The amount of anthropogenic activity in the environment has significantly increased due to urbanization and industrialization. Toxic metals and other contaminants have become more concentrated as a result, appearing in wastewater released by many sectors. Aquatic animals suffer as a result of tainted wastewater entering water bodies. As a result, damage also occurs in the crops contaminating the agricultural ecology. Although several techniques have been used to detoxify contaminants in wastewater, the current situation necessitates environmentally acceptable and economically viable techniques for wastewater treatment. To fulfill this objective, this review is aimed at exploring the major sources of metals in wastewater. The traditional techniques for treating wastewater take a lot of time and are not environmentally or financially sustainable. Utilizing microorganisms, plants, and biomass leftovers to break down metal poisons is a proven biotechnology strategy that is environmentally friendly. Hence, the review highlights the drawbacks of conventional techniques with importance of bioremediation for sustainable ecosystem. Also, phytoremediation—the process of removing metals from the environment using plants is discussed as a successful strategy. Plants are thought to be the most effective option for wastewater remediation because they contain a variety of microorganisms and enzymes that aid in the detoxification of metals from wastewater. Overall, to gain a better understanding of environmentally friendly and sustainable ways, the buildup and detoxification of metals through the use of plants, microorganisms, and biomass residues in environmental remediation is highlighted.
Bioremediation, Metal Pollution, Microbes, Phytoremediation, Plants
Water in all of its forms is the only thing that sustains life on Earth. Many living species, including aquatic and microbes, are thought to call water home.1 Water has various qualities that make it useful for a variety of uses, including drinking, irrigation, industrial processes, agriculture, and more.2 The wastewater released from residential and commercial activities degrades the water quality, which has an impact on aquatic life, plants, and people. About 70% of rivers have water quality that has deteriorated due to prevailing pollutants, making it unfit for human consumption and other commercial activities, according to organizations like the World Health Organization (WHO), Indian Council of Medical Research (ICMR), and Central Pollution Control Board (CPCB).3 Numerous sources of contaminants have deteriorated the water due to increased industrialization, urbanization, and human activity.4 With an increasing population comes exponential growth in both domestic and industrial activities, resulting in massive wastewater production.5,6 The World Health Organization estimates that between 80 and 85 percent of human diseases are caused by toxins found in water, which makes water quality essential for managing water safety and maintaining public health.7,8
As the production of chemicals, fertilizers, pharmaceuticals, industrial items, and other valuable goods has surged, so too has the concentration of heavy metals and nanomaterials in the environment. The most dangerous, poisonous, and non-biodegradable substances that pose a major risk to human safety are thought to be metals. The environment is contaminated by a number of metals, including lead, cadmium, mercury, uranium, zinc, arsenic, and other metals that may be dangerous to humans.9,10 While some conventional treatment approaches have been successful in eliminating these heavy metals from the environment, the current situation calls for further developments in treatment strategies, sophisticated physio-chemical and chemical separation methods,11,12 and biotechnological approaches,13 used widely in today’s scenario to eliminate metal toxicity. Thus, the purpose of this review is to address the origins of metal toxicity in wastewater. In light of this, the bioremediation approach is emphasized to highlight the significance of biomass residues, microorganisms, and plants in resolving the heavy metal problem and cleaning contaminated environments, such as soil, groundwater, and aquatic ecosystems. Microorganisms and plants may be able to remove heavy metals from wastewater. Finally, the reuse of metals after bioremediation process is advocated for sustainable environment.
Sources of metals and their toxicity
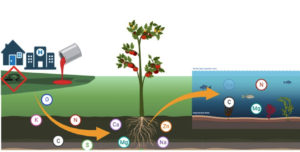
The distinctive chemical and physical characteristics of heavy metals make them valuable components of electronics, equipment, and artefacts. Rocks and other man-made materials can enter the aquatic food chain that supports both people and animals through geochemical weathering. Runoff from rivers, agricultural practices, industrial effluent from metal-finishing businesses, mining wastes, and wastewater from landfills are the main sources of metals14 (Figure 1). Water quality standards intended to preserve the environment are being compromised by the negative effects of metals leached from various sectors into the aquatic environment. Therefore, it is thought that the most prevalent contaminants in wastewater are heavy metals.15 Wastewater contains a variety of metals and their ions, such as lead, cadmium, mercury, chromium, cobalt, arsenic, nickel, zinc, selenium, and others that have long-term effects on people. In order to prevent these harmful metals from combining with surface water, it is imperative that they be removed from the source. Certain metals, when discharged into wastewater, can also cause cancer and other health problems.14 Therefore, one crucial type of therapy for getting rid of harmful metals is bioremediation, which uses materials based on plants and microbes.
Environmentally harmful and poisonous impacts result from metals emitted from industrial exposure and other business facilities. For instance, long-term exposure to lead in the atmosphere can be harmful to humans and can hinder the synthesis of proteins and reduce the body’s amounts of the antioxidant sulfhydryl.16 By swapping zinc elements in proteins and decreasing their affinity to DNA, lead may potentially change the expression patterns of genes. Humans may also consume mercury in various organic, inorganic, and elemental forms through food, drink, and the air.17 Additionally able to pass the blood-brain barrier, adult exposure to mercury has been associated with increased antinuclear autoantibodies. Mercury exposure can result in a number of conditions, such as cerebral palsy, blindness, dysarthria, and mental retardation in children.18 Due to emissions from mining and smelting processes, cadmium can be hazardous even at very low concentrations.19 Given that smoking is the main human source of cadmium, it is thought to be a carcinogen.20 Cadmium interacts and accumulates in the kidney, liver, brain, and lungs, among other biological systems, causing toxicity. Cadmium affects cell function and homeostasis by obstructing epithelia and cell membrane transit.21 Arsenic is released when fossil fuels are burned and pesticides are used in agriculture. This accumulates in humans and is linked to conditions like liver fibrosis, diabetes mellitus, hypertension, and cancer.22 Exposure to arsenic can change the gut microbiome, which can then affect the metabolic profile at a functional level.23 Absorbance of chromium in wastewater is largely due to the weathering of rocks and sediments, volcanic eruptions, and other human-caused sources such as burning fossil fuels, the tanning and leather industry, and plastic manufacturing.24 Below is a detailed description of the sources of environmental contamination caused by heavy metals and how they were remedied.
