The increase of anthropogenic activities has led to the pollution of the environment by heavy metals, including chromium (Cr). There are two common oxidative states of Cr that can be found in industrial effluents the trivalent chromium Cr(III) and the hexavalent chromium Cr(VI). While the hexavalent chromium Cr(VI) is highly toxic and can trigger serious human health issues, its reduced form, the trivalent chromium Cr(III), is less toxic and insoluble. Leather tanning is an important industry in many developing countries and serves as a major source of Cr(VI) contamination. Globally, tannery factories generate approximately 40 million m3 of Cr-containing wastewater annually. While the physico-chemical treatments of tannery wastewater are not safe, produce toxic chemicals and require large amounts of chemical inputs, bioremediation using chromium-resistant bacteria (CRB) is safer, efficient and does not produce toxic intermediates. Chromium-resistant bacteria (CRB) utilise three mechanisms for Cr(VI) removal: biotransformation, biosorption and bioaccumulation. This review will evaluate the three Cr(VI) detoxification mechanisms used by bacteria, their limitations and assess their applications for large-scale remediation of Cr(VI). This can be helpful for understanding the nature of Cr(VI) remediation mechanisms used by bacteria, therefore, bridging the gap between laboratory findings and industrial application of microorganisms for Cr(VI) removal.
Bioremediation, Chromium-Reducing Bacteria, Contamination, Tannery Effluents, Toxicity
The unprecedented growth of human populations, coupled with the increase of industrial activities, has led to the pollution of the environment by different organic and inorganic substances. Chromium (Cr) is a metal abundant in natural environments and in toxic concentrations in effluents generated from industrial activities, including in the fabricated metal industry, leather tanning, dying, coal combustion, oil combustion, the metal mining industry and so forth1. Notably, for the last two decades, Cr has been classified as one of the top 20 highly toxic metallic elements on the superfund priority list of hazardous substances2. In a report released in 2015, by the Blacksmith Institute (Pure Earth), Cr was considered as one of the top six toxic contaminants and was estimated to impact 16 million people and cause 3 million disability-adjusted life years (DALYs)3. Understanding the nature of Cr is, therefore, critical to developing strategies to ensure removal of this metal from the environment.
In the environment, there are two stable forms of Cr: the trivalent chromium Cr(III) and the hexavalent chromium Cr(VI). Cr(VI) has a high level of toxicity and can trigger serious human health issues, including cancers, liver damage and pulmonary congestion illness4-7. Meanwhile, Cr(III) is relatively less toxic and can be removed from wastewater8. The devastating effect of Cr(VI) results from the fact that it can use sulphate transport channels to enter into living cells9. Within the cells, the reduction of Cr(VI) to Cr(III) leads to the formation of reactive oxygen species (ROS), which can damage living organisms through interacting with nucleic acids and proteins10,11.
In the conventional leather tanning industry, crude animal skin is treated to create various leather products and is a major source of Cr(VI) pollution. Skin treatment involves four main stages, including pre-tanning, chrome tanning, post-tanning and finishing. During these processes, Cr(III) is oxidised into Cr(VI), which is then discharged into the environment alongside other metals. As reviewed by Dabai & Mohammed, on a global scale tanning factories generate approximately 40 million m3 of Cr-containing wastewater annually12. Leather tanning is an important industry in many developing countries, including India. In India, the massive leather tanning industry annually leaks between 2000 and 3000 tonnes of Cr into the surroundings, causing an estimated final concentration of 2000 to 5000 mg/L Cr in the environment13. A single operating tannery factory can create groundwater pollution in a 7 to 8 km radius14. Therefore, the removal of Cr is paramount to protect human health as well as to prevent the long-term irreversible damage that can occur to the environment.
The most common way of treating tannery wastewater is conventional methods (or physicochemical treatment), which include electrochemical treatment, reverse osmosis and ion exchange. Nevertheless, the use of such methods is usually associated with a high energy input and generates toxic by-products that cause secondary pollution15,16. Therefore, there is a dire need for an alternative, cost-effective and environmentally friendly approach to remove Cr(VI) contamination.
Bioremediation is a microbially driven approach which uses microorganisms to remove toxic pollutants from the environment. Certain microorganisms, such as bacteria, fungi, archaea and algae, are capable of tolerating and reducing Cr(VI), hence remediating Cr contamination17,18. This review will primarily focus on the use of bacteria to detoxify Cr(VI) contamination. The mechanism used to remove Cr(VI) can vary on the basis of biogeochemical conditions, means of nutrient utilisation by bacteria and the presence/absence of oxygen in the environment. Understanding these mechanisms used by bacteria to reduce the toxicity of Cr(VI) will enhance future applications of microbial communities to remove Cr contamination from an environment. This review will evaluate the three Cr(VI) detoxification mechanisms used by bacteria, their limitations and assess their applications for large-scale remediation of Cr(VI).
Microbial Remediation of Hexavalent Chromium
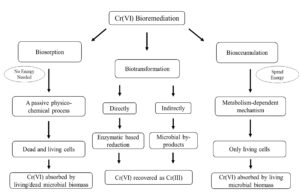
Bioremediation of Cr(VI) using bacteria is a promising approach owing to the fact that it is safe, efficient and does not produce toxic intermediates. In numerous research studies, bacteria have shown the potential to detoxify Cr(VI) by three different mechanisms, including biotransformation, biosorption and bioaccumulation19-21. Biotransformation involves direct and indirect reduction of toxic Cr(VI) to Cr(III)9. The transformation process principally relies on the availability of oxygen and an appropriate electron donor9. Biosorption is a passive physico-chemical process between Cr(VI) and bacteria in which both dead and living cells can participate. Nevertheless, the dead cells are proven to be more effective20. Bioaccumulation is active uptake of Cr(VI) by live bacteria that depends on the concentration of the metal and the time of contact with the microbe22. CRB using any of the three mechanisms can be a potential biological agent for large-scale microbial remediation of Cr(VI). A large number of bacteria were isolated and tested for their ability for Cr(VI) bioremediation (Table 1). In the following subsections, the three major bacterial mechanisms for Cr(VI) detoxification are scrutinised, and their use in large-scale remediation in bioreactors is critically evaluated.
Table (1):
List of bacteria and their mechanisms for Cr(VI) detoxification.
