ISSN: 0973-7510
E-ISSN: 2581-690X
This study investigated the storage temperature effects on Polygonati Rhizoma probiotics (PRP) activity by establishing storage conditions at different temperatures and regularly detecting the viable bacteria counts and value of pH. To further analyze the metabolic changes, the metabonomics analysis was carried out by using UHPLC-QE-MS/MS. Multivariate analysis used principal component analysis and orthogonal partial least squares discriminant analysis. When the storage temperatures were 25 °C and 37 °C, 229 and 307 different metabolites were identified respectively. The levels of most compounds such as glutamine, asparagine, and citrate decreased. This change affected both the flavor and nutritional benefits of PRP. In addition, the count of viable bacteria at 20 °C and 4 °C is very high, and there is no significant difference. Therefore, considering the product quality and energy saving, we recommend 4 °C as the storage temperature. This research provides important information on the storage stability and shelf-life setting of PRP products.
Polygonati Rhizoma, Traditional Chinese Medicine, Probiotic, Stability, Shelf Life, Metabolomics, Storage Method
Polygonati Rhizoma is a medicinal and edible homolog of Polygonatum in the Liliaceae family, which has a pleasant, sweet aroma and flavor. The rhizomes of Polygonati Rhizoma are rich in polysaccharides, proteins, alkaloids, lignans, amino acids, and other components,1 which have important medicinal and nutritional uses. Studies have extensively elucidated the potential of Polygonati Rhizoma in areas such as anti-fatigue, anti-aging, metabolic regulation, neuroprotective, and anti-cancer, among others.1,2 In the food development field, Polygonati Rhizoma is applied as an ingredient or additive in a range of products, such as alcohol, beverages, pastries, confectionery, and noodles.3
Probiotics are live microorganisms whose consumption in sufficient quantities is beneficial to the health of the host,4 aid in balancing the intestinal flora, improving the body’s immunity, anti-allergic, etc.5 Probiotic-related foods and dietary supplements are popular among a wide range of consumers. To ensure that probiotics can maintain their activity and utility to the maximum extent and exert their health benefits, it is very crucial to choose the correct storage method. The number of viable bacteria in probiotic products is susceptible to decrease during the shelf life due to factors including environmental temperature, humidity, dosage form, production environment, and packaging.6-8 Among these conditions, probiotic survival is extremely sensitive to temperature.
Although probiotic product stability has been studied by many researchers, there is no data about the storage stability of probiotic products with Chinese herbal medicine extracts of Polygonati Rhizoma. The Polygonati Rhizoma probiotic (PRP) used in this study is a powder preparation comprising Polygonati Rhizoma extract and various probiotics. In this study, we evaluated the viable bacteria counts and pH of PRP at different temperatures and storage durations. We further analyzed the metabolomic characteristics of PRP at each storage temperature, using an untargeted metabolomics approach to decipher the metabolic profiles, screened metabolites whose levels differed significantly at different temperatures, and analyzed the potential effects of the changes of these metabolites from the perspective of flavor and nutrition. This study offers insights into the storage stability and predicting the shelf life of PRP.
Experimental groups
Eighty aluminum foil bags of the PRP product (Polygonati Rhizoma compound probiotic freeze-dried powder from the Lvzhiyun Bioengineering Group Co., Ltd., the main components include Polygonati Rhizoma powder, Bifidobacterium longum subspecies BL21, Bifidobacterium brevis BBr60, Lactobacillus plantarum Lp90, Lactobacillus rhamnosus LRa05, Bifidobacterium animalis subspecies BLa36, Lactobacillus casei LC89, Pediococcus lactis CCFM7902, Bifidobacterium adolescentis BAC30, etc.), each containing 2.0 g per bag, were placed in a constant temperature incubator (Ningbo Yanghui Instrument Co., Ltd.) at 4 °C, 25 °C, and 37 °C, with 75% humidity, and in a refrigerator at -20 °C, and stored for 90 days, set as PRP-20, PRP4, PRP25, PRP37 groups.
