Cervical cancer (CC) contributes to 6%-29% of all cancers in women. In India, 527,624 new cases of CC are added every year. India contributes to ¼ of deaths worldwide to CC it occurs often in women around the age of 30 yrs which is contributed by a specific kind of human papillomavirus causes long-term infection and inflammation (HPV) which result in morphological changes in the cells of cervix the region that connect vagina and uterus. Cervicovaginal microbiome is observed to be highly diverse among patients with CC where there is reduced number of Latobacillus spp that leads to dysbiosis and decrease in pH and eventually colonised by other anaerobic bacteria. The shift in community state types (CST) is highly associated with the Human Papillomavirus infection and its further progression to cervical dysplasia or CIN i.e. ‘Cervical intraepithelial neoplasia’ and malignant tumour of the cervix uteri. The purpose of this research is to figure out if there’s a link between the cervico-vaginal microbiota and gynaecological cancer and the review also focuses whether cervical microbiome signatures can predict the severity of infection leading to development of CC? Probiotics can be used as a potential alternative to balance the dysbiosis of the cervicovaginal environment. Hence the review summarizes the current knowledge and the interaction of different bacterial groups with Human Papilloma Virus infection and development of CC.
Cervical Cancer (CC), Vaginal Microbiome (VMB), Probiotics, Human Papilloma Virus
Tumour in the cervix region is now the leading frequent malignancy in the world.1 Early sexual behaviour, poor sexual hygiene, frequent use of contraceptive pills, multiple sexual partners, smoking, drinking are some of the causes of cervical cancer. According to the latest Globocan statistics, there are 569, 847 cases of CC and 311, 365 deaths.2 Human papillomavirus (HPV) is the causative organism of this infection and currently there are over 200 different strains of HPV that are known to infect the epithelial layer and mucosal tissues. HPV strains are categorized into two based on the risk status: higher risk (hr-HPV) and lower risk (lr-HPV). Anogenital warts are related with lr-HPV types, while cervical dysplasia and malignant tumour of the cervix,3 are connected with higher risk – Human Papilloma Virus types. Approximately 99 percent of CC cases had high risk DNA of the higher risk – Human Papilloma Virus,4 with Human Papilloma Virus -16 and Human Papilloma Virus -18 accounting for roughly 70% of Cervix tumour cases worldwide.3,4
The development of CC is dependent on the persistence of the HPV infection, which leads to cervical lesion progression and unrestricted cell proliferation, eventually leading to cancer. Most women who have HPV infection do not progress to CC because the host immune system is capable of regulating the infection and preventing the development of cervical lesions and subsequent cancer.5 Yet only few women infected with HPV develop CC, which are influenced by multiple factors such as host immune response, cervical microenvironment dysbiosis that progresses to CIN and to CC further.6 The cervicovaginal microbiota has been linked to HPV infectivity, as well as CIN and CC in recent investigations.7 8 The landscape of the vaginal Microbiome and its impact on immunological responses in CC, as well as its acts as a bioindicator for predicting Human Papilloma Virus infectivity along with the development of tumour, are the topic of this note.
A study published in March 2022, revealed an association between genital inflammation and low Lactobacillus concentration and reduction in virome diversity. Further, association of other viral entities affecting bacteria known as bacteriophages also contribute to disease prognosis. The findings of the study further emphasized the importance of virome study and its complex interactions with other constituents of the human microbiome.9
Genome of HPV
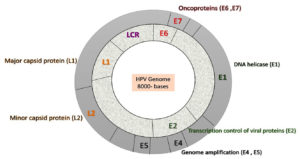
HPV is a circular, non-enveloped, small double stranded DNA which is approximately 55 nm in diameter and size of 8 kb in length. HPV is complex in nature and has three regions. 1) The ‘E1, E2, E4, E5, E6, and E7’ are open reading frames found in early proteins. E1, E2, and E4 are regulatory proteins, while E6, E5, and E7 are oncoproteins. Early protein has a property of replication, transcription, translation, cell signalling, cell control and immune modulation. 2) Late protein has two capsid structural proteins, L1 and L2, which stand for chief and small protein coat, respectively. Viral capsid is responsible for the transmission, spread, and survival of the virus. 3) The “Locus control region (LCR) or Upstream regulatory region (URR) ” has a role in the viral replication and transcriptional regulatory element.10 Upstream regulatory region has the DNA recognition site for both viral and host transcription factor which are essential for viral pathogenesis and are prominent targets for small molecule-based approaches for the treatments. HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 subtypes are higher – risk (HPV) infections. HPV 16 and 18 are highly carcinogenic. The low risk HPV infection are 6,11,40,42,43,44,54,61,70,72,81 subtypes (Figure 1).11
Figure 1. Genomic organization of the HPV genome representing oncogenes (E6/E7) and other viral genes (E1, E2 coding for viral proteins required for viral replication).