Arsenic
Many human activities, such as mining, refining, burning petroleum derivatives, making glass and semiconductors, composting, and using chemotherapy to treat illnesses, release arsenic (As) into the environment. As is created in its whole at a rate of between 36,000 and 45,000 t/year, with Morocco and China playing a major role.25 Regardless, normal activities such as rock erosion, microbial colonization, and volcanic emission are the first to cause significant As pollution in the climate.26 Over 150 million people worldwide are at risk due to drinking water contaminated with arsenic, which affects approximately 70 countries.27 Because of the growing medical issues, As pollution in many Asian countries is more complex. The most heavily contaminated areas of As tainting, with fixations ranging from 0.5 to 4600 µg/L, are in Bangladesh and a few areas of West Bengal, India.28 Approximately 20–45 million people in Bangladesh are at risk due to As-contaminated groundwater. Bangladesh’s basic situation was described by WHO as “the biggest harming of a populace on planet.” In the soil and groundwater of 14 out of 20 countries in Latin America—mostly Chile, Mexico, and Argentina—there is a contamination with As.29 It has also been determined that the USA, Taiwan, Vietnam, Ghana, Australia, Nepal, Hungary, Thailand, and Cambodia have high concentrations of As in their groundwater.30
Microorganisms are typically found in arsenic geochemical ambient variables and have an impact on the biochemical pattern of arsenic. This process converts arsenic into different structures that vary in terms of their development, poisonousness, and bioavailability. Therefore, the most effective technique for reducing the concentration of arsenic in soil and water is bioremediation. Four isolates belonging to the genera Pseudomonas, Acinetobacter, Klebsiella, and Comamonas are capable of oxidising As (III) and exhibiting As (III)-oxidase protein mobility. Pseudomonas sp. ASR1, ASR2, and ASR3, Geobacillus sp. ASR4, Bacillus sp. ASR5, Paenibacillus sp. ASR6, Enterobacter sp. ASR10 and Comamonas sp. ASR11, and ASR12 possessed some or all of the concentrated attributes that advance plant development, such as phosphate-solubilization, siderophore, and particles that resemble indole acetic acid (IAA). This analysis of the boundaries of plant development advancement revealed these organisms.31
Cadmium
The main sources of cadmium (Cd) supply include phosphate manure, waste cremation, stabilizers for plastics, coatings and plating, and non-renewable energy source combustion. The production of ferrous and nonferrous metals, concrete, zinc, lead, copper mining, and other activities all contribute to the concentration of Cd in the atmosphere. The estimated annual global consumption of Cd is between 20,000 and 24,000 t/year.25 The extended amount of Cd in the climate is also largely provided by regular cycles such as residual storms, ocean salt splash, volcanic workouts, enduring, disintegration, and out-of-control fires. In countries like China, the Republic of Korea, and Japan’s abandoned metal mining sites, there is an exceptionally high absolute Cd confirmation and environmental testimony.25 There are other areas with high levels of Cd pollution in a few districts of the Czech Republic, Romania, Slovakia, Poland, Kazakhstan, Mexico, Australia, USA, Belgium, Germany, Namibia, Vietnam, and India. Approximately 150 locations have been identified globally where the estimated population of approximately 5 million people is at risk of contracting HIV. Known as one of Japan’s four biggest contamination illnesses, the Itai-Itai virus caused widespread Cd injury due to mining and purification.32
In soil depleted of cadmium, it is critical to support remediation methods that eradicate or neutralize its toxic effects. The use of microbial bioremediation is a viable treatment for soils depleted of heavy metals. For instance, the Cd-resistant Raoultella sp. strain X13 that was isolated from soil contaminated with heavy metals is able to primarily absorb Cd through particle exchange and chelation, which firmly confines it to cell dividers. Additionally, this resulted in the development of natural plant growth-promoting characteristics that support the synthesis of siderophores, indole acetic acid, and the ability to solubilize phosphates. X13 may reduce the bioavailability of Cd in soils that are Cd-focused. Furthermore, by immunizing soils contaminated with Cd with strain X13, bioavailable Cd was mainly transferred to the inorganic-bound portion. Thus, strain X13 is an effective remedy that may also be used for Cd2+ cleanup.33
Chromium
Chromium (Cr) is frequently used in many sectors. An estimate of the absolute Cr creation is between 18,000 and 30,000 x 103 t/year.34 90% of this total creation is employed in metallurgical projects, such as the production of Cr metal and chrome amalgam. Cr is used in synthetic industries for tanning calfskin, preventing metal consumption, coloring materials, and protecting wood.34 Tanneries are the particular source of the Cr pollution among all of them. Because tanneries’ wastewater control and treatment facilities are unable to adequately remove waste, natural waste entering the atmosphere causes significant Cr damage.35 Low centralization of Cr (VI) can also be liberated from the world’s covering by regular cycles, such as structural and aquatic events. About 75% of the world’s Cr (VI)-contaminated travel locations are primarily in South Asian countries, namely Bangladesh, India, and Nepal. Other incredible sullied locations include China, Central Mexico, the Thiva Basin in Central Greece, the Aromas Red Sands Aquifer in California, and Australia’s Kooragang Island.36
The discharge of hexavalent chromium [Cr (VI)] into the environment has resulted in multiple regrettable interactions with organic structures due to its deleterious capacity and carcinogenic nature. One essential method for detoxifying Cr (VI) to trivalent forms that are safe is chromium reduction by chromium reductase (ChrR). Therefore, the E. coli strain FACU is among the bacterial isolates that are able to detoxify Cr (VI). The rapid development of the E. coli FACU strain augmented with chromate led to its arrival at the log stage, after which it considerably decreased, demonstrating its ability to decrease chromate under stressful conditions and suggesting the presence of genes imparting chromium resistance in the strain.37
Mercury