Name of the bacteria |
Bioremediation mechanism |
Reference |
|---|---|---|
Bacillus sphaericus OT4b31 |
Biosorption/Bioaccumulation |
20 |
Bacillus sphaericus IV(4)10 |
Biosorption/Bioaccumulation |
20 |
Bacillsu cereus IST105 |
Biosorption |
38 |
Pseudomonas aeruginosa |
Biosorption |
40 |
Bacillus subtilis |
Biosorption |
40 |
Acinetobacter haemolyticus |
Biotransformation |
27 |
Enterococcus casseliflavus |
Biosorption |
41 |
Corynebacterium paurometabolum |
Biosorption/Biotransformation |
42 |
Bacillus megaterium |
Biosorption/Bioaccumulation |
21 |
Bacillus coagulans |
Biosorption/Bioaccumulation |
21 |
Pseudomonas gessardii LZ-E |
Biotransformation |
19 |
Shewanella putrefaciens |
Biotransformation |
43 |
Thiobacillus ferrooxidans DSM 11477 |
Biotransformation |
44 |
Methanothermobacter thermautotrophicus |
Biotransformation |
45 |
Staphylococcus xylosus |
Biosorption |
36 |
Bacillus sp. MGG-83 |
Biosorption |
46 |
Bacillus amyloliquifaciens |
Biotransformation |
47 |
Microbacterium spp. |
Biotransformation |
48 |
Staphylococcus aureus K1 |
Biotransformation |
49 |
Stenotrophomonas maltophilia |
Biotransformation |
50 |
Escherichia coli FACU |
Biotransformation |
51 |
Acidiphilium cryptum JF-5 |
Biotransformation |
52 |
Cellulosimicrobium funkei strain AR6 |
Biosorption/Bioaccumulation |
53 |
Mesorhizobium amorphae |
Biosorption |
54 |
Arthrobacter rhombi |
Biotransformation |
55 |
Acinetobacter baumannii L2 |
Biosorption/Biotransformation |
56 |
Pseudomonas stutzeri L1 |
Biosorption/Biotransformation |
56 |
Leucobacter sp. G161 |
Biotransformation |
57 |
Bacillus amyloliquefaciens |
Biotransformation |
58 |
Stenotrophomonas sp. |
Biotransformation |
59 |
Bacillus endophyticus |
Biotransformation |
60 |
Virgibacillus sp. |
Biotransformation |
61 |
Pediococcus acidilactici |
Biotransformation |
62 |
Providencia sp. UTDM314 |
Biotransformation |
63 |
Serratia sp. |
Biotransformation |
64 |
Acinetobacter junii |
Biosorption |
65 |
Pseudomonas aeruginosa A2Chr |
Biotransformation |
66 |
Burkholderia cepacian MCMB-821 |
Biotransformation |
31 |
Desulfovibrio vulgaris Hildenborough |
Biotransformation |
24 |
Tenotrophomonas sp. MB339 |
Bioaccumulation |
37 |
Klebsiella pneumoniae MB361 |
Bioaccumulation |
37 |
Staphylococcus sp. MB371 |
Bioaccumulation |
37 |
Pannonibacter phragmitetus LSSE-09 |
Biotransformation |
28 |
Bacillus thuringiensis |
Biosorption |
34 |
Biotransformation
In the biotransformation approach, the highly toxic hexavalent Cr(VI) is chemically transformed to the less toxic and more stable form of trivalent Cr(III) via the reduction reaction of Cr-reducing bacteria (CRB) using a gene called chrR. The reduced Cr(VI) is then expelled from the cells by a mechanism called “efflux pumps” by the actions of the chrA gene23. Bacterial transformation of Cr(VI) can occur either directly through an enzymatic reduction reaction or indirectly by the metabolic end products of other microbes, such as Fe(II) and H2S of sulfate-reducing bacteria (SRB) and iron-reducing bacteria (IRB)24,25. The efficiency of Cr(VI) reduction differs on the basis of the carbon source available to the bacteria26.
Under aerobic conditions, some CRB have shown a unique ability to detoxify Cr(VI), using industrial waste as a carbon source. A study demonstrated that a locally isolated CR-resistant bacterium known as Acinetobacter haemolyticus was able to resist 100 mg/L of Cr(VI) and reduce more than 90% of Cr(VI) as it grew on sugarcane bagasse waste, compared to Luria Bertan medium, which showed only 25% reduction27. Bacterial species have different preferences for carbon sources. Although glucose can promote the growth of many species, it can also limit the Cr(VI) reduction ability of some CRB. In a study that tested the detoxification ability of Pannonibacter phragmitetus LSSE-09 using different carbon sources noted the high growth rate of the species on glucose; however, limited reduction was observed compared to with using acetate as the carbon source28. On the contrary, glucose can enhance Cr(VI) reduction for other species. Evidently, the addition of 1% of glucose to a culture of Bacillus sp. (strain XW4) increased the reduction of Cr(VI) significantly29. Although the Bacillus sp. showed a complete reduction under a concentration of 40 mg/L CR(VI), it was not able to reduce one of 100 mg/L of Cr(VI)29. Apparently, 100 mg/L of Cr(VI) was high enough to halt the metabolic activity of the bacterium leading to the failure of the reduction pathway.
Under anaerobic conditions, Cr(VI) is used as terminal electron acceptor30. In an experiment that used Burkholderia cepacia MCMB-821 to detoxify Cr(VI) found that the addition of 2% of lactose facilitated the reduction of 75 mg/L of Cr(VI) by 98%31. Meanwhile, other electron donors such as ethanol, methanol and sodium acetate decreased the transformation ability of the species31. In addition, anaerobic species such as sulphate- and iron-reducing bacteria (SRB and IRB) are significant contributors to the reduction of Cr(VI). The combination of IRB and SRB is estimated to provide reduction that is roughly 100 times faster than when the CRB are the only species used for bioremediation24,32. Nevertheless, the activities of many important hydrogenases enzymes required for the reduction were inhibited under high concentrations of Cr(VI)24. Altogether, the efficiency of the biotransformation approach is based on the availability of an appropriate electron donor and is significantly affected under high concentration of Cr(VI) in the environment.
Biosorption
The removal of Cr(VI) through biosorption mechanism is based on a passive physico-chemical process between the heavy metal species and the bacteria in which microorganisms can trap Cr(VI) and make it immobile and unavailable for biological uptake33. In the biosorption mechanism, no energy is needed for metal uptake. Furthermore, the uptake of Cr(VI) continues until it reaches equilibrium between absorbed ions and the ions in the solution30,33.
The ability of dry microbial biomass to take up Cr(VI) from the environment is more efficient in dead than in living cells. Evidently, a comparative analysis of uptake of Cr(VI) ions in dead and living cells of Bacillus sphaericus in a controlled environment found that metabolically inactive cells were 13 to 20% better than living cells at pH 2.5 at absorbing Cr(VI)20. In this study, B. sphaericus OT4b31 showed a 44.5% uptake of 30 mg/L of Cr(VI), compared to a 25% uptake for living cells, and B. sphaericus IV(4)10 showed 32% and 45% for living and dead biomass, respectively20. In addition to this, using dead microbial biomass to take up Cr(VI) is the most effective approach to overcome the pH barrier. In a study that used dried biomass of Bacillus thuringiensis under 250 mg/L of Cr(VI) and at pH 2 revealed that B. thuringiensis was able to absorb 24.1% of Cr(VI)34.