Determination of viable bacterial count and pH
On days 0, 30, 60, and 90 of storage, six bags of samples kept at different storage temperatures were randomly selected and viable bacterial count and pH were determined. Bacterial culture and counting were conducted according to Sang et al.9 The pH value of PRP was measured with the pH meter manufactured by Shanghai INESA Scientific Instrument Co., Ltd.
UHPLC-MS/MS analysis
Metabolomics analysis was conducted utilizing a UHPLC-MS/MS apparatus (Shimadzu Nexera X2 LC-30AD, Japan) in conjunction with Q-Exactive Plus (Thermo Scientific, USA). Separation of the samples on ACQUITY UPLC® HSS T3 column (2.1 × 100 mm, 1.8 µm, Waters Corp., USA). The mobile phases consisted of: 0.1% formic acid in water (A) and 100% acetonitrile (B), and the flow rate was 0.3 mL/min. Gradient elution proceeded in a stepwise manner, with the solvent B percentage increasing linearly from 0% for 2 min to reach 48%, then to 100% for a further 2 min. Thereafter, the percentage was held at 100% for 2 additional minutes, before being decreased linearly to 0% solvent B within 0.1 min, the recalibration time was 3 min. The ESI setting parameters and mass spectrometry acquisition conditions were set by Wang et al.10 For every six samples collected in the collection process, a blank (consisting of a 75% aqueous acetonitrile-CN solution) and a QC sample were injected.
Data preprocessing and filtering
Using MS-DIAL software, the original MS data was subjected to peak alignment, peak area extraction, and retention time correction. The mass accuracy and MS/MS data were determined by comparing with the Human Metabolome Database, Mass Bank, and other public databases, and the standard library of metabolites built by Shanghai Bioprofle Biotechnology Co., Ltd. Only variables with more than half of the non-zero measurements in at least one group were retained among the extracted ion features.
Multivariate statistical analysis
All multivariate data were analyzed and modeled using Python software. Pareto scaling was used to center the data. The models were established according to PCA and OPLS-DA. Mathematically, the variable importance (VIP) score for each variable was calculated as a weighted sum of the squared partial least square weights. The VIP values >1 were generally regarded as significant. The discriminatory metabolites were identified by statistically significant VIP value thresholds through univariate analysis of normalized raw data. Adopting one-way ANOVA of multi-group analysis to calculate the p-value. Metabolites with OPLS-DA VIP value >1 and P-value < 0.05 were regarded as statistically significant.
KEGG enrichment analysis
The altered biological pathways were identified through KEGG pathway analysis of the differential metabolite data. Using Fisher’s exact test to perform KEGG enrichment analysis, and false discovery rate correction of multiple tests was carried out. The pathway enriched in KEGG is statistically significant when P < 0.05.
Viable bacterial count and pH
Viable bacterial count is an important indicator for the evaluation of the function and quality of probiotic products. The counts of viable bacteria at four tested temperatures showed a decreasing trend during the storage time (Figure 1). After 90 days of storage, the active bacteria counts in the four groups, including PRP-20, PRP4, PRP25, and PRP37, were 1.67 × 109, 1.94 × 109, 5.67 × 107, and 3.33 × 106 CFU/mL, respectively. These results indicate that the freezing or refrigerated conditions can maintain the viability of PRP better. In a previous study, Bifidobacterium A04 powder exhibited good stability when stored at 4 °C.10 During storage, the pH of the PRP-20 and PRP4 groups were similar, with the same trend of change, and both decreased after storage for 90 days. However, the pH values of the PRP25 and PRP37 groups did not change significantly after 90 days of storage.
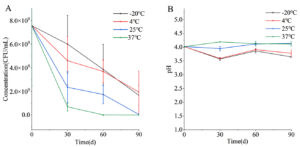 Figure 1. The cell concentration (A) and pH (B) of Polygonati Rhizoma probiotic at different storage temperatures.
Figure 1. The cell concentration (A) and pH (B) of Polygonati Rhizoma probiotic at different storage temperatures.
