ISSN: 0973-7510
E-ISSN: 2581-690X
In the current era there are huge quantities of waste organic matter available, creating a big burden to the environment. To address these issues, researchers started to apply effective and microbial induced biotechnological processes that can mitigate these waste matters. In this context, different nature of microbial systems are involved in hydrolysing the waste organic material into fermentable sugar. These can be easily consumed by specific microbial systems like Saccharomyces cerevisiae MTCC 3821 and Clostridium acetobutylicum that produced bioethanol and biobutanol, respectively. Saccharomyces cerevisiae was cultured in specific media and incubated at rotary shaker with 150 rpm at 30°C for 72 to 96 hours. Ethanol concentrations from different waste matters were found in the range of 1.2-1.5 g.L-1. Ethanol synthesis was done by shake flask experiment with addition of glucose (50 g.L-1) to waste organic hydrolyzed solution. Non-glucose media produced less than 3 g.L-1 ethanol but glucose media produced 4.5 g.L-1. Next, Clostridium acetobutylicum was grown in culture media containing waste organics as sole carbon substrate with pH 7 and then was incubated in anaerobic conditions at 35°C for 72 hours, produced butanol (0.7 to 1.25 g.L-1). This research work promoted biofuels synthesis by keeping a waste mitigation strategy.
Waste Matter, Biotechnological Process, Biofuels, Plant Residues, Saccharomyces cerevisiae
The global energy demand has increased to 1.3% in 2022 and more than 82% of this energy supply demand is fulfilled by fossil fuels sources. The major global energy requirement can be fulfilled by non-renewable energy sources like crude oil, coal and natural gas. However, these resources are non-renewable, take millions of years for formation and could be exhausted in near future.1 Rising price of crude oil, various environmental challenges and political instability are the other factors that forced the replacement of fossil fuel with other eco-friendly alternative energy sources. Since the dawn of time, biofuels have been used as an energy source. Biofuels marked their presence from the last few decades with firing and heating energy source, being discovered. During the early 20th century, Henry Ford proposed automobiles, using bioethanol as their fuel source for future needs.2
In 1906, the New York Times published a description of ethanol as the fuel source for automobiles and published a report in ″Auto Club Aroused Over Alcohol Bill″. During World War I and II, decrease in raw materials and natural resources forced the production of ethanol as an alternative to fossil product derived petrol. The production of bio-ethanol was started and now increased to 600 million gallons with sequential improvement in yield/ titer.2,3 Biofuels can be produced from any photosynthetic living organisms like photosynthetic bacteria (e.g., Cyanobacteria, Proteobacteria), algae and vascular land plants. Biofuels produced can be solid, liquid and gaseous nature’s fuels.4 Liquid biofuels include ethanol, methanol, biodiesel and gaseous fuels (include biohydrogen and methane). Liquid biofuels are used as fuel for vehicles, fuel cells and also in fuel engines. Biofuels are classified as primary secondary biofuels and others.5 The energy derived from plant products like food grains and fruits juices can be reported as primary biofuel. Secondary biofuels are produced indirectly derived from plant waste matters.4,5 Biofuels are further classified based on origin and technology aspects as first, second, third and fourth generation biofuel. In 2022, global production of biofuels was increased by 46% (i.e., 1.9 million barrels of oil equivalent per day.6 This research has discussed the biofuel synthesis from waste biomass conversion and microbial fermentation.