With 38% and 25% of total anthropogenic outflows, respectively, coming from the unique and small-scale activities of coal burning and gold mining, significant mercury (Hg) contamination begins there.38 Various sources of mercury include the manufacturing of ferrous and non-ferrous metals, concrete, large-scale gold mining, waste from urban and clinical settings, army bases, use of pesticides and compost, waste from products containing mercury, and so forth.39 Globally, the majority of severe mercury is released by Asian countries (which account for 47.5% of all anthropogenic mercury discharges), mostly due to strong support from China and India’s regions. Additionally, regions of Africa have a high amount of anthropogenic mercury runoff (16.8%).38 Largely due to coal-terminated power plants, North America and the European Union emit 43.4 t/year and 44.1 t/year Hg, respectively, into the atmosphere. A variety of Hg mine-smelter districts in Mexico, Kyrgyzstan, Peru, Russia, Slovenia, Spain, Alaska (USA), the Philippines, Tajikistan, and Ukraine are among the other outstanding debased locations.25
Mercury changes its substance structures in the climate and goes from one spot to another and lastly it gets deposited into soil and silt. As mercury remediation through customary methodologies is expensive and actually troublesome, bioremediation is a more economical, eco-accommodating strategy and acknowledged by administrative specialists. The disengaged marine biofilm shaping mercury-safe bacterium B. thuringiensis PW-05 has been viewed as an appropriate competitor animal variety as an option for current remediation of inorganic mercury. However, much progression has been occurred in the field of bioremediation of mercury, utilizing cell chelation and recuperation of mercury must be applied to the modern squanders and the shut bodies. Notwithstanding, the seclude
B. thuringiensis PW-05 can volatilize mercury from the debased climate and can be utilized in-situ to different natural conditions to decrease the harmful impacts. As the detach is fit for eliminating >90 % of remaining mercury by a blend of mercury volatilization and sequestration with shifted physico-synthetic boundaries like pH, saltiness and temperature, it will be of extraordinary use in unfriendly ecological conditions sullied with inorganic mercury.40
Currently used conventional methods for removal of metals
The elimination of heavy metal contaminants from wastewater is the main environmental issue. The most widely used methods of treating wastewater to remove heavy metals include ion-exchange, physical adsorption, coagulation, chemical precipitation, filtration, and adsorption.9 These techniques use chemical or physical processes to precipitate the metals out of the water, entrap them in absorbent materials or filters, or swap them for other ions. The kind and concentration of metals, the amount of water, and the intended degree of treatment are the major factors that influence the method choice. To highlight the mechanisms of these techniques, Table 1 is presented that list the methods used to remove heavy metals along with their benefits and drawbacks.
Table (1):
List of conventional metal removal techniques with advantages and disadvantages
Name of Technique |
Advantage |
Disadvantage |
Reference |
|---|---|---|---|
Electrochemical method |
Recovery of metals from wastewater, good reduction yields, reduced chemical requirements, and decreased sludge production |
Costly electricity supply, initial capital investment is high |
41 |
Coagulation and flocculation |
Dewatering and enhanced settling of sludge |
Increased sludge volume generation, consumption of chemicals |
41 |
Adsorption |
Heavy metal-containing wastewater with low concentrations can be removed effectively and economically. Adsorbents, such as tree fern, are inexpensive, have simple operating conditions, a wide pH range, and a high affinity for metals, making them suitable for a variety of industrial applications. |
Production of waste products, low selectivity |
42 |
Membrane technology |
High efficiency, space requirement is less, low pressure, high separation selectivity |
Membrane fouling causes high operational cost, complex process, low permeability flux |
43 |
Chemical precipitation |
Low capital investment, simple process |
Mass production of sludge, reliant on pH, ineffective in treating wastewater with low metal concentrations, costly, and needing hazardous chemicals |
44 |
Ion-exchange |
Less concentration of sludge produced |
High operational cost of experiment |
45 |
Bioremediation for detoxification of metals
The traditional methods mentioned above are not environmentally friendly and have high running costs, which prevents them from working normally and from effectively detoxifying metals from wastewater. Therefore, there is a critical need for an environmentally benign method of employing bioremediation to remove heavy metals from wastewater. Hence, using biological agents to remove and transform pollutants such as heavy metals and metalloids, hydrocarbons, oil, dye, pesticides, etc. into a less hazardous and poisonous state is known as bioremediation. Through the use of biomass, organic materials are broken down and mineralized into nitrogen, water, carbon dioxide, and other elements.12,41 A further definition of bioremediation is the process of biological degradation under controlled conditions to levels below the concentration limits set by regulatory authorities.42
The three primary elements of the bioremediation triangle are nutrients, food, and microbes. In addition to providing a carbon source for microbial development, contaminants in soil or water also provide energy to the bacteria through their ability to carry out the redox process that results in electron transfer.43 Microorganisms must receive the right amounts of vital nutrients and chemicals for them to be able to detoxify the contaminants.44 Microbial enzymes have the ability to take up pollutants as food thus making them suitable for this process. To enable microbial growth and activity to occur at a faster rate, environmental conditions must be altered.45 Additionally, the interaction between metals and microbes is determined by a number of factors, such as pH, temperature, ions, colloidal substances, and other living things that aid in the formation of biofilms and microbial colonization.46 Biodegradation, which entails the total removal and breakdown of pollutants into harmless forms that are safe for the ecosystem, forms the basis of the bioremediation process.47 There are various factors affecting bioremediation such as:
- Microbial Population: Concentration of biomass, Population diversity, Enzymatic activities.48
- Environmental Factors: pH, Temperature, Moisture content, Redox potential, Availability of electron acceptors, Carbon and energy sources.49
- Chemical Factors: Bioavailability of pollutants, Biodegradability of pollutants.