Principal component analysis (PCA)
PCA analysis is effective in showing the dispersion within groups and the differences between groups.11 As shown in Supplementary Figure S1, PCA of the metabolomics data at different temperatures yielded two PCs, explaining more than 51.48% of the total variance (36.53 and 14.95% through PC1 and PC2, respectively). The metabolite profiling data for PRP-20 and PRP4 groups overlapped, suggesting no obvious difference between the PRP at -20 °C and 4 °C. Interestingly, the PRP37 group was clearly distinct and differed significantly from the PRP4 and PRP-20 groups. PRP25 group overlapped partially with the other three groups. In the scoring chart, nearly all samples are included in 95% of Hotelling’s T2 ellipse, with QC samples closely gathered (Supplementary Figure S2), suggesting that the metabonomics method employed in this experiment has good reproducibility and stability.12
Orthogonal partial least-squares discriminant analysis (OPLS-DA)
The metabolic changes were further examined through OPLS-DA analysis. It is an improved supervised identification method that employs different prediction and orthogonal components to account for the differences between groups and within groups, which is used for optimal classifications and building discriminant models.13 To maximize within-group separation, metabolomics data from the four methods were compared pairwise employing OPLS-DA. The OPLS-DA model quality was validated and evaluated through the permutation test. The OPLS-DA score plots comprising samples from differential treatments are shown in Figure 2, clearly showing the separation between groups. Except for the PRP-20 and PRP4 groups, almost all OPLS-DA models exhibited high R2 and Q2 (>0.5) values, indicating that the models were stable and had good prediction ability. These data further verified that there were different components in the PRP at varying temperatures after 90 days of storage.
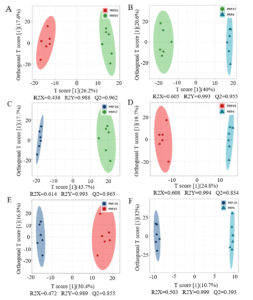 Figure 2. Profiling data of Polygonati Rhizoma probiotic at different storage temperatures. PRP-20, PRP4, PRP25 and PRP37 correspond to the storage temperature of -20 °C, 4 °C, 25 °C, and 37 °C, respectively, for Polygonati Rhizoma probiotic.
Figure 2. Profiling data of Polygonati Rhizoma probiotic at different storage temperatures. PRP-20, PRP4, PRP25 and PRP37 correspond to the storage temperature of -20 °C, 4 °C, 25 °C, and 37 °C, respectively, for Polygonati Rhizoma probiotic.
Differential metabolite selection
A total of 2209 metabolites were identified by UHPLC (Supplementary Table S1). The volcano plots of different treated samples can be seen in Figure 3, a lot of metabolite differences were noted between the two groups. From the PRP37 group, 244, 377, and 406 differential metabolites were identified compared to those in the PRP25, PRP4, and PRP-20 groups, respectively. Compared to the PRP4 and PRP-20 groups, respectively, the RPP25 group had 277 and 343 significantly differential metabolites identified and visualized. Furthermore, comparisons of PRP4 vs. PRP-20 revealed 76 significantly differential metabolites. These data confirmed that the metabolites varied in PRP stored at different temperatures.
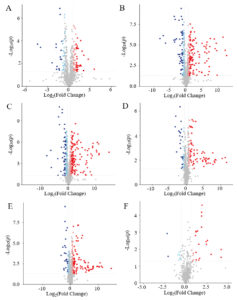 Figure 3. The volcano plots of the contrast group PRP37 vs. PRP25 (A); PRP37 vs. PRP4 (B); PRP37 vs. PRP-20 (C); PRP25 vs. PRP4 (D); PRP25 vs. PRP-20 (E); PRP4 vs. PRP-20 (F). FC > 1.5 or <0.67 with a P-value < 0.05 was used as a screening standard. PRP-20, PRP4, PRP25, and PRP37 correspond to -20 °C, 4 °C, 25 °C and 37 °C storage temperatures of Polygonati Rhizoma probiotic.