Biofuels and its synthesizing parameters
Biofuel is the fuel, extracted from food grains or waste biomass that comes from plant or animal sources. It can be found as a solid, liquid or gaseous nature. Based on the source of waste biomass/organic matter, biofuels originate with their production as primary and secondary biofuels or other types. Primary biofuels can be biosynthesized from the edible plant foods/ products and its waste biomass. But other forms of biofuel can be synthesized from animal waste, wood or crop waste and these can be used as raw carbon sources with high potential substrates for energy production (heat or electricity production).7 Biofuels are further classified as first, second, third and fourth generation biofuel, on the basis of feedstock used. Produced biomasses can come from living organisms especially from plants, and microorganisms, which harness solar energy and convert into chemical form and stored organic matter in their body. This energy is harnessed and converted into electrical or heat energy. Bioethanol and biodiesel are the most studied biofuels and also utilized in transportation tasks.8
First generation bioethanol (C2H5OH/ EtOH) is produced from the fermentation process of wheat grain and sugarcane juices. This generation of bioethanol is primarily used as transportation fuel in its pure form or blended with gasoline (e.g., gasoline blended with 10% ethanol, as E10). Replacing petroleum products with this generation of bioethanol can reduce the emission of carbon dioxide in two ways like heat, steam and carbon dioxide production after combustion of bioethanol.7,8 And the carbon dioxide present in the atmosphere is utilized for growing crops as raw material. Therefore, bioethanol is considered to be a carbon neutral fuel source. In 2022, at worldwide level bioethanol production has reached 28 billion gallons, with the trend of an increasing level (850 billion) from previous year and it is the highest in the last seven years.9 First generation bioethanol can be produced either by sugar or its products containing crops such as palm juice, sugarcane or starch containing crops such as wheat, barley, rice. As starch can’t be directly converted into ethanol due to long chain polymer structure. The starch biomass is degraded by using two enzymes. Starch is converted into dextrin and oligosaccharides with the help of amylases. Dextran is converted into glucose by enzymes like glucoamylase and yeast can be added for fermentation process.10
Second generation biofuels can be synthesized by hydrolysing plant waste biomass and it needs an effective pretreatment and microbial fermentation system. In this fuel bioproduction, yeast and bacterial strains are utilized for fermenting the glucose to ethanol or other alcoholic fuels. And environment reports for high availability waste organics are reports. For utilization of algal biomass for third generation biofuel is necessary.9,10 The algae are cultivated in open shallow raceway ponds attached with wheels for better aeration and nutrient circulation. These ponds are easy to operate and require less operating cost. Unfavorable photosynthetic microbes can enter the pond through atmosphere or waterways and result in low yield of algae.9,11 Table 1 discusses some algal species that have contributed to biodiesel and Table 2 discusses the different generation biofuels that used in transport engine fuels.
Table (1):
Different microalgal biomass utilization for third generation biofuels production
Microalgae |
Culture conditions |
Growth parameters |
Biomass/lipid yield |
Ref. |
|---|---|---|---|---|
Coelastrella sp. F169 |
Bubble column reactors requires 12:12 hour light and dark cycles |
Phototrophic aerated with 1.5% CO2 |
(3.5 g.L-1)/(1.42 mg.L-1.d-1) |
12 |
Asterarcys quadricellulare |
Batch culture needed 12:12 hour light to dark cycles for 7-10 days |
Phototrophic |
(1.44 g.L-1)/(15 mg.L-1.d-1) |
13,14 |
Scenedesmus sp. |
Batch culture in MSW reported with AnMBBR reactor. And light: dark is 16:8 hour ratios |
Phototrophic aeration rate is 0.3 vvm |
(0.106 g.L-1.d-1)/(48.5 mg.g-1) |
15 |
Parachlorella kessleri 211-11G |
Batch culture with brewery waste water |
Mixotrophic |
(0.879 g.L-1.d-1)/(0.34 mg.L-1.d-1) |
16 |
Chlorella pyrenoidosa FACHB-9 |
Batch culture is reported with light: dark cycle 12:12 hour |
Phototrophic |
(0.145 g.L-1.d-1)/(64.44 mg.L-1.d-1) |
17 |
Chlorella sp. |
Algal culture in growth chamber is reported using wastewater from dairy |
Phototrophic |
(2.6 g.m-2.d-1)/(230 mg.m-2.d-1) |
18 |
C. kessleri |