Using both in-situ and ex-situ bioremediation techniques, the bioremediation process can be effectively applied to soil and water.
In-situ bioremediation
Biological treatment is administered at the contamination site in in-situ bioremediation with the goal of eliminating chemicals or pollutants that are subsurface.50,51 This technique doesn’t require excavation that leads to little or no disturbance to the structure of the soil. Application of in-situ bioremediation is greatly influenced by the soil porosity.52,53 Water content, temperature, pH, nutrients, and the state of electron acceptor (oxygen) are other critical variables for this approach. The terms “intrinsic” and “engineered” refer to two different kinds of in-situ bioremediation. A totally natural method of eliminating pollutants without changing the environment is called intrinsic bioremediation. In the context of designed in-situ bioremediation, physiochemical parameters are modified to augment and promote microorganism growth.54
Ex-situ bioremediation
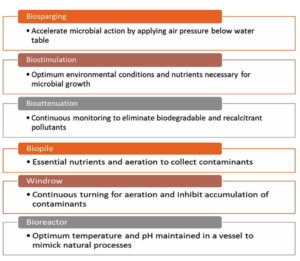
With this kind of bioremediation procedure, a sample is taken from a contaminated site and then moved to another location so that the pollutant can be removed. Should the pollutant be present in the soil, it can be removed through excavation; if it has infiltrated the groundwater, it is pumped out and the tainted soil and water are eliminated.55 The ex-situ method is determined by the extent of contamination and the treatment expenses. In Figure 2, the ex-situ and in-situ techniques are briefly described.
Mechanism of bioremediation
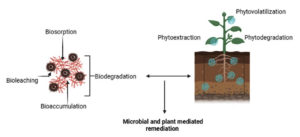
By being adapted to microbes, heavy metals found in contaminated locations are limited. Extracellular polymeric materials found on cell biomass can attach themselves to heavy metals with the aid of proton exchange and micro-precipitation. The bioremediation process is initiated by microbes that immobilize metals and then oxidize and reduce them. Microorganisms including bacteria, fungus, and algae are used in microbial-mediated bioremediation to interact with heavy metal pollutants and help remove them from water. To sequester or detoxify heavy metals, microorganisms can use a variety of strategies, such as biosorption, bioaccumulation, and biotransformation.41 Through enzymatic modification, biotransformation systems change heavy metal ions into less mobile or hazardous forms.56 Microbial-mediated bioremediation is a popular method for treating heavy metal-contaminated water because it is adaptable, affordable, and environmentally safe. Different enzymes found in microorganisms can degrade a wide range of environmental pollutants.44 Microbial bioremediation can take place in an anaerobic or aerobic environment, depending on the kind of contamination and the surrounding circumstances.57 Thus, an insoluble element can be changed into its mobile and soluble phase to aid in bioremediation. The discharge of metals from their solid phase into the solution phase might potentially have a negative impact on mobilization.58 This penetrates microbial metabolic systems and aids in increasing the bioavailability of metals. Therefore, the reduction of hazardous metal ions into their reduced counterparts is aided by microbial reduction.
For instance, Hg (II) is reduced by bacteria to Hg, the volatile form, As (V) and Fe (III) are likewise reduced to As (III) and Fe (II).59 Different microbes are used for heavy metal detoxification in addition to direct microbial activities. Aspergillus niger, for instance, aids in the elimination of As (III) and As (II)60 but Schizophyllum commune aids in the removal of both organic matter and heavy metals.61 Additionally, the reduction of Cr (VI) to Cr (III) and related hydroxides can be aided by Methanothermobacter thermautotrophicus, Bacillus cereus, and Shewanella sp.62 In addition to immobilisation, biosorption and bioaccumulation can aid in the removal of heavy metals from the environment. Although very hazardous metals can accumulate in cells and impair their ability to function metabolically, bioaccumulation refers to the uptake of heavy metals from the contaminated location by living biomass. Therefore, dead biomass is used, which requires no energy to adsorb heavy metals from their surface.63
Notwithstanding the many benefits of bioremediation, such as its high selectivity and specificity, economic viability, and environmental friendliness, these procedures also have a number of disadvantages. Time is of essence for the breakdown of harmful compounds, and bioremediation at highly contaminated sites can no longer be used. Seasonal variations in microbial activity, issues with the use of chemicals for treatment, and occasionally an uncontrollable and challenging-to-manage process are some of the drawbacks associated with in-situ bioremediation. Furthermore, heavy metal and chlorinated hydrocarbon cleanup using the ex-situ approach is ineffective. This means that in order to detoxify metals, more synergistic ways must be used, such as biomass residues and phytoremediation procedures.
Phytoremediation approaches for remediating metal toxicity
To safeguard the environment, heavy metal removal from wastewater must be done effectively.64,65 Several scientists are investigating the potential of different plants for phytoremediation of metals.66,67 This efficient green technology is used to get rid of pollutants found in the air, water, and soil.68 A cheap, low-cost, and environmentally benign method of eliminating pollutants without endangering the ecosystem is called phytoremediation. Many plants have the capacity to absorb metals through their large-surface-area of roots, which makes it easier for pollutants to be mobilised and detoxified for environmental cleanup. Additionally, the microbial community that resides on the roots, stem, and leaves of plants serves as a home for a range of bacteria that stimulate the release of hormones promoting plant development and preserve the health and nutrition of plant.69 Various plants used for Phytoremediation to remove metals are summarized in Table 2.