Earlier studies shows that genital tract dysbiosis and cytokines expressions might have a crucial role in the progression of HPV infection and cervical intra-epithelial neoplasia (CIN) which results in cervical cancer. Lactobacillus depletion and microbiome composition alterations may lead to increased expression of pro-inflammatory cytokines that can increase malignant cell proliferation and HPV E6 and E7 oncogene expression.12
Staging of Cervical Cancer (CC)
The staging system for cervical cancer was established by the International Federation of Obstetrics and Gynecology (FIGO) in 1950, with various changes based on current trends. The staging was published in 2018 and it is categorised based on the image diagnostic identification (Ultrasound or Magnetic resonance imaging [MRI]) and histopathological findings. Basically, cancer is classified into four stages.
STAGE I- Cancer present within the cervix and there is no spread of the lesion beyond the cervix. It includes:
Stage IA- This type of cancer cells are viewed under microscope. Evaluation is done based on the size of the tumour; Stage IA 1-Tumour size is less than 3 mm in depth. Stage IA 2-Tumour size is less than 5 mm in depth, Stage IB-The size of tumour is larger than stage IA. Stage IB 1-Tumour size is more than 5 mm in depth of stromal invasion and 2 cm in wide, Stage IB 2-Tumour size is more than 2 cm in depth and 4 cm in wide, Stage IB 3-Tumour size is more than 4 cm.
STAGE II- The cancer has spread to the cervix’s closer region, yet it is still present in the pelvic wall. It is not contagious and does not spread to other sections of the body. It includes:
Stage IIA- Cancer spreads to the upper part of vagina. It does not spread to next region which is called parametrium, Stage IIA 1-Tumor size is less than 4 cm, Stage IIA 2-Tumour size is more than 4 cm, and Stage II B-Tumour has reached the parametrium region nut not up to the pelvic wall.
STAGE III- The cancer has progressed to the lower vaginal wall. Swelling of kidney takes place termed as hydronephrosis, it involves pelvic and paraaortic lymph nodes. It stops the kidney from functioning. It includes:
Stage III A-Tumour does not affect the wall of pelvic region; Stage IIIB- Tumour affects the pelvic wall and the kidney, Stage IIIC-In this stage the tumour affects the regional lymph nodes irrespective of tumor size and extent. It is unclassified into III C1 -involves pelvic lymph node metastasis only & IIIC2 – involves paraaortic lymph node metastasis.
STAGE IV- Last stage of cancer which spreads throughout the body and affects different organ. It includes:
Stage IV A-Tumour affects the bladder or rectum and adjacent organs, Stage IV B-Tumour has spread to distant organs and entire region.13
Types of Cervical Cancer (CC)
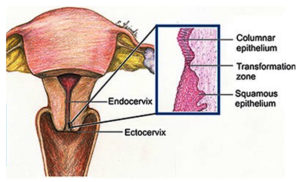
Depending on clinical examination done, cancer cells are divided into two types. Squamous cell carcinoma and adenocarcinoma. The cervix is divided into two parts: the ectocervix and the endocervix. The cells present in the endocervix are glandular cells and the cells present in the ectocervix are squamous cell. Transformation is the zone, where these cells meet each other. Initially there will be only intraepithelial lesion in the transformation zone. After a period up to even 10 years it can turn malignant. The terms for the transformation of normal cells into malignant cells are cervical dysplasis(CIN), squamous intraepithelial lesion (SIL), and dysplasia. (Figure 2)
Figure 2. Structure of female reproductive organ indicating Ectocervix & Endocervix with transformation zone.
https://www.jaypeedigital.com/book/9788180617423/chapter/ch3
Cervicovaginal Microbiome
Human body is made up of trillions of microorganisms that exists together and interact with each other and with the host.14 Lederberg and McCaray explained the word “microbiome” which describes about the genes of commensal (bacteria), mutualistic organisms, and harmful bacteria that distribute the same living area and interact in a complicated way.15 The microbial genome project or HMP, are starts in 2008, investigated about the diversity of microbial makeup in various body areas. The vaginal microbiome of 396 females of various nationalities was studied, and five Community state types (CST) were found and labelled as ‘CST I, II, III, IV, and V’.16 Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii dominate CST I, II, III, and V, respectively. CST IV, on the other hand, is created by a decrease in lactobacillus and a large variety of vaginal bacteriosis (BV) – related bacteria. Most of this bacteria are belongs to anaerobic category. The species “Gardnerella vaginalis, Megasphaera, Sneathia, and Prevotella” are among the most commonly found bacteria. In vaginal microbiome (VMB) composition, there is a repeated evolution of microbiota even in same women at various stages of her life, especially between CST III & IV,17 which is inclined by a variety of external and internal factors such as civilization, hormonal changes, sexual activities, cleanliness, lactation, diabetes and stress factors.18 CST IV in the cervicovaginal niche is strongly linked to HPV infection persistence and development into cervical lesions.8
Generally ‘Lactobacillus crispatus’ dominates ‘CST I’, ‘L. gasseri’ dominates ‘CST II’, and ‘L. jensenii’ dominates ‘CST V’ in healthy CSTs. An abundance of anaerobic bacteria characterize common dysbiotic CSTs; ‘CST III’ is dominated by ‘L. iners’, CST VI by Gardnerella vaginalis, and CST IV by a large concentration of non-aerobic bacteria and a low number of Lactobacillus species. CST IV-A (Anaerococcus, Peptoniphilus, Prevotella, and Streptococcus); CST IV-B (Atopobium vaginae, Megasphaera) and others; CST IV – C (Anaerococcus, Peptoniphilus, Prevotella and Streptococcus); CST IV-D (Anaerococcus, Peptoniphilus, Prevotella) and others (Table). Previously, these cervicovaginal CSTs were linked to considerably varied prevalence of infecting HPV genotypes.19,20
Table:
Classification of CST and dominant species in vaginal microbiota.