Figure 3. The volcano plots of the contrast group PRP37 vs. PRP25 (A); PRP37 vs. PRP4 (B); PRP37 vs. PRP-20 (C); PRP25 vs. PRP4 (D); PRP25 vs. PRP-20 (E); PRP4 vs. PRP-20 (F). FC > 1.5 or <0.67 with a P-value < 0.05 was used as a screening standard. PRP-20, PRP4, PRP25, and PRP37 correspond to -20 °C, 4 °C, 25 °C and 37 °C storage temperatures of Polygonati Rhizoma probiotic.
Classification and KEGG enrichment
The difference between the PRP-20 and PRP4 groups was not significant, therefore, we chose 4 °C among -20 °C and 4 °C storage temperatures, considering the cost and convenience in the actual storage process. Then the PRP4 group paired comparisons with the PRP25 and PRP37 groups.
We observed 274 significantly differential metabolites in PRP37 vs. PRP25, 377 in PRP37 vs. PRP4, and 277 in PRP25 vs. PRP4. Upon matching with the corresponding database, 216 metabolites were identified in PRP37 vs. PRP25, 307 in PRP37 vs. PRP4, and 229 in PRP25 vs. PRP4. Next, KEGG analysis was employed to categorize the metabolites with significantly different levels between the two groups. In PRP25 vs. PRP4, there were 43 carboxylic acids and derivatives (15.87%), 29 fatty acyls (10.7%), 26 organooxygen compounds (9.59%), and other differential metabolites. In PRP37 vs. PRP4, there were 65 carboxylic acids and derivatives (17.71%), 40 fatty acyls (10.9%), 35 organooxygen compounds (9.54%) and other differential metabolites (Figure 4). The KEGG analysis, thus, showed a higher content of carboxylic acid and its derivatives in the higher storage temperature groups, which may be because heat accelerates the metabolism of PRP. One study on the metabolism of probiotics in milk showed that fermentation at 37 °C and 25 °C produced higher levels of acetic acid than at 20 °C and 30 °C.14
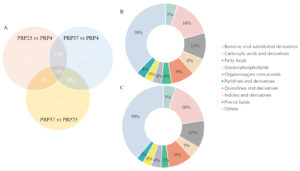 Figure 4. Different metabolites in comparison groups PRP37 vs. PRP4, PRP25 vs. PRP4, and PRP25 vs. PRP37 (A); classification of significant differential metabolites at storage temperatures of 25 °C and 4 °C (B) 37 °C and 4 °C (C). PRP4, PRP25, and PRP37 respectively correspond to the storage temperature of Polygonati Rhizoma probiotic at 4 °C, 25 °C and 37 °C.
Figure 4. Different metabolites in comparison groups PRP37 vs. PRP4, PRP25 vs. PRP4, and PRP25 vs. PRP37 (A); classification of significant differential metabolites at storage temperatures of 25 °C and 4 °C (B) 37 °C and 4 °C (C). PRP4, PRP25, and PRP37 respectively correspond to the storage temperature of Polygonati Rhizoma probiotic at 4 °C, 25 °C and 37 °C.
Further KEGG analysis was carried out to find metabolic pathways and explain the metabolic mechanism changes induced at different temperatures. As shown in Figure 5, the differential metabolites in both the PRP4 vs. PRP25 and PRP37 groups could be categorized into five top classes. In the PRP25 vs. PRP4 group, metabolism primarily includes alanine, aspartate, and glutamate metabolism and pyrimidine metabolism pathways; genetic information processing, including aminoacyl-tRNA biosynthesis pathway; environmental information processing, including ABC transporters and two-component system pathways; human disease processing involving pathways such as cancer-related central carbon metabolism and Parkinson disease; and protein digestion and absorption and mineral absorption pathways were involved in organismal systems. In the PRP37 vs. PRP4 groups, metabolism involves amino acid biosynthesis and alanine, aspartate, and glutamate metabolism pathways; genetic information processing, involving aminoacyl-tRNA biosynthesis pathways; environmental information processing, involving ABC transporters and cAMP signaling pathways; human disease processing, involving pathways such as cancer-related central carbon metabolism and Parkinson disease; and organismal systems includes pathways involves protein digestion and absorption and mineral absorption. Contrasted with the PRP4 group, the significantly differential metabolic pathways in the PRP25 and PRP37 groups were predominantly enriched in cancer-related central carbon metabolism and ABC transporters.