Table (2):
Plants acting as potential sources of Phytoremediation
Plant Species |
Accumulation Part |
Process |
Reference |
|---|---|---|---|
Ricinus communis |
Shoots or roots |
Phytoextraction |
75 |
Puccinellia frigida |
Shoots or roots |
Phytoextraction |
76 |
Iris sibirica |
Rhizosphere |
Phytostabilization |
77 |
Helianthus annuus |
Shoots |
Phytoextraction |
78 |
Pennisetum annuus |
Shoots |
Phytoextraction |
79 |
Hordeum vulgare |
Rhizosphere |
Phytostabilization |
80 |
Salix matsudana |
Shoots |
Phytofiltration |
81 |
Micranthemum umbrosum |
Shoots |
Phytofiltration |
82 |
Brassica sp. (wild type) |
Release in atmosphere |
Phytovolatilization |
83 |
Chara canescens |
Release in atmosphere |
Phytovolatilization |
84 |
The physiologic properties of metals exhibit a variety of absorption and accumulation within plants and soil, which determines how well they are absorbed by plants. After metals enter the plant, they can pass through tissues via the apoplast and symplast.65 Unlike symplastic transport, which happens within the cytoplasm of neighbouring cells via specialised structures called plasmodesmata and sieve plates, apoplastic transport takes place outside the plasma membrane via extracellular spaces, neighbouring cell walls, and xylem vessels.61 This is in contrast to symplastic transport. For metals to travel radially throughout plant tissues, they must pass through the apoplastic route, which also allows them to pass through vascular tissues and the root central cylinder before continuing to the aerial section.54 It is necessary to cross the Casparian strip, a barrier to the apoplastic pathway, in order to reach the xylem through the root. To do this, one must take the symplastic route via endodermal cells.70 It is also possible for another important symplastic transport to occur using sieve tube components in the phloem, which would allow distribution to organs and tissues that are not photosynthetic.71 To travel via the symplastic route, MNPs must be absorbed by the plant cell and pass through the plasma membrane. Phytoremediation technique is based on several methods that plants employ to promote the uptake of heavy metals from soil, including phytoextraction, phytostabilization, phytodegradation, and phytovolatilization. Below is a description of the several processes that phytoremediation employs:
Phytoextraction
This is the method that is most frequently used to extract heavy metals from wastewater. Metals are absorbed by plants through their roots, whereupon they build up in their tissues. After the metals are in the plant, the pollutants in the wastewater can be successfully removed by harvesting and discarding the metals. By giving plants a high limit for metal collection in shoots, this interaction reduces soil metal fixations. Large groups of heavy metals should be divided by the plants into their underlying foundations, moved to above-ground shoots or leaves, and produced in large quantities that are easily gathered, along with the removal of foreign materials from the soil. It might be feasible to recover expensive metals from the gathered plant material (e.g. Phyto mining of Ni, Tl or Au).72 If not, it is possible to consume the dry substance and dispose of the detritus in a regulated manner. Other names for phytoextraction include phytoaccumulation, Phyto sequestration, and Phyto absorption.73 Continuous phytoextraction calls for the use of plants that accumulate dangerous foreign substances in particularly high concentrations over the course of their lives (hyperaccumulators), whereas initiated phytoextraction techniques improve poison aggregation at a single time point by adding more catalysts or chelators to the soil.74
Phytostabilization
Certain plants have the ability to immobilise heavy metals, lowering their bioavailability and mobility in the soil or sediment. Several strategies are used to do this, including altering the pH of the soil, generating organic acids that attach to the metals, and encouraging the development of microorganisms that have the ability to immobilise the metals. Immobilising pollutants in soil, water, plant roots, or shoots lowers their bioavailability in the environment, a process known as phytostabilization, also called phyto immobilisation.73 Phytostabilization is often referred to as Phyto immobilisation or in situ inactivation. Sorption, precipitation, complexation, or a decrease in metal valence can all lead to phytostabilization. Because metals do not corrode strongly, removing them in situ can be the best option in some circumstances, especially in places with low pollution levels or in highly contaminated areas where in situ remediation or large-scale extraction would be absurd. Grass, scavenging plants, and reeds are among the high-pollutant plants that are beneficial for phytostabilization because they reduce the amount of groundwater that is carried away from the site carrying pollutants.
Phytovolatilization
Through a process called phytovolatilization, hazardous metal pollutants are converted into less hazardous and more volatile forms by means of metabolism of plants and soil microorganisms, which allows them to be released into the atmosphere.73 It involves using plants to absorb pollutants from the soil, transforming them into structures with unpredictable structures, and modifying the climate. Certain microscopic species have the ability to absorb and dissipate mercury (Hg). These transgenic plants, which have the bacterial properties of mindfulness transferred to Nicotiana or Brassica species, may prove useful in decontaminating soil tainted with mercury.75 During their migration from roots to leaves, metal contaminants are changed by the process of phytovolatilization into a water-soluble and non-poisonous form, which causes pollution to become volatile.
Phytodegradation
Using enzymes like oxygenases, nitroreduc-tases, and dehydrogenases, phytodegradation-also known as phyto transformation-is a technique for breaking down organic pollutants.76 Different enzymes aid in the process of phytodegradation: peroxidase converts phenolic compounds; nitrilase transforms aromatic compounds; phosphatise transforms organophosphate pesticides; and nitro reductase converts explosives. Dehalogenase is used to convert chlorinated compounds. In other words, it involves the uptake, metabolization, and corrosion of foreign materials inside the plant, or alternatively, it involves the delivery of foreign materials by the plant to soil residue, groundwater, or surface water. For instance, it was thought that crossing poplar trees (Populas deltoids nigra) absorbed the important water and soil pollutant trichloroethylene (TCE), separating the foreign material into its metabolic components.77
Phytoremediation is an environmentally benign and sustainable method of eliminating heavy metals from aquatic ecosystems because of these combined mechanisms. However, a number of variables affect how efficient phytoremediation is: the kind and concentration of heavy metals present, the length of the treatment period, and the ecosystem’s surrounding conditions. Because of this, a variety of microbes and plants function as effective remediators due to their unique ability to take in pollutants and break them down using a variety of bacteria and enzymes secreted by plant tissues (Figure 3).78 Plants have the ability to accumulate metal contaminants, making them a viable source for cleanup. For this reason, phytoremediation and related methods are thought to be the most affordable and effective remediation solution for metal detoxification.