Nevertheless, the use of the biosorption approach might not be an effective solution when Cr(VI) is not the only metal in the effluents. Alongside with Cr(VI), industrial effluents contain large amounts of cadmium, or Cd(II)35. The presence of Cd(II) in the environment can reduce the biosorption of Cr(VI), as Cd(II) is preferred by some types of microbial biomass. In a binary system experiment in which Staphylococcus xylosus and Pseudomonas sp. were grown on a medium enriched with Cr(VI) and Cd(II) demonstrated a profound selectivity for Cd(II) ions against Cr(VI) ions36. Therefore, biosorption is an effective approach when Cr(VI) is the dominant metal in the effluents. This can be problematic for in situ applications, as the biosorption might shift towards another metal.
Bioaccumulation
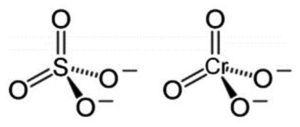
Bioaccumulation of Cr(VI) from the environment is a metabolism-dependent mechanism that requires energy to be spent for the transportation of Cr(VI) reversibly across the membrane. Therefore, only living microbial cells can be used to take up Cr(VI) from an environment. As the chemical structure of Cr(VI) ions resembles that of other ions such as tetrahedral sulphate (SO42-) (Fig. 1), Cr(VI) ions move across the bacterial membrane by utilising the SO42- transport pathways9.
Several studies documented the use of microbial communities to take up Cr(VI) from an environment through the bioaccumulation approach. Certain bacterial species have a distinct surface protein referred to S-layer that can entrap metallic ions on the cell membrane20. In a lab-scale study, two bacterial species – B. sphaericus OT4b31 and B. spaericus IV(4)10 – were able to accumulate 25 to 32 mg/L Cr(VI)20. Bacteria can show a different ability in accumulating Cr(VI) ions. In an experiment that used indigenous microbial strains to remove Cr(VI) from the environment showed that Klebsiella pneumoniae MB361 was able to accumulate an 83.51% Cr(VI) concentration between 500 and 1000 ug/ml under a neutral pH condition37. In another study, two species of bacteria isolated from tannery effluents which contain 0.96 mg/L of Cr(VI), – Bacillus megaterium (strain A) and Bacillus coagulans (181) – showed remarkable capacity, accumulating 15.7 and 23.8 mg Cr/g of the microbial dry weight, respectively, in just 24 hr21. Some metabolically active microbial cells can tolerate a high concentration of Cr(VI) when it is incubated with 5% of industrial effluents. B. cereus strain IST105 isolated from electroplating effluent demonstrated >75% removal of Cr(VI) within a five-day period when living cells were used38.
Nonetheless, the physiological activity of many metabolically active microbial cells can be negatively impacted at high concentrations of the pollutant as well as at low pH levels. In a study that used living cells of Acinetobacter junii VITSUKMW2 under pH levels between 5 and 11 to remove Cr(VI) reported that the growth and the reduction ability of the bacterium were significantly reduced when the pH was adjusted to 5 and reached a peak at a pH of 939. Furthermore, as the bioaccumulation mechanism is energy-dependent, under high concentrations of the pollutant, the metabolic activity can be significantly affected29. Therefore, the low level of pH as well as the high concentration of pollutants in the environment can lead to the failure of the bioaccumulation approach.
Advantages and Limitations of Cr(VI) Remediation Using Microbes
Bioremediation using bacteria offers a cost-effective, efficient and sustainable approach to clean the environment from wastewater containing Cr(VI) through biotransformation, biosorption and bioaccumulation mechanisms.
The biotransformation approach is the most-utilised method for bioremediation of Cr(VI). Given that the reaction in the biotransformation mechanism is based on the carbon source available for microbes, it needs to be chosen carefully according to the preference of the inoculum26. There are many advantages for using the biotransformation approach, such as the reusability of the reduced Cr(VI) as well as maintaining the viability of the inoculum for future applications. Another benefit is that it allows the use of microbial consortia to catalyse the reaction9,24. A microbial consortium of CRB alongside IRB and SRB is a powerful tool to ensure not only faster and complete but also sustainable technology for future application9. However, there are disadvantages that need to be addressed, including the loss of cell viability under high concentrations of Cr(VI) (Table 2)24. The reduction pathway is also energy-dependent, and poor selection of an electron donor can limit the biotransformation ability of the bacteria28,29,31.
Table (2):
Comparison of the three Cr(VI) removal mechanisms.
Feature |
Biotransformation |
Biosorption |
Bioaccumulation |
|---|---|---|---|
Definition |
The direct or indirect reduction of Cr(VI) to Cr(III) |
The passive uptake of Cr(VI) ions by the biomass until reaching equilibrium |
The active accumulation of the Cr(VI) ions by living cells |
Reduction efficiency |
High efficiency |
High efficiency |
Low efficiency |
Nature of cells |
Only living cells |
Both living and dead cells |
Only living cells |
Biomass reusability |
Multiple times |
Single time |
Single time |
Cr(VI) recovery |
Recovered as Cr(III) |
Recovered as dry microbial biomass, which is then converted to powder |
Recovered as dry microbial biomass, which is then converted to powder |
Cr(VI) uptake |
– |
Rapid accumulation |
Slower than biosorption |
Energy requirement |
Spend energy |
No energy is needed |
Spend energy |
pH requirement |
Vary based on the biomass used |
Better Cr(VI) removal under low pH |
Mostly neutral pH |
Major limitations |
High concentration of Cr(VI) and poor selection of carbon source can limit the reduction pathway |
Biosorption is a pH dependent approach and can shift towards other metals when Cr(VI) is not the dominant pollutant |
High concentration of Cr(VI) and low level of pH can inhibit the accumulation of Cr(VI) |
When the cost of providing nutrient media is a feasibility issue, the biosorption approach is a preferred alternative remediation approach. In particular, using dead microbial biomass to adsorb Cr(VI) from contaminated matrices is an effective way that does not require energy and thus reduces the cost of Cr(VI) removal, as dead cells are immune to low pH levels and allow the recovery of the metal from microbial biomass33. One disadvantage is that applied biomass cannot be used for future applications because it is generally converted to powder for Cr(VI) recovery20. Given that the behaviour of microbial biomass under low pH values can vary, so does its ability to eliminate Cr(VI) in an acidic environment. To ensure Cr(VI) removal in the biosorption mechanism, metabolically inactive biomass is used under low pH values between 2.0 and 5.0, depending on the physico-chemical characteristic of the biomass used20,34. However, the presence of multiple metal ions in wastewater can pose a major challenge, as some ions show more affinity to microbial biomass than others36.