No |
Community state types (CST) |
Dominant Species |
|---|---|---|
1. |
CST I |
Lactobacillus crispatus |
2. |
CST II |
L. gasseri |
3. |
CST III |
L. iners |
4. |
CST IV |
Large concentration of non-aerobic bacteria and a low number of Lactobacillus species |
CST IV-A |
Anaerococcus, Peptoniphilus, Prevotella, and Sneathia |
|
CST IV-B |
Atopobium vaginae, Megasphaera |
|
CST IV – C |
Anaerococcus, Peptoniphilus, Prevotella & Streptococcus |
|
CST IV-D |
Anaerococcus, Peptoniphilus, Prevotella |
|
5. |
CST V |
L. jensenii |
6. |
CST VI |
Gardnerella vaginalis |
The oestrogen hormone, which is found in the vaginal epithelium, stimulates maturation, proliferation, and glycogen accumulation, all of which are necessary for Lactobacillus species to thrive, resulting in a lactobacillus rich environment. Lactobacillus metabolises glycogen to produce lactic acid, which is responsible for the vaginal acidity. According to recent research, the -amylase enzyme in mature vaginal epithelium under oestrogen control catabolizes glycogen to produce simple sugars including maltose, maltotriose, maltotetraose, and dextrins, which favour Lactobacilli spp colonization.21
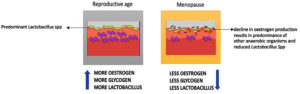
It has been discovered that a healthy vaginal microbiome fluctuates to local immune responses in response to hormonal changes during reproductive age,22 especially variations in oestrogen and progesterone levels have a profound effect on vaginal flora composition during the menstrual cycle.18 During Menopause, decline in oestrogen production results in decrease in Lactobacilli spp and predominance of other anaerobic organisms which can be the primary cause of infections in female reproductive organs (Figure 3). The condition can be reverted by oestrogen replacement therapy.23
Figure 3. Alteration of Lactobacillus spp. between reproductive age and Menopause due to decline in oestrogen production results in predominance of other anaerobic organisms.
According to Di Pietro et al., women with concurrent C.trachomatis and HPV infection had a greater diversity of other species such as G.vaginallis, A. vaginae, and a lower number of Lactobacillus spp in their cervical microbiomes.24 Healthy women, on the other hand, had a higher prevalence of Lactobacillus spp. Women with co infection were more likely to have L.iners. Lactobacillus species can produce two different types of lactic acid isomers: L and D. Antimicrobial peptides (AMP), bacteriocins, and biosurfactants are produced by the latter, which play a greater protective role in vaginal dysbiosis.23 Only lactic acid can be generated by L.iners. Hydrogen peroxide is an antibacterial agent that inhibits bacterial growth. On the downside, L.iners produces inerolysin, a pore-forming cytotoxin comparable to Gardnerella spp secretes vaginolysin which causes pores in the vaginal epithelium. L.crispatus dominated VMB maintains mucosal surface integrity. CST I, II, and V all have dominant Lactobacillus (non-L.iners) species and generate large quantities of lactic acid, H2O2, and bacterial by-products whereas, CST III & IV shows Lactobacillus iners dominant communities including diverse range of other anaerobic species, which produces less lactic acid and exhibit production of interolysin, sialidase and other acidic products which modulates the immune response by activating pro-inflammatory cytokines in cervical region. Hence to conclude it is observed that VMB dominated by L.iners (CST III)is considered to be connected with higher threat of enlargement of precursor lesions and leads to CC, whereas VMB dominated by L.crispatus has lower risk of developing into CC.25
Microbiome & Cervical Alterations in Development of Cervical Cancer
Host Defence Alterations in Cervicovaginal Microenvironment
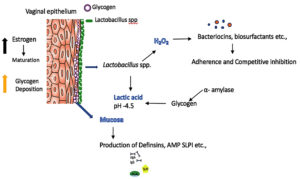
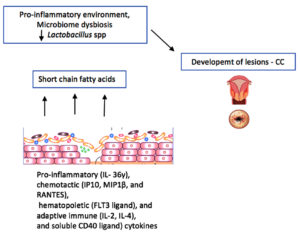
The microbiome composition plays a major role in protective mechanism against female reproductive tract infections. It also helps in maintaining epithelial barrier of mucosal region, production mucous & a-hydroxypropionic acid secretion and eliciting an immune reaction in the cervical microenvironment against pathogens. From this perspective, vaginal mucosa acts as a first line of defence against invading pathogens. Lactobacillus rich microenvironment, produces high amount of lactic acid and maintains the pH of 4.5, which inhibits the colonization of other microbes. It also produces bacteriocins, defensins and other Antimicrobial peptides (AMP) that prevents the colonization of pathogenic organisms (Figure 4). AMP levels and Secretory Leucocyte protease inhibitor (SLPI) are identified to be decreased in women with Bacterial vaginosis (BV) and high in healthy women implying dysbiosis of the microbiome which has got a profound effect on establishment of infection.26,27 The implication of lactic acid depletion leads to reduction in anti-inflammatory effects. Lactic acid reduces inflammation produced by Toll-like receptors (TLR) agonists and it also stimulates the Interleukin -1 pathway by the synthesis of Interleukin-1 receptor antagonist (IL- 1Ra).28 Hence the proposed mechanism of vaginal