To address the heavy metal contamination resulting from both industrial and anthropogenic activities, a number of remediation techniques have been developed over time. In conclusion, methods for removing heavy metals from aquatic ecosystems that show promise include phytoremediation and microbiological processes. By utilizing plants’ and microorganisms’ innate capacities to absorb, accumulate, or change heavy metals, these techniques lower the concentrations of these pollutants in water bodies. Due to its high remediation effectiveness and naturally occurring non-toxic synthetic material, bioremediation has shown itself to be a game-changer. By binding or metabolizing heavy metals and changing them into less hazardous forms or immobilizing them, bacteria, fungus, and algae are used in microbial remediation. By using plants to absorb, collect, and store heavy metals in their tissues, phytoremediation, on the other hand, successfully removes such metals from water. Adopting these environmentally friendly strategies has resulted in a noteworthy decrease in the levels of heavy metal contamination and its harmful impacts, as well as a reduction in the overall expenses and remedial duration. In addition, there is a growing demand for treatment systems that can achieve exceptionally low levels of effluent metal. Comparing microbial and phytoremediation to traditional treatments reveals that they are both economical, sustainable, and kind to the environment. Their efficacy, however, can differ based on the particular pollutants, the surrounding circumstances, and the kinds of microbes or plants employed. Overall, there is a lot of promise for these bioremediation techniques to address heavy metal contamination in aquatic environments; however, more study and application are required to maximize their effectiveness and scalability.
ACKNOWLEDGMENTS
The authors are thankful to the Department of Biotechnology, Delhi Technological University, for providing the facility and infrastructure to carry out the work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Hallsworth JE. Water is a preservative of microbes. Microb Biotechnol. 2022;15(1):191-214.
Crossref - Shoushtarian F, Negahban-Azar M. Worldwide Regulations and Guidelines for Agricultural Water Reuse: A Critical Review. Water. 2020;12(4):971.
Crossref - Khan AS, Anavkar A, Ahmad Ali, Patel N, Alim H. A Review on Current Status of Riverine Pollution in India. Biosci Biotechnol Res Asia. 2021;18(1):9-22.
Crossref - Singh N, Poonia T, Siwal SS, Srivastav AL, Sharma HK, Mittal SK. Chapter 9 – Challenges of water contamination in urban areas. In: Srivastav AL, Madhav S, Bhardwaj AK, Valsami-Jones E, eds. Current Directions in Water Scarcity Research. Urban Water Crisis and Management, Elsevier. 2022;6:173-202.
Crossref - Ezugbe EO, Rathilal S. Membrane Technologies in Wastewater Treatment: A Review. Membranes. 2020;10(5):89.
Crossref - Bijekar S, Padariya HD, Yadav VK, et al. The State of the Art and Emerging Trends in the Wastewater Treatment in Developing Nations. Water. 2022;14(16):2537.
Crossref - Peletz R, Kisiangani J, Bonham M, et al. Why do water quality monitoring programs succeed or fail? A qualitative comparative analysis of regulated testing systems in sub-Saharan Africa. Int J Hyg Environ Health. 2018;221(6):907-920.
Crossref - Saleh HM, Hassan AI. Water Quality Standards. Applied Water Science, John Wiley & Sons, Ltd. 2021;1:441-468.
Crossref - Hasanpour M, Hatami M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv Colloid Interface Sci. 2020;284:102247.
Crossref - Dahiya V. Heavy metal toxicity of drinking water: A silent killer. GSC Biol Pharm Sci. 2022;19(1):020-025.
Crossref - Patnaik P. Handbook of Environmental Analysis: Chemical Pollutants in Air, Water, Soil, and Solid Wastes, Third Edition. CRC Press. 2017.
Crossref - Ali M, Song X, Ding D, Wang Q, Zhang Z, Tang Z. Bioremediation of PAHs and heavy metals co-contaminated soils: Challenges and enhancement strategies. Environ Pollut. 2022;295:118686.
Crossref - Lopez-Roldan R, Tusell P, Cortina JL, Courtois S, Cortina JL. On-line bacteriological detection in water. TrAC Trends Anal Chem. 2013;44:46-57.
Crossref - Gautam RK, Sharma SK, Mahiya S, Chattopadhyaya MC. CHAPTER 1 Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. 2014:1-24.
Crossref - Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60-72.
Crossref - Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res. 2016;23(9):8244-8259.
Crossref - Carocci A, Rovito N, Sinicropi MS, Genchi G. Mercury Toxicity and Neurodegenerative Effects. In: Whitacre DM, ed. Reviews of Environmental Contamination and Toxicology. Rev Environ Contam Toxicol. 2014;229:1-18.
Crossref - Boucher O, Muckle G, Jacobson JL, et al. Domain-Specific Effects of Prenatal Exposure to PCBs, Mercury, and Lead on Infant Cognition: Results from the Environmental Contaminants and Child Development Study in Nunavik. Environ Health Perspect. 2014;122(3):310-316.
Crossref - Pretto A, Loro VL, Morsch VM, et al. Alterations in carbohydrate and protein metabolism in silver catfish (Rhamdia quelen) exposed to cadmium. Ecotoxicol Environ Saf. 2014;100:188-192.
Crossref - Feldkamp ML, Srisukhumbowornchai S, Romitti PA, et al. Self-Reported Maternal Cigarette Smoke Exposure during the Periconceptional Period and the Risk for Omphalocoele. Paediatr Perinat Epidemiol. 2014;28(1):67-73.