The bioaccumulation mechanism relies only on using active microbial biomass. Compared to the biotransformation and the biosorption approaches, microbial accumulation of metals received less attention, owing to the fact that it is a slower process than the biosorption mechanism and can be interrupted under low pH levels. In the biosorption mechanism, a low pH value is needed to compensate the lack of protons important for ion exchange. This cannot be done efficiently in living cells for bioaccumulation20. Although the use of metabolically active microbes can present a significant advantage over the use of dead cells by performing different metabolic activities, including formation of an extracellular complex, precipitation and transport, using living cells can complicate the recovery of Cr(VI), which poses a subsequent challenge, particularly if they were precipitated or compartmentalized inside the cells67. A major limitation of the bioaccumulation approach is that cell growth can be inhibited as Cr(VI) accumulates to a toxic level in a cell25,68.
In summary, approaches for Cr(VI) bioremediation are complicated mechanisms and can be affected by many factors, including Cr(VI) concentration in the effluents, surface charge, the physico-chemical properties of industrial waste (such as pH), the interaction between biomass and metal ions and the interaction within metal ions. In the use of dead microbial biomass, the pH barrier does not constitute a problem. However, to maximise the Cr(VI) reduction in using living biomass, the pH level must be adjusted to the optimum level for boosting the physiological activity of the employed microbe. Bioreactor systems that utilise different Cr detoxification mechanisms need detailed characterization of waste and inocula for maximum efficiency of Cr removal.
Large-scale Cr(VI) Bioremediation Using Bioreactors
For large-scale removal of Cr(VI), bioreactors such as fed-batch or continuous reactors are used in which CRB are exploited as biological agents. Bioreactor systems are classified into four different systems based on their applications: a. Stirred-tank reactors; b. Fixed-bed reactors; c. Fluidized-bed reactors; d. Airlift reactors.
Stirred Tank Bioreactors (STRs)
The STR system has a stirrer that works in two modes: batch or continuous. Furthermore, this type of bioreactor makes it possible to study the removal of Cr(VI) at different concentrations. In an experiment to study the Cr(VI) biotransformation ability of Arthrobacter rhombi-RE strain MTCC7048 grown on molasses as a carbon source using three different growth systems, including an anoxic attached growth, aerobic attached and aerobic suspended system, found that the aerobic attached approach was the most effective, reaching 95% reduction of Cr(VI) in an initial concentration of 20 mg/L and chemical oxygen demand (COD) reduction of between 90 and 95% in an initial concentration of 3000 mg/L55. Nevertheless, there are drawbacks which may limit the application of the STRs for large-scale remediation. These drawbacks include the cost of high energy consumption, STRs can only be used to treat small quantities of effluents, loss of the viability of the metabolically active microbes and the fact that mixing of different contaminants might shift the remediation pathway36,69,70.
Fixed-bed Reactors (FXRs)
The FXR system is characterised by its simplicity in construction and operation. In this system, the biosorbent is contained in a bed fixed on a column which passes the industrial effluents to be treated71. In a study that involved using FXR with immobilized agar-agar that contains a consortium of CRB – namely, Pseudomonas aeruginosa and Bacillus subtilis – the bacteria were able to remove a large amount of heavy metals, including Cr(VI), from textile effluents in 15 days and to significantly reduce COD from 1200 mg/L to 200 mg/L40. In another study that involved the use of SRB growing on ethanol in FXR revealed that the bacteria removed 95% of 50 mg/L of Cr(VI) using the biosorption approach72. Yet, there are some challenges that may limit the use of FXRs. These challenges include the need for multiple columns to maintain the optimal conditions for Cr(VI) remediation, and the need to generate a fixed bed when the biosorbent reaches its maximum capacity25.
Fluidized-bed Bioreactors (FBRs)
In FBR system, microbes grow to form a biofilm on a solid surface, and the suspension state of a bed is maintained by continuously movement of the particles the reactor25. In an experiment that used an FBRs, E. coli supported kaolin to remove a number of metal ions, including Cr(VI). The study found that the presence of kaolin increased the biosorption of Cr(VI) to 100% at a lower concentration (8 mg/L) and to approximately 26% at 116 mg/L73. In another study that used an FBR system and ethanol as a carbon source, SRB showed high efficiency in Cr(VI) removal74. In this study, SRB was able to remove up to 93% of 45 gm/L of Cr(VI) from textile wastewater74. Despite all the benefits, there are some limitations for using FBRs for large-scale Cr(VI) remediation, which include the loss of microbial viability as well as potential contamination with other microbes75.
AirLift Reactors (ALRs)
The function of ALR system is based on reducing shear stress by using an air bubble column alongside an airlift. The system has aeration, which ensures that the amount of needed oxygen has been met and enhances aeration mixture as well as mass transfer25. Furthermore, this type of bioreactor works best when the microorganisms used for bioremediation are fungi. In fact, it allows the formation of fungal mycelium, which in turn increases the area of contact. The system has been extensively studied using fungi, including filamentous fungi and yeast, to remove Cr(VI), but it has been poorly studied for bioremediation by bacteria76,77. Additionally, the system offers many benefits, among them lower power consumption, a lack of moving parts, rapid mixing, low risk of contamination and easy sterilization78. However, the use of the system is limited to the low-density liquids.
Advantages and Limitations for Using Bioreactors
Using bioreactors is the most efficient approach to scale up the bioremediation ability of the three microbial mechanisms for Cr-removal. The benefits and drawbacks for four bioreactors are discussed below.
Stirred-tank reactors (STRs) comprise one system that can enhance the ability of microbes to remove Cr(VI) from industrial wastewater via optimising the conditions to boost the microbial performance. There are many advantages to using STRs, including the simplicity of the system, which allows repeatability of experiments, as well as the ability to study the efficiency of the microbial removal approaches at different concentrations of a pollutant78. Yet several disadvantages limit the use of STRs for large-scale applications (Table 3). Poor energy efficiency is among these drawbacks, and thus use of STRs often comes with a high operational cost. Another disadvantage is that the system can reach maximum performance only when a small amount of industrial wastewater is used, thus limiting large-scale application62,79.
Table (3):
Summary of the advantages and disadvantages of bioreactors.
