ISSN: 0973-7510
E-ISSN: 2581-690X
In developing countries, infectious keratitis being the probable cause of preventable blindness, also have a varied epidemiological profile. This study was conducted to know the microbiological profile, risk factors, epidemiology and antimicrobial susceptibility pattern of bacteria isolated from patients with a corneal ulcers. A total of 193 patients who were clinically diagnosed with cases of infective keratitis in the Department of Ophthalmology were included in the study. The sample collected by an ophthalmologist was received in the Microbiology department. All the demographic details and relevant clinical data were noted. The bacterial identification and antimicrobial susceptibility were done using automated methods while the fungal identification was done using the conventional method (Vitek2 Compact system, BioMerieux). Out of 193 patients, 69% were male and 31% % were females. The Majority of cases were from the age group 41- 50 years. Of 193 cases, 83 (43%) showed microbial etiology in culture. Of 83 culture positive cases, 55 (66.3%) were fungal and 28 (33.7%) were bacterial. The most common isolated fungus was Fusarium species detected in 24 (28.9%) cases followed by Aspergillus species in 14 (16.8%) cases. Gram positive bacteria were predominantly isolated from cases of infective keratitis. Staphylococcus aureus was the most common isolated bacteria in 12 (14.4%) out of 83 positive cases followed by Coagulase negative Staphylococcus. Pseudomonas aeruginosa was the most common Gram negative bacteria isolated from the cases. Among the topical antimicrobials, both Gram positive bacteria as well as Gram negative bacteria showed maximum sensitivity to levofloxacin. Proper knowledge of the clinical presentation and etiological agents aided with microbiological examination is necessary in order to effectively treat corneal ulcers and prevent further complications that can lead to blindness.
Keratitis, Fungal, Bacterial, Antibiotic Susceptibility
Infectious keratitis has been labelled as a “silent epidemic” in underprivileged countries.1 As per reports of the World Health Organization (WHO), major causes of vision loss and blindness in the world today is cataract followed by corneal diseases.2-5
According to The National Blindness and Visual Impairment Survey (2015-2019), corneal related diseases are still among the major causes of blindness in India.6
The patient demographic, corneal health, geographic location, and climate have all been found to affect the aetiology and epidemiological patterns of corneal ulcers, which also tend to change over time. Thus, for early detection, prompt initiation of treatment, ideal care, and illness prevention, it is crucial to have a thorough awareness of the epidemiological traits, risk factors, and etiological agents that are present in a particular area. A thorough laboratory investigation is required before beginning a specific course of treatment which includes corneal scraping culture and microscopy to identify the pathogenic agent.7-8
Although viral pathogens like Herpes Simplex Virus type 1 are more commonly associated with the disease, the other common infectious agents leading to infectious keratitis are bacteria, including Streptococcus pneumonia, Staphylococcus aureus, coagulase-negative Staphylococci, Pseudomonas aeruginosa; fungus including Candida albicans, Aspergillus flavus, Fusarium species, Penicillium species and Aspergillus fumigatus as well as parasites like Acanthamoeba.9
Despite having a thorough knowledge of known bacterial pathogens involved in infectious keratitis, there are studies that show increase in the trend of resistance to various antimicrobial drugs over few decades.10,11
This study was conducted to know the epidemiological pattern, risk factors, microbiological profile and antimicrobial susceptibility pattern of bacteria isolated from patients with corneal ulcers.
The study was planned on 193 clinically suspected cases of infectious keratitis as a prospective observational collaborative study in the Department of Microbiology with the department of Ophthalmology at Maharishi Maharishi Markandeshwar Institute of Medical Sciences and Research, Mullana, Haryana. The study was conducted for two years from 1stJune 2020 to 31st May 2022. The ethical clearance was taken from the Institutional ethical committee.
Inclusion criteria
Patients of both gender and all age group, who were clinically diagnosed as cases of infectious corneal ulcer after thorough clinical evaluation by an ophthalmologist were included in this study.
Exclusion criteria
Patients who were already on topical antimicrobial medications were excluded from the study.
The socio demographic data and risk factors of the patients were recorded (Table 1 and Figure 1).