Batch culture is reported with 20:4 hour of light: dark period |
Mixotrophic |
(0.17 g.L-1.d-1)/(NA) |
19 |
Table (2):
Different biofuels from different waste organic sources and its impacts
Type of Fuel |
Wastes |
Examples |
Ref. |
|---|---|---|---|
First Generation Biofuels |
Derived from food crops is wheat, corn, soybeans. Used as feed/ substrates for this generation fuel synthesis |
Biodiesel, corn ethanol, sugar alcohol is utilized that needs specific enzymes for its hydrolysis |
20 |
First Generation Biofuels |
Sucrose and starch rich feedstock can be utilized for substrates |
Bioethanol from edible agricultural crops and products |
21 |
Second Generation Biofuels |
Derived from energy crops such as jatropha, miscanthus, wood or grass is used |
Bioethanol, biobutanol, biodiesel |
22 |
Second Generation Biofuels |
Sila sorghum stalks with varied flow rate of waste biomass is used |
Reduced bioethanol production rate from 21,759.5 to 19,397.6 kg/h. |
23 |
Third Generation Biofuels |
Derived from micro- and macro algae |
Biodiesel, bioethanol, biohydrogen |
24 |
Third-generation biofuel production from microalgae |
Cultivation of microalgae through aquafarming, in wastewater |
Reduce the cost of biodiesel production from $3.90 to $0.54 per litre. |
24,25 |
Fourth Generation Biofuels |
Derived from genetically modified photosynthetic microbes. Still study is going on |
Bioethanol from microbes is reported |
26 |
Fourth Generation Biofuels |
Byproducts obtained from energy extraction step and residual water from the harvesting process |
Fourth generation biofuel (FGB) uses genetically modified (GM) algae for biofuels production |
27 |
Petroleum Products |
Fossil fuel; Non-renewable energy resource |
LPG, CNG, Petrol, Diesel, Kerosene, Jet fuel |
28 |
For this research work, first Saccharomyces cerevisiae MTCC 3821 was incubated in YEPD (yeast extract peptone dextrose) media at 30°C. YEPD media consisted of yeast extract powder (10 g.L-1), peptone (C13H24O4 ~20 g.L-1), D-glucose (dextrose ~20 g.L-1), chloramphenicol (500 mg/L) with then keeps media pH of 5.5. It was adjusted with 1.0 N H2SO4/ HCL solution. For YEPD agar plate development, agar-agar (20 g.L-1) was added. The sub-culturing was done every 15 days. For culture preparation, three loopful of Saccharomyces cerevisiae MTCC 3821, strain into growth medium (100 ml) is done and incubated at 30°C, 200 rpm and 24 hours to obtain exponential phase. Butanol production task was done by adding Clostridium acetobutylicum strain and this strain was isolated from local agricultural land of Vishakhapatnam by using particular media. Single strain is isolated and cultured in CGM (clostridium growth media) under anaerobic conditions. The pure culture was done for gram-staining tasks and microscopic characteristics observation. Rod- shaped and gram-positive strains were selected for further study. For this microbial strain growth, P2 medium was used. The sub-culturing was done every 10 days. Solvent production capabilities were identified by using acetone tests. Positive acetone production was identified by yellow to purple colour change by adding 1 ml of 5% of sodium nitroprusside (SNP ~Na2[Fe(CN)5(NO)]) and a drop of 40% NH4+ solution. Biobutanol production has occurred in P2 medium. All 15 strains are cultured into 10 ml of P2 medium broth and incubated under anaerobic condition for 7 days at 37°C. Butanol concentration was checked by HPLC techniques.
Next, CGM contains glucose (30 g.L-1), yeast extract (6.25 g.L-1), (NH4)2SO4 (2.5 g.L-1), sodium chloride, (1.25 g.L-1), C2H8N2O3 (2.5 g.L-1), KH2PO4 (0.95 g.L-1), K2H2PO4 (0.95 g.L-1), MgSO4.7H2O (0.5 g.L-1), MnH14O11S (13 mg.L-1) and FeH14O11S (13 mg.L-1) with pH 6.4. P2 medium composition is shown to consist of following components like, glucose (30 g.L-1), (0.5 g.L-1), potassium dihydrogen phosphate, (0.5 g.L-1), potassium hydrogen phosphate,
(0.4 g.L-1), magnesium sulphate heptahydrate, (0.01 g.L-1), iron sulfate pentahydrate (1 g.L-1), yeast extract (0.5 g.L-1), cysteine, biotin (80 µg.L-1) and PABA (1 mg.L-1). All the procedure was carried out aseptically and triplicate run. The concentration of glucose, xylose, ethanol, glycerol and acetic acid is determined by HPLC (Shimadzu, Japan) equipped with refractive index detector and Bio-Rad HPX-87H column (300 mm × 7.8 mm) using 5 m.M sulphuric acid as eluent at 45°C, flow rate of 0.6 mL/min and sample volume of 20 µ.L. We have applied DNS (3,5-Dintrosalicylic acid) for determination of total reducing sugars (i.e. residual sugars) consumed during fermentation.