Bioreactors |
Characteristics |
Advantages |
Disadvantages |
|---|---|---|---|
Stirred Tank Bioreactors (STRs) |
The reactor has stirrer to maintain the suspension of the biomass. |
i. Simple system and is easy to operate. ii. Allows the repetition of the experiment. |
High energy consumption leading to high operation cost. |
Fixed-Bed Reactors (FXRs) |
Mostly used for the removal of chromium using biosorption approach. Characterised by containing a column that passes the industrial effluents to be treated. |
i. Simple to construct and operate. ii. Increases the working life of the microbial biomass. |
The reaction conditions need to be closely monitored. It requires more than one inoculum to ensure a new fixed bed is generated when reaching the maximum capacity of the biomass. |
Fluidized-Bed Bioreactors (FBRs) |
This type of bioreactors is maintained in a suspension state through supplying contaminated effluents. Moreover, microbial biofilm is used and is grown on a slide surface. |
i. The clogging effect is reduced significantly. ii. Take short retention hydraulic time. |
Drawbacks of this type of bioreactors include the loss of the viability of the biomass used and microbial contamination. |
AirLift Reactors (ALRs) |
The function of this system is based on reducing the shear stress by using air bubble column alongside with airlift. Furthermore, the system is frequently used in the bioprocesses that rely on gas-liquid contact. |
i. Consume low power, lack of moving parts, rapid mixing, high oxygen solubility and relatively better homogenisation ii. It reduces pellet formation thus allowing efficient surface contact. |
Not efficient when used for effluents that are highly viscous or contain highly dense particles. |
Fixed-bed reactor (FXR) systems are usually used to optimise the Cr(VI) uptake by microbial biomass. There are many positive aspects of using FXRs, including the simplicity of construction and operation, longer working life for microbial biomass and the allowance of Cr(VI) recovery through cycles of desorption (Table 3). Additionally, FXRs facilitates the use of large particles for immobilization of biosorbents 66. Some disadvantages, however, might restrict large-scale applications of FXR system, including the need to maintain the reaction condition and the need for multiple inocula to be supplied when the bed has reached its full capacity80,81.
Fluidized-bed bioreactors (FBRs) support microbial biomass to form a biofilm on a solid surface. An important feature of FBR system is that the clogging effect is reduced considerably, which allows better flow of supplies to the system and thus achieves better results capacity25. Moreover, a shorter retention time makes it more efficient than the other systems (Table 3). Nevertheless, loss of the viability of microbial biomass is a major issue for FBR system. In addition, as the system required constant aeration, there is a high potential of microbial contamination through air flow75.
AirLift reactors (ALRs) work by reducing shear stress via an air bubble column coupled with an airlift. The many advantages of using ALR system include low power consumption (cost- effective), mixing of contents at faster rate than in the other systems, low risk of microbial contamination and a lack of moving parts (Table 3)77. Although there are many benefits for ALR bioreactors, some disadvantages might limit the use of ALRs for large-scale bioremediation. These drawbacks include poor efficiency in dealing with viscous solutions as well as dense particles78.
Future Directions
Several studies were conducted to improve the efficiency of the microbial mechanism for removing Cr. Among these studies, genetic engineering, using microbial consortium, using nano-material and designing higher efficiency bioreactors showed promising results in detoxifying Cr from the environment. In the following subsections, findings of these studies will be highlighted.
Designing Super-reduction Pathway
Employing genetic engineering to improve the microbial ability to tolerate high stress resulting from metallic ions is a promising approach. The capabilities of some microbial species to survive in highly toxic environments are attributed to their genetic adaptation to extreme environmental conditions25. Therefore, the genetic manipulation of CRB to design a super-reduction pathway can be achieved, specifically for plasmid-mediated Cr resistance. In a lab-scale study aimed at developing a recombinant bacterium able to reduce elevated levels of Cr, the gene nemA of E.coli and the gene phaC of Ralstonia eutropha were fused to create a novel Cr reduction system and were transferred to a recombinant microorganism, resulting in the expression of a Cr-reducing enzyme with 200-fold higher reduction efficiency82. Such approaches can be used to construct more efficient microbes that can tolerate multiple metals while reducing Cr(VI).
Using Microbial Consortium
The application of a microbial consortium is one of the most effective approaches to remove Cr(VI) from contaminated sites. The use of microbial consortia for large-scale Cr removal is evident in bioreactors83. One application that uses a microbial consortium to remove Cr(VI) is bioaugmentation, which involves adding metal resistance strain or a consortium to a site of contamination, allowing it to remove contaminants from the site (in suit)84. In a study that used Aeromonas hydrophila strain LZ-MG14 isolated from textile wastewater to develop bioaugmentation strategy in membrane bioreactor revealed that A. hydrophila was able to colonise the activated sludge and improve the ability of other microbes to reduce 0.5 mmol/L of Cr(VI) by 93.71% in just 12 hr85. Therefore, bioaugmentation using microbial consortia can be an effective tool for Cr(VI) remediation because it can activate diverse microbial pathways.
Using Nano-material
Additionally, the use of nanotechnology to improve microbial remediation is another alternative solution. This approach includes using immobilized microbial cells and metabolic enzymes alongside nanotechnology. It not only enhances the stability of the enzymes used for Cr(VI) reduction but can also promote the remediation of contaminated matrices at a nanometre scale by combining CRB with nano-materials that can act as electron donors for the reaction86.
Designing Higher Efficiency Bioreactors
Led by the desire to develop a low-cost, biologically based treatment for Cr-contaminated aquatic environments. Williams et al. developed the foundation for building a fixed film pilot bioreactor, which involved using microbial consortia such as Enterobacter cloacae, Flavobacterium sp. and Ralstonia sp. to remove Cr(VI)87. Notably, this was the first effective illustration of upscaled removal of > 99% of Cr(VI) from 24,000 L of contaminated groundwater87. Another promising means of Cr removal that can be applied is slurry-phase bioremediation. This slurry-phase reactor is characterized by treating soil in a bioreactor88. Adopting these strategies could help in developing an effective large-scale reactor for industrial wastewater treatment.
The widespread discharge of highly toxic Cr(VI) warrants development of efficient and rapid remediation technologies. Bacteria offer a promising solution to tackle Cr(VI) contamination in the environment. Three naturally evolved Cr-reducing mechanisms– namely, biotransformation, biosorption and bioaccumulation – are already being utilised, each with advantages and limitations. For an improved remediation of Cr(VI) using bacteria in bioreactors, multiple factors, including Cr(VI) concentrations, pH levels and carbon sources, need to be adjusted to achieve a considerable reduction level. Microbial consortia, genetically modified bacteria and well-designed bioreactors can be the most efficient ways to ensure the removal of hexavalent Cr from the environment.