dysbiosis is a rise in inflammation promoting cytokines and chemokines associated with pathogenic microbial diversity contributing to the increase in inflammatory responses (Figure 5).29 According to one study, females with Cervical tumour or dysplasia and female without tumour had a non-lactobacillus-dominant microenvironment considered by “proinflammatory (IL-36), chemotactic (IP10, MIP1, and RANTES), hematopoietic (FLT3 ligand), and adaptive immune (IL-2, IL-4, and soluble CD40 ligand) cytokines”, as a result correlating dysbacteriosis, Swelling and CC.30 Another study by Audirac CA. et al., analysed the cervical microbiome and cytokine profiles at diverse stages of dysplasia. In hr HPV infection, the microbiome community shifts from ‘L. crispatus to L.iners’, as the illness progresses to SIL (Squamous intraepithelial lesion) in which a microbiome diversity was observed to be dominated by the species such as Sneathia and Fusobacterium type. In this scenario it is concluded from the study, HPV infection creates an immunosuppressive cervical microenvironment aided by microbiome diversity through expression of Interleukin-10 and macrophage type II induction, which is stimulated by Transforming growth factor beta-1 derived from microbiota, creating encouraging role between microbiota and cytokine profile.7
Figure 4. Host defence alterations by reducing the pH, altering the mucosa layer and production of hydrogen peroxide.
Oxidative Stress and Cervical Alterations
‘Oxidative stress’ is defined as an imbalance in the intracellular reduction-oxidation process caused by the presence of too many reactive oxygen species (ROS), which can be caused by microbiome dysbiosis. In biological systems, ‘superoxide radical (O2.-), hydrogen peroxide (H2O2), and hydroxyl radical (OH*)’ can all play a dual (beneficial and detrimental) role. The build up of reactive oxygen species (ROS) in the cells might potentially cause oxidative damage. As a result, imbalance in the free radicals and antioxidant has been associated with a promotion and advancement of a variety of malignancies, together with CC.31
The accumulation of these ROS causes a high level of cellular injury, which cause HPV incorporation and lead to cell transformation.32 Imbalance in the free radicals and antioxidants plays a major role in Human Papilloma Virus-mediated CC; the build up of these ROS causes a high level of cellular damage, which may allow HPV integration and lead to the changeover of normal cells into the tumorigenic cells. HPV assimilation into the host genome disrupts early genes (E2) and prevents controlled production of oncogenes (E6/E7), resulting in uninhibited function of HPV E6/E7 proteins, which creates the enhanced increased multiplication of cells and reduced cell death.33 Women with BV had ten times higher amounts of H2O2 than healthy individuals, according to Chen et al.33 However, because the cervix-vaginal environment is deficient in oxygen level and lactobacillus need a lot of oxygen to make hydrogen peroxides; few studies have found an agreement on the defensive role of H2O2 in the cervix-vaginal microbiome and its impact on the vaginal microbiota.34 To validate the role of VMB especially dominated by Lactobacilli spp, most studies used aerobic conditions that observed the antimicrobial effects of H2O2, However when Lactobacillus are grown in a hypoxic condition, results in fermentation leading to the production of lactic acid, turning the cervicovaginal environment to an acidic pH, which can in turn inactivate the BV causing bacteria ‘Chlamydia trachomatis’, which was unobserved in the availability of H2O2.35 More research is needed to better understand the link between oxidative stress and cervicovaginal microbiome dysbiosis, which could lead to a better knowledge of how cervical lesions and cancer are generated by microbiome dysbiosis.
Microbiome and Cervical Cancer Development
Cervical cancer is linked to chronic cervicitis (inflammation of the cervix). 36 In women, pelvic inflammatory disease (PID) is caused by a bacterial infection that spreads from the cervix to the uterus and fallopian tubes. Certain bacteria in PID produce antigens that cause a persistent inflammatory response. The formation of a microbiome enriched with swelling-enhanced bacteria near the cervix uteri, causing cervicitis, has been linked to PID and cervical cancer. Studies show there are higher incidence of HPV prevalence in women who have PID contributing to the development of CC when compared to the healthy patients.37
The dysbiosis of bacterial populations has been linked to immunological dysregulation and the development of a tumor-promoting microenvironment.38 BV has been linked to greater rates of HPV infection, implying that a decline in ‘lactobacilli’ may confer to the determination of HPV- infection. It was well established that HPV alone is not enough to cause CC. The host immunological reaction is able to suppress the establishment of severe lesions and tumours in the majority of infected women.39 However, multiple studies have linked distinct VMB with HPV infection to the development of various degrees of CIN and CC.