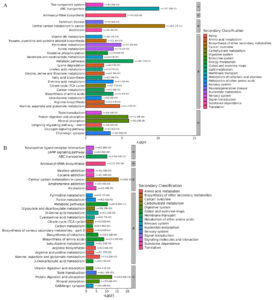 Figure 5. KEGG enrichment pathways of different metabolites in the two comparison groups of PRP25 vs. PRP4 (A) and PRP37 vs. PRP4 (B). PRP4, PRP25, and PRP37 respectively correspond to Polygonati Rhizoma probiotic stored at 4 °C, 25 °C and 37 °C.
Figure 5. KEGG enrichment pathways of different metabolites in the two comparison groups of PRP25 vs. PRP4 (A) and PRP37 vs. PRP4 (B). PRP4, PRP25, and PRP37 respectively correspond to Polygonati Rhizoma probiotic stored at 4 °C, 25 °C and 37 °C.
Determination of major metabolites
Among the PRP4 vs. PRP25 and PRP37 groups, 14 different metabolites are involved in cancer-related central carbon metabolism. At higher storage temperatures, most compounds enriched in ABC transporters, cancer-related central carbon metabolism, and amino acid biosynthesis pathways were notably altered, as shown in Figure 6.
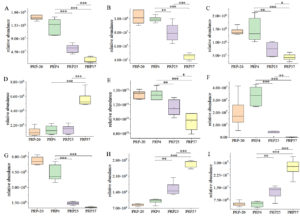 Figure 6. Box plot presenting pathways involved in the biosynthesis of ABC transporter, cancer-related central carbon metabolism, and amino acids biosynthesis pathways in Polygonati Rhizoma probiotic stored at different temperatures. (A) Glutamine, (B) Asparagine, (C) Methionine, (D) Tyrosine, (E) Citrate, (F) NAD+, (G) Deoxycarnitine, (H) Adenosine, and (I) Riboflavin. PRP-20, PRP4, PRP25, and PRP37 respectively correspond to Polygonati Rhizoma probiotic stored at -20 °C, 4 °C, 25 °C and 37 °C. *P < 0.05, ** P < 0.01 and *** P < 0.001
Figure 6. Box plot presenting pathways involved in the biosynthesis of ABC transporter, cancer-related central carbon metabolism, and amino acids biosynthesis pathways in Polygonati Rhizoma probiotic stored at different temperatures. (A) Glutamine, (B) Asparagine, (C) Methionine, (D) Tyrosine, (E) Citrate, (F) NAD+, (G) Deoxycarnitine, (H) Adenosine, and (I) Riboflavin. PRP-20, PRP4, PRP25, and PRP37 respectively correspond to Polygonati Rhizoma probiotic stored at -20 °C, 4 °C, 25 °C and 37 °C. *P < 0.05, ** P < 0.01 and *** P < 0.001
At the end of the storage period, the relative contents of several amino acids changed significantly (P < 0.05) in PRP25 and PRP37 contrasted with those in PRP4. For example, the levels of glutamine, asparagine, and methionine decreased, while that of tyrosine increased, but the level of tyrosine increased significantly only in the PRP37 group. Amino acids are important nutrients and impart flavor. Glutamine, the most abundant amino acid found in blood,15 can be used to generate energy and produce biosynthetic intermediates by driving the tricarboxylic acid cycle.16 In terms of flavor, glutamine confers a fresh taste,17 asparagine has a fresh and sweet taste,18 tyrosine is considered bitter,19 and along with the reduction of citrate content, these amino acids may have reduced PRP palatability and increased its undesirable flavor. The content of nicotinamide adenine dinucleotide (NAD+) and deoxycarnitine was also significantly reduced (P < 0.05). NAD+ is a cozymase substantial for taking part in cell redox reactions,20 enhancing energy metabolism, cardioprotection, DNA repair, and imparting anti-aging properties.21-24 Deoxycarnitine is used as a dietary supplement and a direct precursor of carnitine biosynthesis.25 The synthesized L-carnitine possesses many physiological functions, such as oxidative fat catabolism, weight loss, and anti-fatigue.26,27 The adenosine and riboflavin levels increased significantly (P < 0.05). Adenosine can provide a substrate for the lower step of glycolysis by releasing ribose.28 Besides, adenosine is an important signaling molecule, however, an abnormal increase in adenosine levels raises the risk of death induced by epilepsy.29 Riboflavin, also known as vitamin B, is soluble in water and participates as a coenzyme in the metabolism of nutrients in the body.30
Through analyzing the above significantly different metabolites among the PRP stored at different temperatures, although higher temperatures led to the production of some of the compounds useful to human health, the levels of most beneficial compounds were reduced significantly and the flavor of the product declined. Thus, compared to 25 °C and 37 °C, storage at 4 °C may be more conducive to stabilizing the beneficial components in the PRP and maintaining the flavor of the product, in exerting healthful functions.
