder farms in Ethiopia. Agric Ecosyst Environ. 2018;261:144-152.
Crossref - Khanghahi MY, Ricciuti P, Allegretta I, Terzano R, Crecchio C. Solubilisation of insoluble zinc compounds by zinc solubilising bacteria (ZSB) and optimization of their growth conditions. Environ Sci Pollut Res. 2018;25(26):25862-25868.
Crossref - Sridevi M, Mallaiah KV. Phosphate solubilization by Rhizobium strains. Indian J Microbiol. 2009;49(1):98-102.
Crossref - Menendez E, Perez-Yepez J, Hernandez M, Rodriguez-Perez A, Velazquez E, Leon-Barrios M. Plant growth promotion abilities of phylogenetically diverse Mesorhizobium strains: effect in the root colonization and development of tomato seedlings. Microorganisms. 2020;8(3):412
Crossref - Kumari P, Meena M, Upadhyay RS. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal Agric Biotechnol. 2018;16:155-162.
Crossref - Geneve RL. Vigor testing in small-seeded horticultural crops. Acta Hort. 2008;782:77-82.
Crossref - Rezapour R, Kazemi-arbat H, Yarnia M, Zafarani-Moattar P. Effect of seed size on germination and seed vigor of two soybean (Glycine max L.) cultivars. Int Res J Appl Basic Sci. 2013;4:3396-3401.
- Chanda S, Mridul AM, Sagar A, Sarwar A. Germination and seedling growth of Sesbania species as influenced by seed size. Progressive Agriculture. 2017;28(4):316-322.
Crossref - HasanMZ, Alam MS, Mian MH, Islam MZ. Isolation, Characterization and Selection of Rhizobium Strains for Cultivation of Sesbania sp. J Biosci Agric Res. 2015;03(02):79-86.
Crossref - Senthilkumar M, Krishnamoorthy R. Isolation and characterization of tomato leaf phyllosphere methylobacterium and their effect on plant growth. Int J Curr Microbiol Appl Sci. 2017;6(11):2121-2136.
Crossref - Miljakovic D, Marinkovic J, Tamindzic G, Dordevic V, Tintor B, Milosevic D, Ignjatov M, Nikolić Z. Bio-Priming of Soybean with Bradyrhizobium japonicum and Bacillus megaterium: Strategy to Improve Seed Germination and the Initial Seedling Growth. Plants. 2022; 11(15):1927
- Gopalakrishnan S, Srinivas V, Vemula A, Samineni S, Rathore A. Influence of diazotrophic bacteria on nodulation, nitrogen fixation, growth promotion and yield traits in five cultivars of chickpea. Biocatal Agric Biotech. 2018;15:35-42.
Crossref - Cummings SP, Gyaneshwar P, Vinuesa P, et al. Nodulation of Sesbania species by Rhizobium (Agrobacterium) strain IRBG74 and other rhizobia. Environ Microbiol. 2009;11(10):2510-25.
Crossref - Jensen ES, Peoples MB, Hauggaard-Nielsen H. Faba bean in cropping systems. Field Crops Res. 2010;115(3):203-216.
Crossref - Taurian T, Ibanez F, Fabra A, Aguilar O.M. Genetic diversity of rhizobia nodulating Arachis hypogaea L. in central Argentinean soils. Plant Soil. 2006;282:41-52.
Crossref - Ahmad Z, Malik A, Ahmad S, et al. Isolation, characterization, and effect of phosphate-zinc-solubilizing bacterial strains on chickpea (Cicer arietinum L.) growth. Saudi J Biol Sci. 2019;26(5):1061-1067.
Crossref - Shokri D, Emtiazi G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr Microbiol. 2010;61(3):217-225.
Crossref - Kumar H, Dubey RC, Maheshwari DK. Effect of plant growth promoting rhizobia on seed germination, growth promotion and suppression of Fusarium wilt of fenugreek (Trigonella foenum-graecum L.). Crop Prot. 2011;30(11):1396-1403
Crossref - Nahar N, Begum A, Akhter H. Isolation, identification and molecular characterization of Rhizobium species from Sesbania bispinosa cultivated in Bangladesh. Afr J Agric Res. 2017;12(22):1874-1880.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.