Sample collection
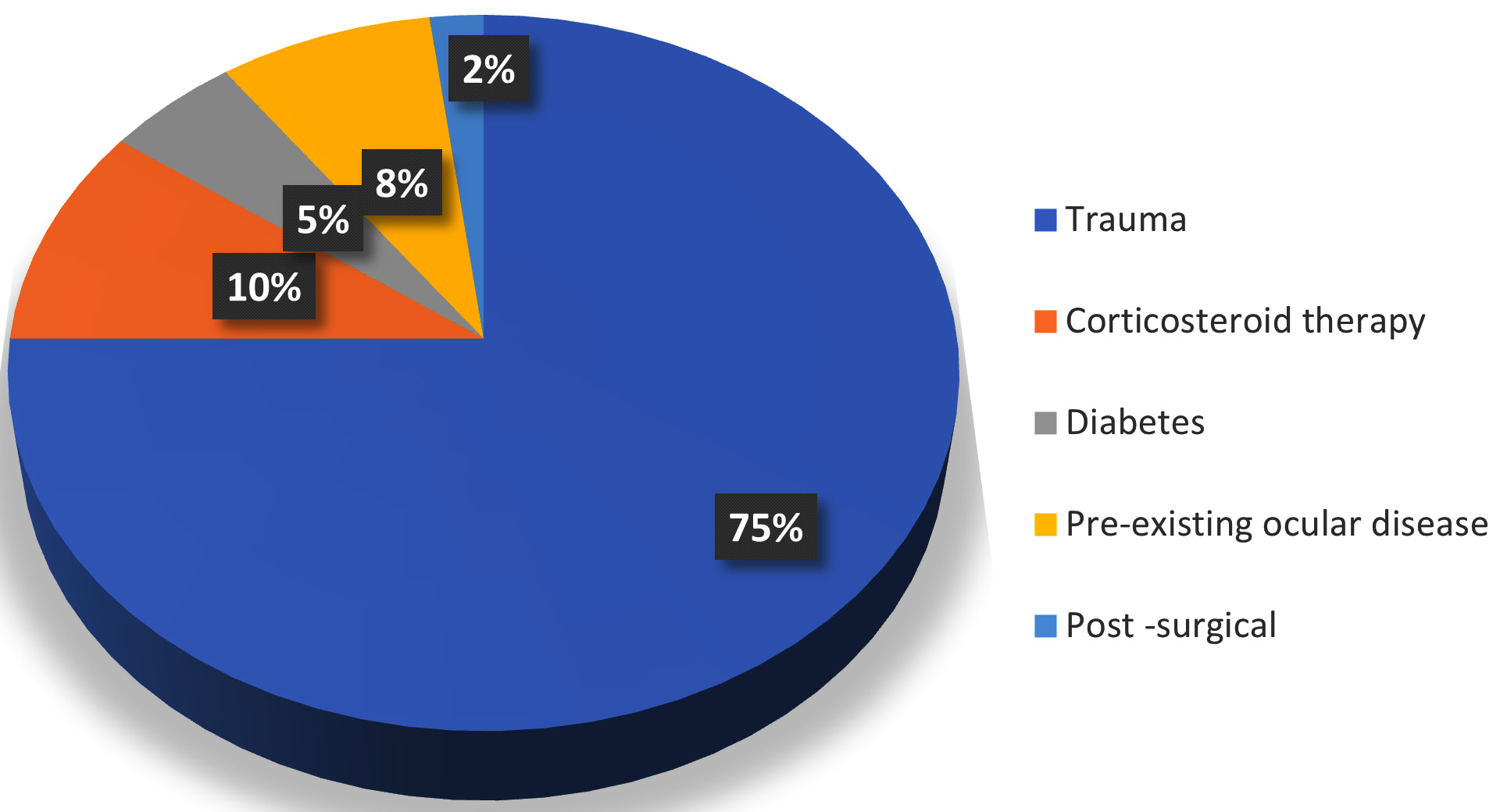
Informed consent of the patient was taken before sample collection. The patient was explained in detail about the procedure before sample collection. After making patient comfortable, the ophthalmologist performed a slit lamp examination. Before collection of the sample, alocal anesthetic agent (topical 4% xylocaine) was instilled into the affected eye. With aid of slit lamp, corneal scrapings were collected from the edge of the cornea using a kimura spatula. Scrapings were taken in sufficient amounts for both staining and culture purposes. Part of the sample was collected on two clean slides for Gram Staining and Potassium Hydroxide (KOH) mount. Another part of the sample was directly inoculated by the ophthalmologist on the blood agar, chocolate agar, MacConkey agar and two Sabouraud dextrose agar (SDA) in multiple C shaped streaks with help of a surgical blade in OPD. (Figure 2) Without any delay, the slides and culture media were sent to the Microbiology department.