Fermentation/biotechnological process conditions for biofuel synthesis
Saccharomyces cerevisiae MTCC 3821 were inoculated in fermentation broth and incubated in a rotary shaker with 200 rpm speed rate under partial anaerobic conditions at 30°C for 48 hours. In every 6 hours, the sample analysis was performed to analyse for ethanol/residual sugars. During the complex organic matter hydrolysis, the effect of inhibitory compounds on ethanol yield was also compared. Biomass concentration was determined by a UV spectrophotometer at 600 nm. Supernatants were obtained by centrifugation of the sample at 5000 rpm for 15 min. Supernatants are filtered by using a 0.22 µm membrane filter. Ethanol and residual sugar concentrations were determined by HPLC technique with Rezex ROA organic acid, 300 × 7.8 mm HPX-87H column, and RI detector. Among many strains of Clostridium acetobutylicum, only one was isolated and it showed highest butanol concentration in ABE production. They were cultured in P2 medium with waste organic hydrolysate as the sole carbon source and incubated in anaerobic conditions at 37°C for 72 hours. To study the effect of inhibitory compounds like formic acid, the fermentation of detoxified pretreated hydrolysate and non-removal was compared. Samples were collected, centrifuged at 6000 rpm for 5 min and supernatant was collected for analysis of acetone, butanol, ethanol and reducing sugar by HPLC.
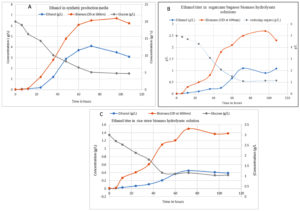
Ethanol production
During experiments, when Saccharomyces cerevisiae MTCC 3821 was inoculated in untreated waste organic hydrolysate, low ethanol yield was obtained. It may be due to the presence of some inhibitory compounds in the hydrolysate media. Different concentration of ethanol was compared for sugarcane bagasse, rice straw and wheat straw hydrolysate solution and the highest ethanol yield was reported by rice straw at pH 5.5. The rice straw at pH 5.5 has produced the ethanol of 2.25 g.L-1 while the sugarcane bagasse based hydrolysate ethanol yield was found to 2.12 g.L-1 which is comparatively low. The wheat straw at the same pH has produced 1.98 g.L-1 of ethanol. In general, S. cerevisiae is an acidophilic organism and it requires an acidic medium for its growth and development. The optimum pH for S. cerevisiae growth was 4.0-6.0, depending on the temperature, oxygen availability and the type of strain. Optimum pH is important for all cellular activities especially for enzymes and transport proteins bound to plasma membranes. During growth and metabolism of S. cerevisiae, this microbe needs to maintain a constant pH during fermentation period. The environmental pH (pH of media) changes needs to maintain the H+ ion concentration by diffusion. When outside pH can be varied too much from the optimum range, then the cell cannot maintain the intracellular pH, affecting the functioning of enzymes. Furthermore, it results in denaturation of enzymes and cells entering into the decline phase. It can result in low ethanol yield. The temperature below 30°C was unable to produce sufficient amounts of ethanol. S. cerevisiae requires a temperature range of
27°C-40°C for the fermentation process that results in the best yield and titer. Maximum ethanol yield (2.24 g.L-1) was obtained from plant origin waste organic matter at 72 hours. Fermentation of rice and wheat straw hydrolysis solution has produced ethanol of different titers (1.85 g.L-1 and 1.0 g.L-1 respectively). Figure 1 shows ethanol production from waste matter in crops.