ACKNOWLEDGMENTS
All the listed author(s) are thankful to their representative universities/institutes for providing the related support to compile this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All the listed author(s) have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Cheng H, Zhou T, Li Q, Lu L, Lin C. Anthropogenic chromium emissions in China from 1990 to 2009. PLoS ONE. 2014;9(2):e87753.
Crossref - Chrysochoou M, Johnston CP. Reduction of chromium (VI) in saturated zone sediments by calcium polysulfide and nanoscale zerovalent iron derived from green tea extract. In GeoCongress 2012:3959-3967.
Crossref - Pure Earth, Green Cross Switzerland. World’s worst pollution problems 2015: The new top six toxic threats priority list for remediation. 2015:46-48. https:// www.worstpolluted.org/docs/WWPP_2015_Final.pdf
- Stout MD, Herbert RA, Kissling GE, et al. Hexavalent chromium is carcinogenic to F344/N rats and B6C3F1 mice after chronic oral exposure. Environ Health Perspect. 2009;117(5):716-722.
Crossref - Tagliari KC, Vargas VMF, Zimiani K, Cecchini R. Oxidative stress damage in the liver of fish and rats receiving an intraperitoneal injection of hexavalent chromium as evaluated by chemiluminescence. Environ Toxicol Pharmacol. 2004;17(3):149-157.
Crossref - Beaver LM, Stemmy EJ, Schwartz AM, et al. Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environ Health Perspect. 2009;117(12):1896-1902.
Crossref - Deng Y, Wang M, Tian T, et al. The effect of hexavalent chromium on the incidence and mortality of human cancers: a meta-analysis based on published epidemiological cohort studies. Front Oncol. 2019;9:24.
Crossref - Wu D, Sui Y, He S, Wang X, Li C, Kong H. Removal of trivalent chromium from aqueous solution by zeolite synthesized from coal fly ash. J Hazard Mater. 2008;155(3):415-423.
Crossref - Thatoi H, Das S, Mishra J, Rath BP, Das N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. J Environ Manage. 2014;146:383-399.
Crossref - Ray RR. Adverse hematological effects of hexavalent chromium: an overview. Interdiscip Toxicol. 2016;9(2):55-65.
Crossref - DesMarias TL, Costa M. Mechanisms of chromium-induced toxicity. Curr Opin Toxicol. 2019;14:1-7.
Crossref - Dabai AI, Mohammed K. Chromium removal from tannery wastewater: a review. Platform: A J Sci Tch. 2020;3(1):63-73.
- Rameshraja D, Suresh S. Treatment of tannery wastewater by various oxidation and combined processes. International Journal of Environmental Research. 2011;5(2):349-360.
- Gowd SS, Govil PK. Distribution of heavy metals in surface water of Ranipet industrial area in Tamil Nadu, India. Environ Monit Assess. 2008;136(1-3):197-207.
Crossref - Witek-Krowiak A. Kinetics and equilibrium of copper and chromium ions removal from aqueous solutions using sawdust. Environmental Engineering & Management Journal (EEMJ). 2013;12(11).
Crossref - Aarthy M, Rajesh T, Thirunavoukkarasu M. Critical review on microbial fuel cells for concomitant reduction of hexavalent chromium and bioelectricity generation. J Chem Technol Biotechnol. 2020;95(5):1298-1307.
Crossref - Bhattacharya A, Gupta A, Kaur A, Malik D. Alleviation of hexavalent chromium by using microorganisms: insight into the strategies and complications. Water Sci Technol. 2019;79(3):411-424.
Crossref - Leong YK, Chang JS. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour Technol. 2020;303:122886.
Crossref - Huang H, Wu K, Khan A, et al. A novel Pseudomonas gessardii strain LZ-E simultaneously degrades naphthalene and reduces hexavalent chromium. Bioresour Technol. 2016;207:370-378.
Crossref - Velasquez L, Dussan J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J Hazard Mater. 2009;167(1-3):713-716.
Crossref - Srinath T, Verma T, Ramteke PW, Garg SK. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere. 2002;48(4):427-435.
Crossref - Timkova I, Sedlakova-Kadukova J, Pristas P. Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Separations. 2018;5(4):54.
Crossref - Juhnke S, Peitzsch N, Hubener N, Grosse C, Nies DH. New genes involved in chromate resistance in Ralstonia metallidurans strain CH34. Arch Microbiol. 2002;179(1):15-25.
Crossref - Chardin B, Giudici-Orticoni MT, De Luca G, Guigliarelli BA, Bruschi M. Hydrogenases in sulfate-reducing bacteria function as chromium reductase. Appl Microbiol Biotechnol. 2003;63(3):315-321.
Crossref - Fernandez PM, Martorell MM, Blaser MG, Ruberto LAM, Figueroa LIC, Mac WP. Phenol degradation and heavy metal tolerance of Antarctic yeasts. Extremophiles. 2017;21(3):445-457.
Crossref - Kathiravan MN, Karthick R, Muthu N, Muthukumar K, Velan M. Sonoassisted microbial reduction of chromium. Appl Microbiol Biotechnol.2010;160(7):2000-2013.
Crossref - Ahmad WA, Ahmad WHW, Karim NA, Raj AS, Zakaria ZA. Cr(VI) reduction in naturally rich growth medium and sugarcane bagasse by Acinetobacter haemolyticus. Int Biodeterior Biodegradation. 2013;85:571-576.
Crossref - Xu L, Luo M, Li W, et al. Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 stimulated with external electron donors under alkaline conditions. J Hazard Mater. 2011;185(2-3):1169-1176.
Crossref - Liu YG, Xu WH, Zeng GM, Li X, Gao H. Cr(VI) reduction by Bacillus sp. isolated from chromium landfill. Process Biochemistry. 2006;41(9):1981-1986.
Crossref - Jobby R, Jha P, Yadav AK, Desai N. Biosorption and biotransformation of hexavalent chromium [Cr (VI)]: a comprehensive review. Chemosphere. 2018;207:255-266.
Crossref - Wani R, Kodam KM, Gawai KR, Dhakephalkar PK. Chromate reduction by Burkholderia cepacia MCMB-821,isolated from the pristine habitat of alkaline crater lake. Appl Microbiol Biotechnol. 2007;75(3):627-632.
Crossref - Retnaningrum E, Yulianti DM, Wilopo W. Chromium Precipitation Activity and Molecular Characterization of Sulfate-Reducing Bacteria. Journal of Applied Geology. 2019;4(1):15-19.
Crossref - Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH. Biosorption of heavy metals: a review. Journal of Chemical Science and Technology. 2014;3(4):74-102.
- Sahin Y, Ozturk A. Biosorption of chromium(VI) ions from aqueous solution by the bacterium Bacillus thuringiensis. Process Biochemistry. 2005;40(5):1895-1901.