Staining
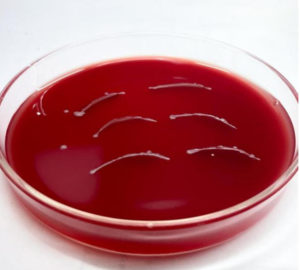
Two Slides were received from the ophthalmology department, one slide was heat fixed and stained with Gram staining to observe the presence of bacteria.12
KOH mount
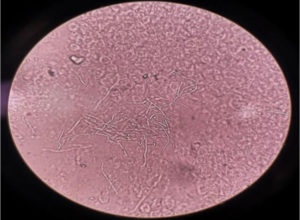
From other slide with corneal scraping a KOH mount was prepared by adding 10% KOH on the slide, which was covered with coverslip and observed under microscope for presence of any fungal elements.13 (Figure 3).
Culture
The culture media that was inoculated in the ophthalmology department were received in the bacteriology as well as the mycology section of the Microbiology laboratory.
Bacterial Culture
All the bacterial culture media (blood agar, chocolate agar and MacConkey agar) were incubated overnight at 37°C. If growth was not observed after overnight incubation, the plates were further incubated for 48 hours. Once growth was observed along the C streaks in culture media, colony morphology was observed followed by Gram staining, catalase, oxidase, coagulase and motility test which were carried out as per standard procedures. (Figure 2) Final identification of the bacteria and its antimicrobial susceptibility testing was done by automated method (Vitek2 Compact system, bioMerieux).12
Fungal Culture
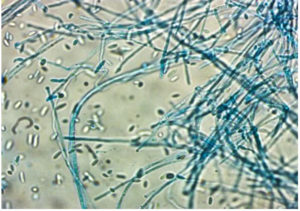
The inoculated media for fungal culture (two inoculated Sabouraud Dextrose Agar containing 0.05mg/mL of chloramphenicol) was incubated at 37°C and 22°C for 14 days and were checked at regular intervals (3rd day, 7th day & 14th day) for any evidence of any fungal growth. When there was fungal growth, identification was done based on colony characteristics and microscopically by observing the fungal morphology in lactophenol cotton blue (LPCB) mount (Figure 4).8,11 Diagnosis of fungal keratitis was made only when the KOH mount and fungal culture were both positive or when the same growth was observed in both Sabouraud Dextrose Agar media).
In patients presenting with corneal ulcers, microbial (bacterial and fungal) etiology was seen in 43% (83/193). There was no organism isolated from the rest of the 110 samples. Bacteria were isolated in 33.7% (28/83) samples, and in 66.3% (55/83) samples fungal growth. No Gram-positive bacilli were observed.
The predominant fungal species was Fusarium species followed by Aspergillus species. In a relatively smaller incidence Curvularia species and Bipolaris species were also isolated.
Infective keratitis is still one of the leading causes of blindness especially in developing countries like India. Although both bacteria and fungus are known to cause infective keratitis, the etiological agents vary from region to region depending upon geographical area depending on environmental conditions.14
In the present study, male subjects were more affected than the female which is in accordance with the study conducted by Tewari et al. in India (Table 1).15 However, there is a study in China that has reported women as predominantly affected by gender.16 This could be due to the higher employability of women particularly in the agricultural sector in China, while in India, it is males who work in the agriculture sector.
Table (1):
Epidemiological characteristics of patients.
Demographics |
Indicator |
No. (%) (n=193) |
|---|---|---|
Age (In years) |
<20 years |
1 (0.5%) |
21–30 years |
3 (1.5%) |
|
31–40 years |
38 (19.7%) |
|
41- 50 years |
77 (39.9%) |
|
51-60 years |
48(24.9%) |
|
61-70 years |
10 (5.2%) |
|
71-80 years |
12 (6.3%) |
|
>80 years |
4 (2%) |
|
Sex |
Male |
133 (69%) |
Female |
60 (31%) |
|
Residence |
Rural |
135 (70%) |
Urban |
58 (30%) |
|
Occupation |
Farmer |
78 (40.5%) |
Housewife |
60 (31.2%) |
|
Laborers |
35 (18.1%) |
|
Carpenter |
10 (5.1%) |
|
Other |
10 (5.1%) |
In the present study, corneal ulceration was seen in all age groups with preponderance among the age group 41-50 years of age followed by 51-60 years (Table 1). In the results of studies conducted by Cameron et al.17 and Das et al. in Kolkata,18 41-60 years was the predominant age group affected by this disease. This could be because this age group is actively engaged in outdoor activities and work.