Figure 1. Ethanol production from synthetic media (A) and biomass hydrolysate containing media (B) sugarcane waste and (C) rice waste
Figure 2. Biobutanol production from plant wastes matter with contribution of Clostridium species and fermentation process (A-Sugarcane waste) and (B-Rice wastes)
Biobutanol synthesis
High concentration of butanol (1.2 g.L-1) and ABE (1.9 g.L-1) in 50 ml of biomass hydrolysate solution was achieved in production /fermentation media that was detoxified before start to fermentation. A low concentration of butanol (0.35 g.L-1) and ABE (0.8 g.L-1) in 10 ml of biomass hydrolysate solution was found in non-detoxified solution also. But, some improvement in concentration of butanol (1.4 g.L-1) and ABE (2.1 g.L-1) in 50 ml of biomass hydrolysate solution in production/fermentation media that was systematically detoxified. Figure 2 shows the titers of butanol from waste matter in crops. From these experiment results, it has indicated low concentration of fermentation production was due to low initial concentration of biomass hydrolysates and it was attributed low amount of carbon or glucose sources as impact of mass action. Clostridium species has shown the capability of clostridial solvent production and it occurs in a biphasic process. In this process, the first phase (i.e., acidogenic phase) is reported to acid forming pathways with full activation and it is responsible for production of acetate, butyrate, hydrogen and carbon dioxide as major products. In the first phase, the clostridium growth period is found during the exponential growth phase. And then the second phase is started as a solventogenic phase and this phase is characterized by assimilation of acids and then this process is responsible for production of acetone, butanol and ethanol (ABE).
This research work was done on a laboratory scale and authors have done some sets of experiments on biofuel production, such as ethanol. During this work, systematic pretreatments and enzymatic hydrolysis tasks were performed in the laboratory. This work focussed on the mitigation of waste organic matter in the environment that was generated during crop production and food processing. Saccharomyces cerevisiae MTCC 3821 was inoculated in a different nature of waste organic hydrolysate and low ethanol yield was obtained in non-detoxified biomass hydrolysates. Maximum ethanol yield (2.24 g.L-1) was obtained from plant origin waste organic matter at 72 hours in detoxified broth. Ethanol of different titers in fermentation of rice (1.85 g.L-1) and wheat straw (1.0 g.L-1) hydrolysed solution were produced. Clostridium species has shown the capability of clostridial solvent production and it occurs in a biphasic process. Better improvement in concentration of butanol (1.4 g.L-1) and ABE (2.1 g.L-1) in 50 ml of biomass hydrolysate solution in production/fermentation media that was systematically detoxified.
ACKNOWLEDGMENTS
The authors would like to thank GITAM (Deemed to be University), Visakhapatnam, Andhra Pradesh, India, for providing necessary help and support for this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Das S. The early bird catches the worm – first mover advantage through IoT adoption for Indian public sector retail oil outlets. Journal of Global Information Technology Management. 2019;22(4):1-29.
Crossref - Abel CR, Kamyria C, Johnson C, Thornton MJ, Zigler BT, McCormick RL. Global Ethanol-Blended-Fuel Vehicle Compatibility Study. Golden, CO: National Renewable Energy Laboratory. NREL/TP-5400-81252. 2021.
Crossref - Busic A, Mardetko N, Kundas S, et al. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol Biotechnol. 2018;56(3):289-311.
- Ivancic Santek M, Miskulin E, Beluhan S, Santek B. New trends in the ethanol production as a biofuel. Kem Ind. 2016;65(1-2):25-38.
Crossref - Gunatilake H, Holst DR, Sugiyarto G. Energy security for India: biofuels, energy efficiency and food productivity. Energy Policy. 2014;65:761-767.
Crossref - Hao H, Liu Z, Zhao F, et al. Biofuel for vehicle use in China: current status, future potential and policy implications. Renew Sustain Energy Rev. 2018;82:645-653.
Crossref - Barman A, Bhattacharjee S, Dutta T, et al. Biofuel from organic waste- a smart solution to conserve non- renewable resources – A review. J Phys: Conf Ser. 2022;2286:012028.
Crossref - Akın M, Bartkiene E, Ozogul F, et al. Conversion of organic wastes into biofuel by microorganisms: A bibliometric review. CleanCircul Bioecon. 2023;6:100053.
Crossref - Dutta SK, Chakraborty S. Mixing effects on the kinetics and the dynamics of two-phase enzymatic hydrolysis of hemicellulose for biofuel production. Bioresour Technol. 2018;259:276-285.
Crossref - Ferrari G, Pezzuolo A, Nizami A-S, Marinello F. Bibliometric analysis of trends in biomass for bioenergy research. Energies. 2020;13(14):3714.