Mitra et al. looked at 169 women who were referred for colposcopy and found that there was more bacterial variety and less lactobacilli, which was strongly linked to the severity of the cytological abnormalities. According to the study, CST- IV was widespread in 40 percent of the females with dysplaxia, with just ten percent having normal cytology. The activation of pro-inflammatory cytokines and the employment of CD4+ cells are both elevated in CST IV. Instead, the CST I was found in 50 percent of cytological exams but only in 20 percent of CC instances. The presence of L. jensenii was strongly linked to the severity of the lesion.40
Diversity in microbiota composition especially ‘L. crispatus and L. Iners’ were the principal strains for Human papilloma virus negative and Human papilloma virus positive females with absence of cytological alterations, cervical lesions including cancer. Sneathia species and Fusobacterium spp. be leading in ‘squamous intraepithelial lesions and cervical cancer women’.7 Lactobacillus dominance decrease mutually with cervix tumour with increasing harshness with Sneathia spp, which are contributing to lower or higher grade pre-cancerous lesion and invasive cervical cancer.30 Similarly CST IV (highly diverse microbiota with less Latobacillus spp) has direct impact on cervical abnormalities leading to severity. The cluster was more common in “low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL), and cervical cancer”. However, HSIL samples had a higher incidence of vaginal microbiome ‘Sneathia sanguinegens, Anaerococcus tetradius, and Peptostreptococcus anaerobius’ than LSIL samples, which implies that there exists a shift in micro-biome community depends on the disease nature.40
Cervical precancerous lesions that regress have a distinct immunological microenvironment than those that proceed to malignancy.41 Specific bacteria, such as Gardnerella, and a high level of microbial diversity can be utilized as remarks of cervical alterations to recognize women at threat of acquiring persistent HPV infection, CIN, and cancer.42 As a result, the environment of the cervical microbiota has a direct impact on the wellbeing of the lower part female reproductive region, as well as its capability to protect against dysbiosis and infectivity.
Cervical Microbiome as Biomarkers
It is largely unforeseeable, Why some women get chronic cervical HPV infections and are at the highest risk of acquiring invasive cervical lesions. Amsel’s clinical criteria and direct staining of secretions from vagina is based on Nugent score which are used in modern diagnosis.43 The Nugent score ranges from ‘0 – 3’- normal, ‘4–6’ – intermediate, and ‘7–10’- BV, and is based on the amount of big ‘Gram (+) rod shaped (Lactobacillus morphotypes), Gram negative, or Gram-variable (BV flora) bacteria’ found in secretion collected during a Papanicolaou test. Owing to certain limitation now a day’s molecular diagnostic techniques are much reliable,44 where the sensitivity of detection (90-94%) is much higher than conventional procedures. By Comparing the Nugent score and Amsel’s clinical criteria the ‘real-time PCR, semi-quantitative, or quantitative multiplex real-time PCR’ assays can differentiate bacterial species such as ‘A. vaginae, BVAB-2, Megasphaera type 1 and 2, L. acidophilus, L. crispatus, L. jensenii, and G. Vaginalis’ with increased sensitivity (90–99%) and unpredictable specificity (70.2–95%).45,46
With regards to Cervicovaginal microenvironment, which varies highly based on CST indicating the presence of dominant variants. ‘CST III & IV’ is the most infective communities that can lead to the development of persistent infection with HPV and development of CC. CST IV is the most diverse type, including ‘Gardnerella, Prevotella, and Atopobium’ as major species, as well as other diverse species with varying abundance, such as ‘Anaerococcus sp., Peptostreptococcus sp., Fusobacterium sp., Moryella, Sneathia, and Schlegelella’.47
Women with cervicitis, PID or BV, non-consideration of symptomatic prominence or severity of infection have higher incidence of cervical lesions or viral infections.18 In scientific studies, the current technology for detecting bacterial species is 16S- High Throughput Screening (HTS). This approach is frequently utilized as a clinical diagnostic tool because it can capably notice the complication and specific characteristics of bacteria present in a sample.48 The challenge of doing HTS lies in the data analysis, though in recent years development of various software and user friendly pipelines for 16S analysis has somehow bypass the difficulties.49 Alternative molecular diagnostic kits have been created and are available on the market to detect specific bacteria in the cervicovaginal microenvironment utilizing cervicovaginal fluid (CVF) smear samples, such as the use of direct probes tests in real time PCR.
With the help of above discussed high throughput screening techniques (HTS) can these microbiome signatures used as biomarkers? The findings of earlier studies on various potential biomarkers such as tissue inhibitors of metallo-proteinases ‘TIMP -1 and TIMP-2’, Matrix metallo-proteinases ‘MMP -2 and MMP -8’, p62 and isomer od vacuolar ATPase (a2V) etc., have proved to have an influence to alter the microbial niche in uterine cervix. Path analysis was used in a study to see how the composition of the vaginal microbiota, the amounts of a variety of compound in vaginal secretions, and the length of the cervical canal in pregnant women interacted. Yet the outcome of the study did not give a profound evidence of these microbiomes that can be used as biomarkers, whereas the CST III & IV said to have an influence on pregnancy outcomes it has been observed that vaginal dominance by Latobacillus spp promotes healthy pregnancy.50 Further vaginal microbiome signatures need to be explored and standardized according to the ethnicity and correlated with other biomarkers to understand the role of CST in predetermining the risk of HPV infected women who are vulnerable to develop CIN and progress into CC. No systematic cross sectional studies have been carried out addressing the issue due to several limitations.