There was a higher incidence of keratitis among farmers (40.5%) in this study (Table 1). This is in accordance with studies conducted by Tewari et al.15 and Suwal et al.19 but Ranjini et al. in their study reported a higher incidence of keratitis in housewives.20
In this study, agriculture workers were affected more than the general population (Table 1). This is attributed to the fact that those working in agricultural fields are more prone to vegetative trauma. In northern states of India, the harvesting period is from April to mid-September. In present study, a maximum number of cases and culture positivity were seen from June–September, which happens to be the harvesting season in North India (Table 2). However, a study conducted by Krishna et al.21 reported a maximum number of cases during the month of January, February and June which happens to be harvesting months in South India.
Table (2):
Month wise distribution of cases and culture positive cases.
Months |
Cases (2020) |
Culture positive cases |
Cases (2021) |
Culture positive cases |
Cases (2022) |
Culture positive cases |
|---|---|---|---|---|---|---|
June |
8 |
6/8 (75%) |
20 |
11/20 (55%) |
– |
– |
July |
10 |
6/10 (60%) |
24 |
12/24 (50%) |
– |
– |
August |
7 |
4/7 (57.1%) |
16 |
8/18 (44.4%) |
– |
– |
September |
4 |
2/4 (50%) |
10 |
6/10 (60%) |
– |
– |
October |
3 |
1/3 (33.3%) |
8 |
4/8 (50%) |
– |
– |
November |
2 |
0/2 (0%) |
6 |
3/6 (50%) |
– |
– |
December |
2 |
1/2 (50%) |
2 |
1/2 (50%) |
– |
– |
January |
– |
– |
1 |
0/1 (0%) |
5 |
1/5 (20%) |
February |
– |
– |
3 |
0/1 (0%) |
6 |
1/6 (16%) |
March |
– |
– |
6 |
1/6 (16%) |
8 |
2/8 (25%) |
April |
– |
– |
8 |
2/8 (25%) |
12 |
4/12 (33.3%) |
May |
– |
– |
10 |
3/10 (30%) |
12 |
4/12 (33.3%) |
Total |
36 |
20/36 |
114 |
51/114 |
43 |
12/43 |
Trauma was the most common predisposing factor matching other studies conducted in South India by Ranjini et al.20 but in other countries like Barhaim in the Middle East, a study conducted by Yusuf et al.22 reported wearing of contact lenses as the major risk for developing corneal ulcers. In our study, none of the patients with keratitis wore lenses. (Figure 1)
Table (3):
Distribution of isolates.
Type |
Isolate |
No. of isolates (%) (n=83) |
|---|---|---|
Bacterial 28 (27.7%) |
Staphylococcus aureus (S.aureus) |
12/83 (14%) |
Coagulase negative Staphylococii (CONS) |
8/83 (9.6%) |
|
Pseudomonas aeruginosa (P.aeruginosa) |
4/83 (4.8%) |
|
Klebsiella pneumonia (K. pneumonia) |
2/83 (2.4%) |
|
Enterobacter cloacae (E. cloacae) |
1/83 (1.2%) |
|
Escherichia coli (E. coli) |
1/83 (1.2%) |
|
Fungal 55 (66.3%) |
Fusarium spp. |
24/83 (28.91%) |
Aspergillus spp. |
14/83 (16.86%) |
|
Curvularia spp. |
7/83 (8.43%) |
|
Bipolaris spp. |
7/83 (8.43%) |
|
Alternaria spp. |
3/83 (3.61%) |
In the present study, out of 193 cases, 83 (43%) were culture positive. In culture positive samples, fungi (66.3 %) were recovered more frequently than bacteria (33.7%) (Table 3 and 4). Srinivasan et al.23 reported an almost equal number of bacterial (47.1%) and fungal (46.8%) etiological agents with 5.1 % of mixed infection in their study. A comparison of prevalence rates of etiological agents of microbial keratitis reported in various studies, with the present study is shown in Table 5.