Central carbon metabolism and amino acid biosynthesis pathway are the main enrichment pathways of PRP differential metabolites at different storage temperatures. The visualization and relative abundance of metabolic pathways are shown in Supplementary Figure S3.
In conclusion, after 90 days of storage, PRP at lower storage temperatures could still maintain good viability. Higher storage temperatures lead to a notable decrease in the activity of PRP and significantly downgrade the levels of many beneficial metabolites, including glutamine, asparagine, methionine, citrate, NAD+, deoxycarnitine, etc, significantly affecting the nutritive value and taste of PRP. The findings for storage at -20 °C and 4 °C were similar; considering product quality, energy consumption, and convenience, we recommend 4 °C as the storage temperature option.
Additional file: Additional Table S1 and Figure S1-S3.
ACKNOWLEDGMENTS
The authors would like to thank Shanghai Bioprofile Technology Co., Ltd., Shanghai, China, for their technical help.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This research was funded by the Science and Technology Innovation Program of Hunan Province (2022RC1224, 2022ZYC010), the Changsha Science and Technology Program (kh2004018), and 2023 National Traditional Chinese Medicine Specialty Technology Inheritance Talent Training Project (T20234832005).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript and/or in the supplementary files.
ETHICS STATEMENT
Not applicable.
- Wang S, He F, Wu H, et al. Health-Promoting Activities and Associated Mechanisms of Polygonati Rhizoma Polysaccharides. Molecules. 2023,28(3):1350.
Crossref - Lin H, Wang W, Peng M, et al. Pharmacological properties of Polygonatum and its active ingredients for the prevention and treatment of cardiovascular diseases. Chin Med. 2024;19(1):1.
Crossref - Li M, Xie B, Li L, et al. A comprehensive review on the potential applications of medicine Polygonatum species in the food sector. Food Biosci, 2024;60:104116.
Crossref - Bianchi L, Laghi L, Correani V, et al. A Combined Proteomics, Metabolomics and In Vivo Analysis Approach for the Characterization of Probiotics in Large-Scale Production. Biomolecules. 2020;10(1):157.
Crossref - Kullar R, Goldstein EJC, Johnson S, McFarland LV. Lactobacillus Bacteremia and Probiotics: A Review. Microorganisms. 2023;11(4):896.
Crossref - Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506-514.
Crossref - Chand P, Kumar MD, Kumar S, et al. Influence of processingand packaging conditions on probiotic survivability rate, physico-chemical andsensory characteristics of low calorie synbiotic milk beverage. J Dairy Res. 2022;89(1):94-99.
Crossref - Ermis E. A review of drying methods for improving the quality of probiotic powders and characterization. Dry Technol. 2022;40(11):2199-2216.
Crossref - Sang Y, Feng H, Meng L, et al. Effects of nitrogen-filled packagingand storage temperature on storage stability of probiotics powder. Food and Fermentation Industries. 2020;46(19):143-147.
Crossref - Wang S, Sang Y, Hou C, Wang P, Ren F, Wang R. Study on the storage activity of freeze-dried Bifidobacterium lactis A04 powder. Chinese Journal of Food Science. 2021;21(09):192-202.