Probiotics to Alter Vaginal Microbiota
Women with vaginal or cervical infections exhibit a high diversity of microbiota and conventional usage of antibiotics such as ‘metronidazole and clindamycin’ as a treatment option may be efficient in controlling the infection yet it does not make certain recolonization of Lactobacillus spp.18 It could in turn lead to relapse or cause side effects or contributing to the emergence of resistant strains and also interferes with normal microbiota leading to dysbiosis.51 ‘Bifidobacterium, Lactobacillus, and Streptococcus’ are able of changing the host microbiome, by modulating the immune response and the inflammatory condition.52 Mechanism of action of these probiotics needs to be further studied but it alters the vaginal pH causing vaginal acidification and prevents bacterial adhesion and colonization. Hence a novel therapeutic strategy could help in improvement of treatment outcomes and efficient management of infection and prevent from further progression of CIN and CC. In this context, the use of probiotics which are live microbes when administered along with antibiotics grant a potential strength benefit to the host and help in proper restoration of vaginal and cervical microbiota. For example, a few studies have previously mentioned the clinical usefulness of probiotics as an antibiotic adjuvant. By comparing the antimicrobial treatment, Heczko et al., in 2015 found that using an oral pro-biotic including ‘L. fermentum 57A, L. plantarum 57B, and L. gasseri 57C’, along with metronidazole, was capable to extend the periods devoid of BV 54.53 In a separate trial, using vaginal tablets containing ‘L. rhamnosus BMX 54’ as an immunologic adjuvant to anti-microbial treatment improved the frequencies of healthiest vaginal microbiota in persons diagnosed with BV as compared to those who just received antibiotics.
Verhoeven V. et al.54 investigated the effect of pro-biotics on changing of the cervix cells on HPV infection. Women with PCR-proven HPV infection were given the probiotic L. paracasei strain, and after 3 months, Human papillomavirus had cleared in 25 percent of the females who intake the pro-biotic, compared to 7.7 percent of the control category. The clearance status in the pro-biotic and control groups after 6 months of duration was found to be 29.2 percent and 19.2 percent, respectively. Similarly, in the probiotic group, elimination of cytological abnormalities was twice as great as in the control group.54 These initial studies shed a promising aspect on probiotics can aid in efficient management of vaginal and cervical infections and possibly prevent the onset of formation of cancerous lesions.
Microbiome as Biomarkers
Numerous studies have been carried out in recent years for understanding human microbial diversity. It is revealed that microbiota present in healthy and diseased individuals are quite distinct. Microbial alterations are considered to be the profound reason behind various gene expressions directly related to diseased conditions especially Cancers. Recent advances in technology (metagenomic studies) highlights the dominant species during healthy and disease states.
Review study by Meenakshi R et al.55 highlights, similar profiles exist between inflammatory bowel disease (IBD) and type 2 diabetes mellitus (T2DM), two multifactorial diseases that show a pattern of increasing global incidence following industrialization. Altered gut bacterial composition and changes in host processing of bacteria-derived metabolites are implicated in IBD and T2DM and provide a shared underlying pathogenetic mechanism. Hence the review study gives a broad insight on microbiome signature linked with inflammatory and metabolic disorders.55
Recent understanding on the complexity of microbiome pertaining to disease condition, due to microbial dysbiosis (microbial imbalance), is very critical, as many of these diseases are multifactorial can cause changes in the microbial community structure. Obtaining molecular microbiome heterogeneity associated with the disease is the important step for a successful diagnostics strategy. Systematic addition of effective targeted interventions to the treatment options contribute toward the main goal of personalized (genomic) medical diagnostics (point of care predictors), by identification of microbiome quantitative traits or QTLs that predict the risk for a clinical condition.56
Similar to other conditions, a recent study by Manzanares-Leal GL et al., carried out a study to determine the association between the bacterial communities of aerobic vaginitis and cervicovaginal neoplasia in Mexican women population, the study lacked to discuss about the base of elements to clarify, if aerobic vaginitis promotes carcinogenesis or if it is established as a consequence of cancer development due to the deprivation of the immune system when trying to counteract the neoplasia. They have reported based on previous data and studies that there seems to be an strong link between cervico-aerobic microbial alterations accompanied by inflammation leads to progression of neoplasia into cancer.57
Finally, while vaginal dysbiosis is understudied, it is a significant risk factor in HPV and cervical cancer epidemiology, which has been investigated in the last decade. Evidence suggests that the varied and complex composition of VMB, as well as constant infectivity with hr-HPV, is a major role in the growth of CIN and CC. According to recent research, the suitable niche of the vaginal and cervical microbiome, which contains a large number of CST I, II, and V, contributes to viral clearance, limiting the formation of cancer precursor lesions at anatomic sites. Microbiome modification may aid in HPV clearance and reverse the natural course of HPV infection. As a result, research into the impact of VMB composition and alterations (dysbiosis) on HPV infection and persistence could help researchers better understand the pathogenesis. HTS and other currently available technologies are widely used in longitudinal research to better recognize the responsibility of a variety of bacterial strains in the aetiology of HPV-related cervical cancer.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
TRD conceptualized the study. AD wrote the original draft. NP and DV reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
DATA AVAILABILITY
Not applicable.