Table (4):
Antibiotic Sensitivity pattern of Bacterial isolates.
| Bacterial isolate | Antibiotic Sensitivity (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | OX | CIP | LEV | LZ | VA | CD | TE | AZ | COT | GEN | |
| S. aureus (12) | 66.67 | 100 | 58.33 | 83.33 | 100 | 100 | 100 | 50 | 100 | 66.67 | 50 |
| CONS (8) | 87.5 | 100 | 62.5 | 100 | 100 | 100 | 100 | 75 | 100 | 75 | 50 |
| TOB | LEV | NET | CIP | AZT | PIP-TAZ | ME | CAZ | GEN | AK | CEP | |
| P. aeruginosa (4) | 50 | 75 | 25 | 50 | 100 | 100 | 100 | 100 | 25 | 50 | 75 |
| AMC | TE | CIP | GEN | PIP-TAZ | COT | ME | CTX | LE | AK | CEP | |
| K. pneumoniae (2) | 100 | 50 | 50 | 50 | 100 | 100 | 100 | 100 | 100 | 100 | 0 |
| E. cloacae (1) | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| E. coli (1) | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
P: Penicillin, OX: Oxacillin CIP: Ciprofloxacin, LEV: Levofloxacin, LZ: Linezolid, VA: Vancomycin, CD: Clindamicin, TE: Tetracycline, AZ: Azithromycin, COT: Cotrimoxazole, GEN: Gentamicin, TOB: Tobramycin, NET: Netilmicin, A: Ampicillin, AZT: Aztreonam, ME: Meropenem, CAZ: Ceftazidime, AK: Amikacin, CEP; Cefipime, CTX: Cefotaxime, PIP-TAZ: Piperacillin-Tazobactam, AMC: Amoxicillin-Clavulanate
Table (5):
Prevalence rate of various etiological agents causing microbial keratitis in various studies.
Study |
Microbial Keratitis |
Bacterial Keratitis |
Fungal Keratitis |
|---|---|---|---|
Tiwari et al.12, India |
59.3% |
65.1% |
34.9% |
Sharmila et al.16, Nepal |
44% |
56% |
44% |
Ranjini et al.17, India |
38% |
44.44% |
49.57% |
Present study |
43% |
33.7% |
66.3% |
In current study among the fungal isolates, Fusarium species was the predominant species isolated from cases of infective keratitis, followed by Aspergillus species. (Table 3) Similar results were reported in studies conducted by Ranjini et al.18 in South India and Jisha et al.24 in Kerela. However, Alkantan et al.25 from Saudi Arabia and Kartara et al.26 from Gujarat, India, have reported Aspergillus species as the predominant fungus isolated from corneal ulcers in their respective studies. The difference in the predominant fungus isolates from the corneal ulcers could be due to differences in climatic and environmental variations from region to region. While spores of Aspergillus species are ubiquitously present in the environment, Fusarium species are common plant pathogens and can gain easy entry in case of traumatic injury by vegetative matter. Furthermore, it has been seen that keratitis caused due to Fusarium species has a more intrusive course and is comparatively less responsive to treatment than the keratitis caused by Aspergillus species.27
In the present study, Gram positive bacteria were predominantly responsible for bacterial keratitis. The observation is comparable to other Indian studies by Gopinathan et al.28 and Ranjini et al.20 The predominant bacteria isolated in the present study was Staphylococcus aureus followed by Coagulase negative Staphylococcus species. Pseudomonas aeruginosa was the predominant Gram-negative bacteria isolated from the cases of bacterial keratitis, followed by Klebsiella species. (Table 3)
The observation of the present study is comparable to the study conducted in Pakistan by Narsani et al.29 which reported Staphylococcus aureus was the predominantly isolated species from the cases of infective keratitis while in Hong Kong, Lam et al.30 reported Pseudomonas species as predominant bacteria in their study.
The standard treatment of bacterial corneal ulcer varies depending upon the clinician’s presentation, duration and etiological agent the ophthalmologist is suspecting and the culture results. In most eye care clinics and tertiary care setups, topical antibiotics are prescribed. In the present study, both topical as well as systemic antibiotics were tested for bacteria.
The antibiotic sensitivity pattern in the present study showed that all Gram positive bacteria were 100 percent sensitive to vancomycin, azithromycin, linezolid and oxacillin. For the drugs given as topical antibiotics, the sensitivity of Staphylococcus aureus was 83.3 % (10/12) for levofloxacin and clindamycin both, followed by 66.6% (8/12) for cotrimoxazole, 58.3% (7/12) for tetracycline and least 50% (6/12) for tetracycline and gentamicin both. Coagulase negative Staphylococcus also showed 100 % (12/12) sensitivity for both levofloxacin and clindamycin and the least for gentamicin with 50% (4/8) (Table 4).