Crossref - Zhang Y, Ji X, Ku T, Li B, Li G, Sang N. Ambient fine particulate matter exposure induces cardiac functional injury and metabolite alterations in middle-aged female mice. Environ Pollut (Barking, Essex: 1987). 2019;248:121-132.
Crossref - Qu Q, Zhang Z, Li Y, et al. Comparative molecular and metabolic responses of wheat seedlings (Triticum aestivum L.) to the imazethapyr enantiomers S-IM and R-IM. Science Total Environ. 2019;692:723-731.
Crossref - Arendse E, Fawole OA, Magwaza LS, Nieuwoudt H, Opara UL. Evaluation of biochemical markers associated with the development of husk scald and the use of diffuse reflectance NIR spectroscopy to predict husk scald in pomegranate fruit. Sci Hortic. 2018;232:240-249.
Crossref - Hilde MO. Treimo J, Judith AN. Effect of temperature on growth and metabolism of probiotic bacteria in milk. Int Dairy J. 2005;15(10):989-997.
Crossref - Mayers JR, Heiden MGV. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem Sci. 2015;40(3):130-140.
Crossref - Sayin VI, LeBoeuf SE, Singh SX, et al. Activation of the NRF2 antioxidant program generates an imbalance in central carbon metabolism in cancer. eLife. 2017;6:e28083.
Crossref - Ackroff K, Sclafani A. Flavor Preferences Conditioned by Dietary Glutamate. Adv Nutr (Bethesda, Md), 2016;7(4):845s-852s.
Crossref - Liu Z, Wei S, Xiao N, et al. Insight into the correlation of key taste substances and key volatile substances from shrimp heads at different temperatures. Food Chem. 2024;450:139150.
Crossref - Hakimi S, Kari NM, Ismail N, Ahmad F. Evaluation of taste active peptides and amino acids from anchovy proteins in fish sauce by in silico approach. Food Sci Biotechnol. 2022;31(7):767-785.
Crossref - Alegre GFS, Pastore GM. NAD+ Precursors Nicotinamide Mononucleotide (NMN) and Nicotinamide Riboside (NR): Potential Dietary Contribution to Health. Current Nutr Rep. 2023;12(3):445-464.
Crossref - Abdellatif M, Sedej S, Kroemer G. NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation. 2021;144(22):1795-1817.
Crossref - Huang Q, Sun M, Li M, et al. Combination of NAD+ and NADPH Offers Greater Neuroprotection in Ischemic Stroke Models by Relieving Metabolic Stress. Mol Neurobiol. 2018;55(7):6063-6075.
Crossref - Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell metabolism. 2019;30(4):630-655.
Crossref - Yamaguchi S, Franczyk MP, Chondronikola M, et al. Adipose tissue NAD+ biosynthesis is required for regulating adaptive thermogenesis and whole-body energy homeostasis in mice. Proc Natl Acad Sci USA. 2019;116(47):23822-23828.
Crossref - Koeth RA, Levison BS, Culley MK, et al. g-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20(5):799-812.
Crossref - Fielding R, Riede L, Lugo JP, Bellamine A. l-Carnitine Supplementation in Recovery after Exercise. Nutrients. 2018;10(3):349.
Crossref - Savic D, Hodson L, Neubauer S, Pavlides M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2020;12(8):2178.
Crossref - Strefeler A, Blanco-Fernandez J, Jourdain AA. Nucleosides are overlooked fuels in central carbon metabolism. Trends in endocrinology and metabolism: TEM. 2024;35(4):290-299.
Crossref - Purnell B, Bhasin J, Rust B, et al. Disruption of adenosine metabolism increases the risk of seizure-induced death despite decreased seizure severity. Epilepsia. 2024;65(9):2798-2811.
Crossref - Qin Y, Zhou J, Xiong X, et al. Effect of riboflavin on intestinal development and intestinal epithelial cell function of weaned piglets. J Anim Physiol Anim Nutr, 2023;107(2):518-528.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