Crossref - Tariq SR, Shah MH, Shaheen N, Khalique A, Manzoor S, Jaffar M. Multivariate analysis of selected metals in tannery effluents and related soil. J Hazard Mater. 2005;122(1):17-22.
Crossref - Ziagova M, Dimitriadis G, Aslanidou D, Papaioannou X, Tzannetaki EL, Liakopoulou-Kyriakides M. Comparative study of Cd(II) and Cr(VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour Technol. 2007;98(15):2859-2865.
Crossref - Aslam F, Yasmin A, Sohail S. Bioaccumulation of lead, chromium, and nickel by bacteria from three different genera isolated from industrial effluent. Int Microbiol. 2020;23(2):253-261.
Crossref - Naik UC, Srivastava S, Thakur IS. Isolation and characterization of Bacillus cereus IST105 from electroplating effluent for detoxification of hexavalent chromium. Environ Sci Pollut Res Int. 2012;19(7):3005-3014.
Crossref - Pulimi M, Jamwal S, Samuel J, Chandrasekaran N, Mukherjee A. Enhancing the hexavalent chromium bioremediation potential of Acinetobacter junii VITSUKMW2 using statistical design experiments. J Microbiol Biotechnol. 2012;22(12):1767-1775.
Crossref - Ajao AT, Adebayo GB, Yakubu SE. Bioremediation of textile industrial effluent using mixed culture of Pseudomonas aeruginosa and Bacillus subtilis immobilized on agar-agar in a bioreactor. Journal of Microbiology and Biotechnology Research. 2011;1(3):50-56.
- Saranraj P, Stella D, Reetha D, Mythili K. Bioadsorption of chromium resistant Enterococcus casseliflavus isolated from tannery effluents. Journal of Ecobiotechnology. 2010;2(7):17-22.
- Prabhakaran DC, Subramanian S. Studies on the bioremediation of chromium from Aqueous Solutions Using C. paurometabolum. Transactions of the Indian Institute of Metals. 2017;70(2):497-509.
Crossref - Myers CR, Carstens BP, Antholine WE, Myers JM. Chromium(VI) reductase activity is associated with the cytoplasmic membrane of anaerobically grown Shewanella putrefaciens MR-1. J Appl Microbiol. 2000;88(1):98-106.
Crossref - QuiIntana M, Curutchet G, Donati E. Factors affecting chromium(VI) reduction by Thiobacillus ferrooxidans. Chem Eng J. 2001;9(1):11-15.
Crossref - Singh R, Dong H, Liu D, et al. Reduction of hexavalent chromium by the thermophilic methanogen Methanothermobacter thermautotrophicus. Geochim Cosmochim Acta. 2015;148:442-456.
Crossref - Malekzadeh F, Mashkani SG, Ghafourian H, Soudi MR. Biosorption of tungstate by a Bacillus sp. isolated from Anzali lagoon. World J Microbiol Biotechnol. 2007;23(7):905-910.
Crossref - Tharannum S. Bioremediation of hexavalent chromium from electroplating effluents by wild and mutant strains of Bacillus amyloliquifaciens. Indian J Exp Biol. 2020;58(10):722-729.
- Abo Elazm A, El-Rahim A, Mohamed W, Sabbor AT, Moawad H, Sedik MZ. Bioremediation of Hexavalent Chromium Widely Discharged in Leather Tanning Effluents. Egyptian Journal of Chemistry. 2020;63(6):2201-2212.
Crossref - Tariq M, Waseem M, Rasool MH, Zahoor MA, Hussain I. Isolation and molecular characterization of the indigenous Staphylococcus aureus strain K1 with the ability to reduce hexavalent chromium for its application in bioremediation of metal-contaminated sites. Peer J. 2019;7:e7726.
Crossref - Baldiris R, Acosta-Tapia N, Montes A, Hernandez J, Vivas-Reyes R. Reduction of hexavalent chromium and detection of chromate reductase (ChrR) in Stenotrophomonas maltophilia. Molecules. 2018;23(2):406.
- Mohamed MS, El-Arabi NI, El-Hussein A, El-Maaty SA, Abdelhadi AA. Reduction of chromium-VI by chromium-resistant Escherichia coli FACU: a prospective bacterium for bioremediation. Folia Microbiologica (Praha). 2020;65(4):687-696.
Crossref - Cummings DE, Fendorf S, Singh N, Sani RK, Peyton BM, Magnuson TS. Reduction of Cr(VI) under acidic conditions by the facultative Fe(III)-reducing bacterium Acidiphilium cryptum. Environ Sci Technol. 2007;41(1):146-152.
Crossref - Karthik C, Ramkumar VS, Pugazhendhi A, Gopalakrishnan K, Arulselvi PI. Biosorption and biotransformation of Cr (VI) by novel Cellulosimicrobium funkei strain AR6. J Taiwan Inst Chem Eng. 2017;70:282-290.
Crossref - Xie P, Hao X, Mohamad OA, Liang J, Wei G. Comparative study of chromium biosorption by Mesorhizobium amorphae strain CCNWGS0123 in single and binary mixtures. Appl Biochem Biotechnol. 2013;169:570-587.
- Elangovan R, Philip L. Performance evaluation of various bioreactors for the removal of Cr(VI) and organic matter from industrial effluent. Biochemical Engineering Journal. 2009;44(2):174-186.
Crossref - Sathishkumar K, Murugan K, Benelli G, Higuchi A, Rajasekar A. Bioreduction of hexavalent chromium by Pseudomonas stutzeri L1 and Acinetobacter baumannii L2. Ann Microbiol. 2016;67(1):91-98.
Crossref - Ge S, Zheng W, Dong X, Zhou M, Zhou J, Ge S. Distributions of soluble hexavalent chromate reductase from Leucobacter sp. G161 with high reducing ability and thermostability. J Pure Appl Microbiol. 2014;8(3):1893-1900.
- Das S, Mishra J, Das SK, et al. Investigation on mechanism of Cr (VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere. 2014;96:112-121.
- Gunasundari D, Muthukumar K. Simultaneous Cr (VI) reduction and phenol degradation using Stenotrophomonas sp. isolated from tannery effluent contaminated soil. Environ Sci Pollut Res Int. 2013;20(9):6563-6573.
Crossref - Panneerselvam P, Choppala G, Kunhikrishnan A, Bolan N. Potential of novel bacterial consortium for the remediation of chromium contamination. Water, Air, & Soil Pollution. 2013;224(12):1716.
Crossref - Mishra RR, Dhal B, Dutta SK, Dangar TK, Das NN, Thatoi HN. Optimization and characterization of chromium (VI) reduction in saline condition by moderately halophilic Vigribacillus sp. isolated from mangrove soil of Bhitarkanika, India. Journal of Hazardous Materials. 2012;227:219-226.