Among the Gram negative isolates, predominantly isolated bacteria, Pseudomonas aeruginosa showed 100 percent susceptibility to piperacillin/tazobactam, aztreonam, and meropenem, followed by 90% sensitivity to cefotaxime. For antimicrobials that are prescribed for topical use, maximum sensitivity was observed for levofloxacin 75 % (3/4) followed by tobramycin 50% (2/4), amikacin 50% (2/4) and ciprofloxacin 50% (2/4). The least sensitivity was seen for netilmicin 25 % (1/3) and gentamicin 25 % (1/3) (Table 4).
The overall sensitivity of bacterial isolates varies from region to region as reported by various studies.10,18,20 The irrational use of topical antibiotics, non-compliance to the treatment and over the counter availability of these drugs in India could be the reason for developing resistance to topical antibiotics.
Limitation of this study was that it was a single tertiary center study. To get a broader picture of the etiological agents of this disease a multicentric study of the region is required. Since the maximum number of patients in the study belonged to rural areas, it is important to collaborate with rural health setups providing medical services to the population of the region.
Another limitation of the study was that, the study started during the initial waves of the COVID-19 pandemic in India, when there were restrictions and patients hesitated to come to the hospital for treatment. Due to this factor the number of subjects in the study was less than expected.
Another limitation of this study was that we did not include anaerobes, amoebic and viral agents causing keratitis, therefore only aerobic bacterial and fungal aspects of the disease were studied. All the aspects of infectious keratitis were not included as it was not feasible to perform advanced tests. Although not many studies have been conducted to study the anaerobes in cases of a corneal ulcers due to feasibility and financial issues but many years back in 1982, a study was conducted by Perry et al.31 that had reported a prevalence of 16.66% of anaerobes to isolate from cases of corneal ulcers. Further studies which include the isolation of all pathogens associated with infective keratitis would give a better picture of the disease in that region.
Infectious keratitis is still one of the common ocular morbidities that can lead to blindness in developing countries. The etiological agents and the antibiotic sensitivity of bacterial agents vary from region to region depending upon the environment and exposure to risk factors. Therefore, proper knowledge of changing trends in the region, routine ophthalmological check-up along with the microbiological profile of patients presenting with a corneal ulcersis recommended before formulating treatment protocol.
ACKNOWLEDGMENTS
The author would like to thank Department of Ophthalmology, MMIMSR, for their support during patient screening and sample transportation.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
HK, NK, JC conceptualized and designed the study. HK, JC, RD performed data acquisition. HK, RB, JC performed data analysis and statistical analysis. JC, NK wrote the manuscript. NK, RB reviewed the manuscript. HK, JC, NK edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Maharishi Maharishi Markandeshwar Institute of Medical Sciences and Research, Mullana, Haryana, vide letter no. MMIMSR/IEC/08XP dated 08/02/2020.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Whitcher JP, Srinivasan M. Corneal ulceration in developing world – a silent epidemic. Br J Ophthalmol. 1997;81(8):622-623.
Crossref - Global initiative for the elimination of avoidable blindness. WHO: Geneva; 1997. (unpublished document) WHO/PBL. 97-61 – Rev.1
- Srinivasan M. Infective keratitis: a challenge to Indian ophthalmologists. Indian J Ophthalmol. 2007;55(1):5-6.
Crossref - Pramanick P, Sengupta M, Banerjee M, et al. Microbiological Profile in Patients Having Keratitis in a Tertiary Care Hospital in India. Cureus. 2022;14(11):e31653.
Crossref - Biradar S, Nigudgi A, Doddamani P, Kollur PB. Microbiological profile of infective keratitis in a tertiary care hospital in north Karnataka. Indian J Microbiol Res. 2021;8(2):168-173.
Crossref - Dr. Rajendra Prasad Centre for Ophthalmic Sciences, AIIMS, New Delhi. National Blindness and Visual Impairment Survey 2015-2019. “https://npcbvi.mohfw.gov.in/writeReadData/mainlinkFile/File341.pdf”. Accessed on 30th September, 2022.
- Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22-27.