- Small W, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis: Cervical Cancer: A Global Health Crisis. Cancer. 2017;123(13):2404-2412.
Crossref - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68(6):394-424.
Crossref - Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89(1):101-105.
Crossref - Braaten KP, Laufer MR. Human Papillomavirus (HPV), HPV-Related Disease, and the HPV Vaccine. Rev Obstet Gynecol. 2008;1(1):2-10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2492590/. Accessed July 3, 2021.
- Shulzhenko N, Lyng H, Sanson GF, Morgun A. Menage a trois: an evolutionary interplay between human papillomavirus, a tumor, and a woman. Trends Microbiol. 2014;22(6):345-353.
Crossref - Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity. 2015;42(5):965-976.
Crossref - Audirac-Chalifour A, Torres-Poveda K, Bahena-Roman M, et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLOS ONE. 2016;11(4):e0153274.
Crossref - Kyrgiou M, Mitra A, Moscicki AB. Does the vaginal microbiota play a role in the development of cervical cancer? Transl Res. 2017;179:168-182.
Crossref - Kaelin EA, Skidmore PT, Laniewski P, et al. Cervicovaginal DNA Virome Alterations Are Associated with Genital Inflammation and Microbiota Composition. Chia N, ed. mSystems. 2022;7(2):e00064-22.
Crossref - Jing Y, Wang T, Chen Z, et al. Phylogeny and polymorphism in the long control regions E6, E7, and L1 of HPV Type 56 in women from Southwest China. Mol Med Rep. 2018;17(5):7131-7141.
Crossref - Reid R, Stanhope CR, Herschman BR, Booth E, Phibbs GD, Smith JP. Genital warts and cervical cancer. I. Evidence of an association between subclinical papillomavirus infection and cervical malignancy. Cancer. 1982;50(2):377-387.
Crossref - Kyrgiou M, Moscicki AB. Vaginal microbiome and cervical cancer. Semin Cancer Biol. 2022:S1044579X2200061X.
Crossref - Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynecol Obstet. 2019;145(1):129-135.
Crossref - Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S. The evolution of the host microbiome as an ecosystem on a leash. Nature. 2017;548(7665):43-51.
Crossref - Lederberg J, McCray AT. ‘Ome Sweet ‘Omics- A Genealogical Treasury of Words. Genealogical Treasury of Words. Scientist. 2001;15(7):8.
- Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680-4687.
Crossref - Romero R, Hassan SS, Gajer P, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):4.
Crossref - Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4(1):58.
Crossref - Brotman RM, Shardell MD, Gajer P, et al. Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J Infect Dis. 2014;210(11):1723-1733.
Crossref - Dareng EO, Ma B, Famooto AO, et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect. 2016;144(1):123-137.
Crossref - Spear GT, French AL, Gilbert D, et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis. 2014;210(7):1019-1028.
Crossref - Bradley F, Birse K, Hasselrot K, et al. The vaginal microbiome amplifies sex hormone-associated cyclic changes in cervicovaginal inflammation and epithelial barrier disruption. Am J Reprod Immunol. 2018;80(1):e12863.
Crossref - Amabebe E, Anumba DOC. The Vaginal Micro-environment: The Physiologic Role of Lactobacilli. Front Med. 2018;5:181.
Crossref - Di Pietro M, Filardo S, Porpora MG, Recine N, Latino MA, Sessa R. HPV/Chlamydia trachomatis co-infection: metagenomic analysis of cervical microbiota in asymptomatic women. New Microbiol. 2018;41(1):34-41. PMID: 29313867
- Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017;25(3):182-191.
Crossref - Nasioudis D, Beghini J, Bongiovanni AM, Giraldo PC, Linhares IM, Witkin SS. a-Amylase in Vaginal Fluid: Association With Conditions Favorable to Dominance of Lactobacillus. Reprod Sci Thousand Oaks Calif. 2015;22(11):1393-1398.
Crossref - Balkus J, Agnew K, Lawler R, Mitchell C, Hitti J. Effects of Pregnancy and Bacterial Vaginosis on Proinflammatory Cytokine and Secretory Leukocyte Protease Inhibitor Concentrations in Vaginal Secretions. J Pregnancy. 2010;2010:385981.
Crossref - Hearps AC, Tyssen D, Srbinovski D, et al. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017;10(6):1480-1490.
Crossref - Torcia M. Interplay among Vaginal Microbiome, Immune Response and Sexually Transmitted Viral Infections. Int J Mol Sci. 2019;20(2):266.