Crossref - Van Kerkhove E, Pennemans V, Swennen Q. Cadmium and transport of ions and substances across cell membranes and epithelia. BioMetals. 2010;23(5):823-855.
Crossref - He J, Wang M, Jiang Y, et al. Chronic Arsenic Exposure and Angiogenesis in Human Bronchial Epithelial Cells via the ROS/miR-199a-5p/HIF-1a/COX-2 Pathway. Environ Health Perspect. 2014;122(3):255-261.
Crossref - Lu K, Abo RP, Schlieper KA, et al. Arsenic Exposure Perturbs the Gut Microbiome and Its Metabolic Profile in Mice: An Integrated Metagenomics and Metabolomics Analysis. Environ Health Perspect. 2014;122(3):284-291.
Crossref - Mohan D, Singh KP, Singh VK. Trivalent chromium removal from wastewater using low cost activated carbon derived from agricultural waste material and activated carbon fabric cloth. J Hazard Mater. 2006;135(1-3):280-295.
Crossref - Summaries, Mineral Commodity. “Mineral commodity summaries.” US Geological Survey: Reston, VA, USA 200 (2021).
- Lopez DL, Bundschuh J, Birkle P, et al. Arsenic in volcanic geothermal fluids of Latin America. Sci Total Environ. 2012;429:57-75.
Crossref - Thakur BK, Gupta V, Chattopadhyay U. Arsenic Groundwater Contamination Related Socio-Economic Problems in India: Issues and Challenges. In: Nautiyal S, Rao KS, Kaechele H, Raju KV, Schaldach R, eds. Knowledge Systems of Societies for Adaptation and Mitigation of Impacts of Climate Change. Environmental Science and Engineering. Springer. 2013:163-182.
Crossref - Chakraborty M, Mukherjee A, Ahmed KM. A Review of Groundwater Arsenic in the Bengal Basin, Bangladesh and India: from Source to Sink. Curr Pollut Rep. 2015;1(4):220-247.
Crossref - Bundschuh J, Litter MI, Parvez F, et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci Total Environ. 2012;429:2-35.
Crossref - Basu A, Saha D, Saha R, Ghosh T, Saha B. A review on sources, toxicity and remediation technologies for removing arsenic from drinking water. Res Chem Intermed. 2014;40(2):447-485.
Crossref - Das S, Jean JS, Kar S, Chou ML, Chen CY. Screening of plant growth-promoting traits in arsenic-resistant bacteria isolated from agricultural soil and their potential implication for arsenic bioremediation. J Hazard Mater. 2014;272:112-120.
Crossref - Gomez OA. The Evolution of Official Lessons: The Japanese Experience of the “Big Four” Pollution Diseases Through the Lens of International Aid. Social Science Research Network; 2008. https://papers.ssrn.com/abstract=1308304. Accessed December 20, 2021.
- Xu S, Xing Y, Liu S, Huang Q, Chen W. Role of novel bacterial Raoultella sp. strain X13 in plant growth promotion and cadmium bioremediation in soil. Appl Microbiol Biotechnol. 2019;103(9):3887-3897.
Crossref - Dhal B, Thatoi HN, Das NN, Pandey BD. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J Hazard Mater. 2013;250-251:272-291.
Crossref - Rahman Z, Thomas L. Chemical-Assisted Microbially Mediated Chromium (Cr) (VI) Reduction Under the Influence of Various Electron Donors, Redox Mediators, and Other Additives: An Outlook on Enhanced Cr(VI) Removal. Front Microbiol. 2021;11:3503.
Crossref - Gonzalez AR, Ndung’u K, Flegal AR. Natural Occurrence of Hexavalent Chromium in the Aromas Red Sands Aquifer, California. Environ Sci Technol. 2005;39(15):5505-5511.
Crossref - Mohamed MSM, El-Arabi NI, El-Hussein A, El-Maaty SA, Abdelhadi AA. Reduction of chromium-VI by chromium-resistant Escherichia coli FACU: a prospective bacterium for bioremediation. Folia Microbiol (Praha). 2020;65(4):687-696.
Crossref - Xu J, Bravo AG, Lagerkvist A, Bertilsson S, Sjoblom R, Kumpiene J. Sources and remediation techniques for mercury contaminated soil. Environ Int. 2015;74:42-53.
Crossref - Steenhuisen F, Wilson SJ. Development and application of an updated geospatial distribution model for gridding 2015 global mercury emissions. Atmos Environ. 2019;211:138-150.
Crossref - Dash HR, Mangwani N, Das S. Characterization and potential application in mercury bioremediation of highly mercury-resistant marine bacterium Bacillus thuringiensis PW-05. Environ Sci Pollut Res. 2014;21(4):2642-2653.
Crossref - Kapahi M, Sachdeva S. Bioremediation Options for Heavy Metal Pollution. J Health Pollut. 2019;9(24).
Crossref - Sutar H, Kumar D. A Review on: Bioremediation. Int J Res Chem Environ. 2012;2:13-21.
- Kulshreshtha A, Agrawal R, Barar M, Saxena S. A Review on Bioremediation of Heavy Metals in Contaminated Water. IOSR J Environ Sci Toxicol Food Technol. 2014;8(7):44-50.
Crossref - Othman AR, Hasan HA, Muhamad MH, Ismail N ‘Izzati, Abdullah SRS. Microbial degradation of microplastics by enzymatic processes: a review. Environ Chem Lett. 2021;19(4):3057-3073.
Crossref - Abatenh E, Gizaw B, Tsegaye Z, Wassi M. The Role of Microorganisms in Bioremediation- A Review. Open J Environ Biol. 2017;2(1):038-046.
Crossref - Gadd GM. Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology. 2010;156(3):609-643.
Crossref - Jain P, Bajpai V. Biotechnology of bioremediation-A review. Int J Env Sci. 2012;3(1):535-549.