Crossref - Lytras G, Lytras C, Argyropoulou D, Dimopoulos N, Malavetas G, Lyberatos G. A novel two-phase bioreactor for microbial hexavalent chromium removal from wastewater. J Hazard Mater. 2017;336:41-51.
Crossref - Thacker U, Parikh R, Shouche Y, Madamwar D. Hexavalent chromium reduction by Providencia sp. Process Biochemistry. 2006;41(6):1332-1337.
Crossref - Deng P, Tan XQ, Wu Y, Bai QH, Jia Y, Xiao H. Cloning and sequence analysis demonstrate the chromate reduction ability of a novel chromate reductase gene from Serratia sp. Exp Ther Med. 2015;9:795-800.
Crossref - Yahya SK, Zakaria ZA, Samin J, Raj AS, Ahmad WA. Isotherm kinetics of Cr(III) removal by non-viable cells of Acinetobacter haemolyticus. Colloids Surfaces B Biointerfaces. 2012;94:362-368.
Crossref - Ganguli A, Tripathi A. Bioremediation of toxic chromium from electroplating effluent by chromate-reducing Pseudomonas aeruginosa A2Chr in two bioreactors. Appl Microbiol Biotechnol. 2002;58(3):416-420.
Crossref - Ahluwalia SS, Goyal D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol. 2007;98(12):2243-2257.
Crossref - Donmez G, Aksu Z. The effect of copper(II) ions on the growth and bioaccumulation properties of some yeasts. Process Biochemistry. 1999;35(1-2):135-142.
Crossref - Vendruscolo F, da Rocha Ferreira GL, Antoniosi Filho NR. Biosorption of hexavalent chromium by microorganisms. Int Biodeterior Biodegradation. 2017;119:87-95.
Crossref - Villegas LB, Pereira CE, Colin VL, Abate CM. The effect of sulphate and phosphate ions on Cr(VI) reduction by Streptomyces sp. MC1, including studies of growth and pleomorphism. Int Biodeterior Biodegradation. 2013;82:149-156.
Crossref - Rosca M, Hlihor RM, Cozma P, Comanita ED, Simion IM, Gavrilescu M. Potential of biosorption and bioaccumulation processes for heavy metals removal in bioreactors. In 2015 E-Health and Bioengineering Conference (EHB). 2015:1-4. IEEE.
Crossref - Pagnanelli F, Viggi CC, Cibati A, Uccelletti D, Toro L, Palleschi C. Biotreatment of Cr(VI) contaminated waters by sulphate reducing bacteria fed with ethanol. J Hazard Mater. 2012;199:186-192.
Crossref - Quintelas C, Rocha Z, Silva B, Fonseca B, Figueiredo H, Tavares T. Removal of Cd(II), Cr(VI), Fe(III) and Ni(II) from aqueous solutions by an E. coli biofilm supported on kaolin. Chem Eng J. (Lausanne, Switzerland: 1996). 2009;149(1):319-324.
Crossref - Cirik K, Dursun N, Sahinkaya E, Cinar O. Effect of electron donor source on the treatment of Cr(VI)-containing textile wastewater using sulfate-reducing fluidized bed reactors (FBRs). Bioresour Technol. 2013;133:414-420.
Crossref - Nkhalambayausi-Chirwa EM, Wang YT. Simultaneous chromium(VI) reduction and phenol degradation in a fixed-film coculture bioreactor: reactor performance. Water Research (Oxford). 2001;35(8):1921-1932.
Crossref - Morales-Barrera L, Cristiani-Urbina E. Removal of hexavalent chromium by Trichoderma viride in an airlift bioreactor. Enzyme Microb Technol. 2006;40(1):107-113.
Crossref - Guillen-Jimenez FDM, Netzahuatl-Munoz AR, Morales-Barrera L, Cristiani-Urbina E. Hexavalent chromium removal by Candida sp. in a concentric draft-tube airlift bioreactor. Water, Air, and Soil Pollution. 2009;204(1-4):43-51.
Crossref - Han M, Laari A, Koiranen T. Hydrodynamic and mass transfer characteristics of annulus-rising airlift reactor – the effect of reactor scale. MATEC Web of Conferences. 2016;49:4001.
Crossref - Sharma S, Malaviya P. Bioremediation of tannery wastewater by chromium resistant novel fungal consortium. Ecol Eng. 2016;91:419-425.
Crossref - Camargo F, Okeke B, Bento F, Frankenberger W. Hexavalent chromium reduction by immobilized cells and the cell-free extract of Bacillus sp. ES 29. Bioremediation Journal. 2004;8(1-2):23-30.
Crossref - Konovalova VV, Dmytrenko GM, Nigmatullin RR, Bryk MT, Gvozdyak PI. Chromium(VI) reduction in a membrane bioreactor with immobilized Pseudomonas cells. Enzyme Microb Technol. 2003;33(7):899-907.
Crossref - Robins KJ, Hooks DO, Rehm BH, Ackerley DF. Escherichia coli NemA is an efficient chromate reductase that can be biologically immobilized to provide a cell free system for remediation of hexavalent chromium. PloS One. 2013;8(3):59200.
Crossref - He Z, Yao Y, Lu Z, Ye Y. Dynamic metabolic and transcriptional profiling of Rhodococcus sp. strain YYL during the degradation of tetrahydrofuran. Appl Environ Microbiol. 2014;80(9):2656-2664.
Crossref - Herrero M, Stuckey DC. Bioaugmentation and its application in wastewater treatment: A review. Chemosphere (Oxford). 2015;140:119-128.
Crossref - Ji J, Kulshreshtha S, Kakade A, Majeed S, Li X, Liu P. Bioaugmentation of membrane bioreactor with Aeromonas hydrophila LZ-MG14 for enhanced malachite green and hexavalent chromium removal in textile wastewater. Int Biodeterior Biodegradation. 2020;150:104939.
Crossref - Gutierrez-Corona JF, Romo-Rodriguez P, Santos-Escobar F, Espino-Saldana AE, Hernandez-Escoto H. Microbial interactions with chromium: basic biological processes and applications in environmental biotechnology. World J Microbiol Biotechnol. 2016;32(12):191.
Crossref - Williams PJ, Botes E, Maleke MM, et al. Effective bioreduction of hexavalent chromium-contaminated water in fixed-film bioreactors. Water SA. 2014;40(3):549-554.
Crossref - Kulshreshtha A, Agrawal R, Barar M, Saxena S. A review on bioremediation of heavy metals in contaminated water. IOSR J Environ Sci Toxicol Food Technol. 2014;8(7):44-50.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.