Crossref - Laniewski P, Barnes D, Goulder A, et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci Rep. 2018;8(1):7593.
Crossref - Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des. 2018;24(40):4771-4778.
Crossref - Williams VM, Filippova M, Filippov V, Payne KJ, Duerksen-Hughes P. Human papillomavirus type 16 E6* induces oxidative stress and DNA damage. J Virol. 2014;88(12):6751-6761.
Crossref - Chen Z, Zhang Z, Zhang H, Xie B. Analysis of the Oxidative Stress Status in Nonspecific Vaginitis and Its Role in Vaginal Epithelial Cells Apoptosis. BioMed Res Int. 2015;2015:795656.
Crossref - Vaneechoutte M. The human vaginal microbial community. Res Microbiol. 2017;168(9-10):811-825.
Crossref - Gong Z, Luna Y, Yu P, Fan H. Lactobacilli Inactivate Chlamydia trachomatis through Lactic Acid but Not H2O2. PLoS ONE. 2014;9(9):e107758.
Crossref - Giraud J, Coiffic J, Poulain P, Kerisit J. High prevalence of cervical intra-epithelial neoplasia in women treated for pelvic inflammatory disease. Eur J Obstet Gynecol Reprod Biol. 1998;81(1):51-54.
Crossref - Skapinyecz J, Smid I, Horvath A, Jeney C, Kardos L, Kovacs P. Pelvic inflammatory disease is a risk factor for cervical cancer. Eur J Gynaecol Oncol. 2003;24(5):401-404.
- Garrett WS. Cancer and the microbiota. Science. 2015;348(6230):80-86.
Crossref - Insinga R, Perez G, Wheeler C, et al. Incident Cervical HPV Infections in Young Women: Transition Probabilities for CIN and Infection Clearance. Cancer Epidemiol Biomark Prev. 2011;20(2):287-296.
Crossref - Mitra A, MacIntyre DA, Lee YS, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep. 2015;5(1):16865.
Crossref - Ovestad I, Gudlaugsson E, Skaland I, et al. Local immune response in the microenvironment of CIN2-3 with and without spontaneous regression. Mod Pathol. 2010;23(9):1231-1240.
Crossref - Usyk M, Zolnik CP, Castle PE, et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLOS Pathog. 2020;16(3):e1008376.
Crossref - Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297-301.
Crossref - Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for Detection of Vaginal Bacteria Associated with Bacterial Vaginosis. J Clin Microbiol. 2007;45(10):3270-3276.
Crossref - Coleman JS, Gaydos CA. Molecular Diagnosis of Bacterial Vaginosis: an Update. Kraft CS, ed. J Clin Microbiol. 2018;56(9):E00342.
Crossref - van der Veer C, van Houdt R, van Dam A, de Vries H, Bruisten S. Accuracy of a commercial multiplex PCR for the diagnosis of bacterial vaginosis. J Med Microbiol. 2018;67(9):1265-1270.
Crossref - Curty G, Costa RL, Siqueira JD, et al. Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci Rep. 2017;7(1):17364.
Crossref - Deurenberg RH, Bathoorn E, Chlebowicz MA, et al. Application of next generation sequencing in clinical microbiology and infection prevention. J Biotechnol. 2017;243:16-24.
Crossref - Robertson CE, Harris JK, Wagner BD, et al. Explicet: graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics. 2013;29(23):3100-3101.
Crossref - Yoo HN, Park KH, Jung EY, Kim YM, Kook SY, Jeon SJ. Non-invasive prediction of preterm birth in women with cervical insufficiency or an asymptomatic short cervix (≤25 mm) by measurement of biomarkers in the cervicovaginal fluid. Wilson BA, ed. PLOS ONE. 2017;12(7):e0180878.
Crossref - Bertuccini L, Russo R, Iosi F, Superti F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int J Immunopathol Pharmacol. 2017;30(2):163-167.
Crossref - Li Y, Yu T, Yan H, et al. Vaginal Microbiota and HPV Infection: Novel Mechanistic Insights and Therapeutic Strategies. Infect Drug Resist. 2020;13:1213-1220.
Crossref - Heczko PB, Tomusiak A, Adamski P, et al. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: a randomised, double-blind, placebo-controlled trial. BMC Womens Health. 2015;15(1):115.
Crossref - Verhoeven V, Renard N, Makar A, et al. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: a prospective controlled pilot study. Eur J Cancer Prev. 2013;22(1):46-51.
Crossref - Rajpoot M, Sharma AK, Sharma A, Gupta GK. Understanding the microbiome: Emerging biomarkers for exploiting the microbiota for personalized medicine against cancer. Semin Cancer Biol. 2018;52(Part 1):1-8.
Crossref - Braundmeier AG, Lenz KM, Inman KS, et al. Individualized medicine and the microbiome in reproductive tract. Front Physiol. 2015;6:97.
Crossref - Manzanares-Leal GL, Coronel-Martinez JA, Rodriguez-Morales M, et al. Preliminary Identification of the Aerobic Cervicovaginal Microbiota in Mexican Women With Cervical Cancer as the First Step Towards Metagenomic Studies. Front Cell Infect Microbiol. 2022;12:838491.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.