- Zhang H, Yuan X, Xiong T, Wang H, Jiang L. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chem Eng J. 2020;398:125657.
Crossref - Singh P, Singh VK, Singh R, et al. Chapter 1 – Bioremediation: a sustainable approach for management of environmental contaminants. In: Singh P, Kumar A, Borthakur A, eds. Abatement of Environmental Pollutants, Elsevier. 2020:1-23.
Crossref - Sharma S. Bioremediation: Features, Strategies And Applications. Asian J Pharm Life Sci. 2012.
- Hussain A, Rehman F, Rafeeq H, et al. In-situ, Ex-situ, and nano-remediation strategies to treat polluted soil, water, and air – A review. Chemosphere. 2022;289:133252.
Crossref - Azubuike CC, Chikere CB, Okpokwasili GC. Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J Microbiol Biotechnol. 2016;32(11):180.
Crossref - Alori ET, Gabasawa AI, Elenwo CE, Agbeyegbe OO. Bioremediation techniques as affected by limiting factors in soil environment. Front Soil Sci. 2022;2.
Crossref - Senthil Kumar P, Gunasundari E. Bioremediation of Heavy Metals. In: Varjani SJ, Agarwal AK, Gnansounou E, Gurunathan B, eds. Bioremediation: Applications for Environmental Protection and Management. Energy, Environment, and Sustainability, Springer. 2018:165-195.
Crossref - Kuppusamy S, Palanisami T, Megharaj M, Venkateswarlu K, Naidu R. Ex-Situ Remediation Technologies for Environmental Pollutants: A Critical Perspective. In: de Voogt P, ed. Reviews of Environmental Contamination and Toxicology. Rev Environ Contam Toxicol, Springer. 2016;236:117-192.
Crossref - Cai X, Liu X, Jiang J, et al. Molecular Mechanisms, Characterization Methods, and Utilities of Nanoparticle Biotransformation in Nanosafety Assessments. Small. 2020;16(36):1907663.
Crossref - Murillo-Tovar MA, Saldarriaga-Norena H, Saeid A. Trace Metals in the Environment: New Approaches and Recent Advances. BoD – Books on Demand. 2021.
- Fomina M, Gadd GM. Biosorption: current perspectives on concept, definition and application. Bioresour Technol. 2014;160:3-14.
Crossref - Bachate SP, Khapare RM, Kodam KM. Oxidation of arsenite by two b-proteobacteria isolated from soil. Appl Microbiol Biotechnol. 2012;93(5):2135-2145.
Crossref - Pokhrel D, Viraraghavan T. Arsenic removal from an aqueous solution by a modified fungal biomass. Water Res. 2006;40(3):549-552.
Crossref - Wengel M, Kothe E, Schmidt CM, Heide K, Gleixner G. Degradation of organic matter from black shales and charcoal by the wood-rotting fungus Schizophyllum commune and release of DOC and heavy metals in the aqueous phase. Sci Total Environ. 2006;367(1):383-393.
Crossref - Chen Z, Huang Z, Cheng Y, et al. Cr(VI) uptake mechanism of Bacillus cereus. Chemosphere. 2012;87(3):211-216.
Crossref - Velasquez L, Dussan J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus. J Hazard Mater. 2009;167(1):713-716.
Crossref - Lourenco J, Mendo S, Pereira R. Rehabilitation of Radioactively Contaminated Soil: Use of Bioremediation/Phytoremediation Techniques. In: Gupta DK, Voronina A, eds. Remediation Measures for Radioactively Contaminated Areas. Springer. 2019:163-200.
Crossref - Bansal M, Santhiya D, Sharma JG. Mechanistic understanding on the uptake of micro-nano plastics by plants and its phytoremediation. Environ Sci Pollut Res. 2024;31(6):8354-8368.
Crossref - Rahbar A, Farjadfard S, Leili M, Kafaei R, Haghshenas V, Ramavandi B. Experimental data of biomaterial derived from Malva sylvestris and charcoal tablet powder for Hg2+ removal from aqueous solutions. Data Brief. 2016;8:132-135.
Crossref - Rezania S, Taib SM, Md Din MF, Dahalan FA, Kamyab H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater. 2016;318:587-599.
Crossref - Sarwar N, Imran M, Shaheen MR, et al. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere. 2017;171:710-721.
Crossref - Kumar V, Shahi SK, Singh S. Bioremediation: An Eco-sustainable Approach for Restoration of Contaminated Sites. In: Singh J, Sharma D, Kumar G, Sharma NR, eds. Microbial Bioprospecting for Sustainable Development, Springer. 2018:115-136.
Crossref - Roberts AG, Oparka KJ. Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 2003;26(1):103-124.
Crossref - Raliya R, Franke C, Chavalmane S, Nair R, Reed N, Biswas P. Quantitative Understanding of Nanoparticle Uptake in Watermelon Plants. Front Plant Sci. 2016;7:1288.
https://doi.org/10.3389/fpls.2016.01288. Accessed October 18, 2022. - Mahar A, Wang P, Ali A, et al. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol Environ Saf. 2016;126:111-121.
Crossref - Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, Mukhlisin M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int J Chem Eng. 2011;2011:e939161.
Crossref - Bian F, Zhong Z, Zhang X, Yang C, Gai X. Bamboo – An untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere. 2020;246:125750.
Crossref - Lasat MM. The Use of Plants for the Removal of Toxic Metals from Contaminated Soils. U S Environmental Protection Agency. 2000.
- Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals-Concepts and applications. Chemosphere. 2013;91(7):869-881.
Crossref - Dietz AC, Schnoor JL. Advances in phytoremediation. Environ Health Perspect. 2001;109(suppl 1):163-168.
Crossref - Chirakkara RA, Cameselle C, Reddy KR. Assessing the applicability of phytoremediation of soils with mixed organic and heavy metal contaminants. Rev Environ Sci Biotechnol. 2016;15(2):299-326.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.