Crossref - Chauhan J, Kaur N, Kumar H, Bala R, Chauhan S. Mycological profile of acute invasive fungal rhinosinusitis during COVID-19 pandemic at a tertiary care hospital. Med J Babylon 2022;19(4):595-600.
Crossref - Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001;85(7):842-847.
Crossref - Zhang C, Liang Y, Deng S, Wang Z, Li R, Sun X. Distribution of bacterial keratitis and emerging resistance to antibiotics in China from 2001 to 2004. Clin Ophthalmol. 2008;2(3):575-579.
Crossref - Ray KJ, Prajna L, Srinivasan M, et al. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmol. 2013;131(3):310-313.
Crossref - Mackie, McCartney. Practical Medical Microbiology. Collee JG, Fraser AG, Marmion BP, Simmons A. 14th edition. New York: Churchill and Livingstone; 1996.
- Leck AK, Thomas PA, Hagan M, et al. Aetiology of suppurative corneal ulcers in Ghana and south India and epidemiology of fungal keratitis. Br J Ophthalmol 2002;86(11):1211-1215.
Crossref - Larone DH. Medical Important Fungi: A guide to identification. 5th Edition. ASM press; 2011.
Crossref - Tewari A, Sood N, Vegad MM, Mehta DC. Epidemiological and microbiological profile of infective keratitis in Ahmedabad. Indian J Ophthalmol. 2012;60(4):267-272.
Crossref - Cao J, Yang Y, Yang W, et al. Prevalence of infectious keratitis in Central China. BMC Ophthalmol. 2014;14:43.
Crossref - Cameron NL, Pham JN, Paul BR, et al. Bacteria commonly isolated from Keratitis specimen retain antibiotic susceptibility to Fluoroquinolones and Gentamicin plus Cephalothin. Clin Exp Ophthalmol. 2006;34(1):44-50.
Crossref - Das S, Konar J. Bacteriological profile of corneal ulcer with references to Antibiotic susceptibility in a tertiary care hospital in West Bengal. IOSR J Dent Med Sci. 2013;11(6):72-75.
Crossref - Suwal S, Bhandari D, Thapa P, Shrestha MK, Amatya J. Microbiological profile of corneal ulcer cases diagnosed in a tertiary care ophthalmological institute in Nepal. BMC Ophthalmol. 2016;16(1):209.
Crossref - Ranjini CY, Waddepally VV. Microbial Profile of Corneal Ulcers in a Tertiary Care Hospital in South India. J Ophthalmic Vis Res. 2016;11(4):363-367.
Crossref - Krishna S, Shafiyabi S, Sebastian L, Ramesha R, Pavitra D. Microbial keratitis in Bellary district, Karnataka, India: Influence of geographic, climatic, agricultural and occupational risk factors. Int J Pharm Biomed Res. 2013;4:189 193.
- Al-Yusuf N. Microbial Keratitis in Kingdom of Bahrain: Clinical and Microbiology Study. Middle East Afr J Ophthalmol. 2009;16(1):3-7.
Crossref - Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol. 1997;81(11):965-971.
Crossref - Jisha K, Sreekumari PK, Rajesh PS, Jacob KK, Jayalekha B. Fungal corneal ulcers: a prospective study on the causative fungus and the response to the present treatment protocol. J Evolution Med Dent Sci. 2016;5(33):1822-1826.
Crossref - Alkatan H, Athmanathan S, Canites CC. Incidence and microbiological profile of mycotic keratitis in a tertiary care eye hospital: A retrospective analysis. Saudi J Ophthalmol. 2012;26(2):217-21.
Crossref - Katara RS, Patel ND, Sinha M. A Clinical Microbiological Study of Corneal Ulcer Patients at Western Gujarat, India. Acta Med Iran. 2013;51:399-403.
- Thomas PA. Fungal infections of the cornea. Eye. 2003;17:852-862.
Crossref - Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J Ophthalmol. 2009;57(4):273-279.
Crossref - Narsani AK, Jatoi SM, Lohana MK, Dabir SA, Gul S, Khanzada MA. Hospital based epidemiology, risk factors and microbiological diagnosis of bacterial corneal ulcer. Int J Ophthalmol. 2009;2:362-366.
- Lam D, Houang E, Fan DSP, et al. Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye. 2002;16(5):608-18.
Crossref - Perry LD, Briner JH, Colander H. Anaerobic corneal ulcers. Ophthalmology. 1982;89(6):636-642.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.