Crossref - Subhadra B, Edwards M. An integrated renewable energy park approach for algal biofuel production in United States. Energy Policy. 2010;38(9):4897-4902.
Crossref - Lee YC, Lee K, Oh YK. Recent nanoparticle engineering advances in microalgal cultivation and harvesting processes of biodiesel production: a review. Bioresour Technol. 2015;184:63-72.
Crossref - Melcher F, Paper M, Bruck TB. 9 Photosynthetic conversion of CO2 into bioenergy and materials using microalgae. Photosynth. 2021:227-254.
Crossref - Ren HY, Song X, Kong F, Song Q, Ren N-Q, Liu B-F. Lipid production characteristics of a newly isolated microalga Asterarcys quadricellulare R-56 as biodiesel feedstock. Environ Sci Pollut Res. 2023;30(16):48339-48350.
Crossref - Kumar N, Banerjee C, Jagadevan S. Identification, characterization, and lipid profiling of microalgae Scenedesmus sp. NC1, isolated from coal mine effluent with potential for biofuel production. Biotechnol Rep. 2021;30:e00621.
Crossref - Nawkarkar P, Chugh S, Sharma S, Jain M, Kajla S, Kumar S. Characterization of the Chloroplast Genome Facilitated the Transformation of Parachlorella kessleri-I, A Potential Marine Alga for Biofuel Production. Curr Genomics. 2020;21(8):610-623.
Crossref - Das SK, Sathish A, Stanley J. Production of Biofuel and Bioplastic From Chlorella Pyrenoidosa. Materials Today: Proceedings. 2018;5(8-3):16774-16781.
Crossref - Kuo C-M, Sun Y-L, Lin C-H, Lin C-H, Wu H-T, Lin C-S. Cultivation and Biorefinery of Microalgae (Chlorella sp.) for Producing Biofuels and Other Byproducts: A Review. Sustainability. 2021;13(23):13480.
Crossref - Salama E-S, Kurade M, Abou-Shanab RA, et al. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renewable and Sustainable Energy Reviews. 2017;79:1189-1211.
Crossref - Bertrand E, Vandenberghe LPS, Soccol CR, Sigoillot JC, Faulds C. First Generation Bioethanol. Green Energy and Technology. 2016:175-212.
Crossref - Muktham RK, Bhargava S, Bankupalli S, Ball AS. A Review on 1st and 2nd Generation Bioethanol Production-Recent Progress. J Sustain Bioener Syst. 2016;6(3):72-92.
Crossref - Lopes TF, Cabanas C, Silva A, et al. Process simulation and techno-economic assessment for direct production of advanced bioethanol using a genetically modified Synechocystis sp. Bioresour Technol Rep. 2019;6:113-122.
Crossref - Ngigi W, Siagi Z, Kumar A, Arowo M. Predicting the techno-economic performance of a large-scale second-generation bioethanol production plant: a case study for Kenya. Int J Energy Environ Eng. 2023;14(1):95-108.
Crossref - Rafa N, Ahmed SF, Badruddin IA, Mofijur M, Kamangar S. Strategies to Produce Cost-Effective Third-Generation Biofuel From Microalgae. Front Energy Res. 2021;9:749968.
Crossref - Vasistha S, Khanra A, Clifford M, Rai MP. Current Advances in Microalgae Harvesting and Lipid Extraction Processes for Improved Biodiesel Production: A Review. Renewable and Sustainable Energy Reviews. 2021;137:110498.
Crossref - Cavelius P, Engelhart-Straub S, Mehlmer N, Lercher J, Awad D, Bruck T. The potential of biofuels from first to fourth generation. PLoS Biol. 2023;21(3):e3002063.
Crossref - Trost D, Polcar A, Boldor D, Nde DB, Wolak A, Kumbar V. Temperature Dependence of Density and Viscosity of Biobutanol-Gasoline Blends. Appl Sci. 2021;11(7):3172.
Crossref - Abdullah B, Syed Muhammad SAF, Shokravi Z, et al. Fourth generation biofuel: A review on risks and mitigation strategies. Renewable and Sustainable Energy Reviews. 2019;107:37-50.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.