Ghana, a country in the African continent experienced its first ever outbreak of Marburg Virus disease on July 2022. Prior to this, Democratic Republic of Congo and Angola were the two most severely affected countries to be affected by the same disease. Marburg Virus disease is a lethal and serious disease with an average mortality of 50% and has been seen to go as high as 90% in some cases. But despite the propensity to cause fast epidemics, the Ghana government acted swiftly and controlled its spread saving many lives. Simultaneously the country was also facing the Monkeypox outbreak and was doing its best to control the outbreak. This brief article is about the containment measures taken by the country to effectively prevent both the diseases to spread.
Marburg Virus, Monkeypox Virus, Containment, Ghana
Africa accounts for the largest burden of infectious and communicable diseases globally. The increased connectivity via travel and transport increases risk of spread of emerging or re-emerging infections across countries in other parts of the world. The recent outbreaks of the two Marburg virus disease (MARVD) and Monkeypox (MPV) in different parts of Africa were posing continuous threat to the public health.
MARVD, also called as Marburg hemorrhagic fever, is a lethal and often serious infection in human beings and primates. The Marburg virus (MARV) which belongs to the Filoviridae family and genus Marburgvirus is responsible for the hemorrhagic fever.1 Ebola virus also is in the same filoviridae family and is responsible for causing Ebola fever. Even though these two are different viruses, the diseases caused by them are more or less similar. The name of the virus originated in the town of Marburg (Germany) in August 1967, where the earliest ever outbreak of the disease was detected.2,3 The infection might have been spread from Uganda through infected African green monkey tissue. The virus is pleomorphic in morphology, observed in circulars, U, rod-like shapes, and commonly in filamentous forms.4 The virus’s natural reservoir is animals, particularly bats. A fruit bat (Rousettus aegyptiacus) is the commonest reservoir of the virus; Sundevall’s roundleaf bat (Hipposideros caffer) and some Chiroptera as minor sources.5
MARV is transmitted to humans via persistent contact with bat licked fruits, bat saliva and faeces in mines and caves colonized by bat colonies. It can also be transmitted human to human via direct contact (broken skin or mucosa) with infected patient’s blood, secretions or various bodily fluids, also via materials (linen, clothes, etc.) contaminated with blood, fluids and secretions.6,7 In addition, humans can get infected by non-human primates by direct contact or through the consumption of bushmeat.8 There were reports about the virus being detected in semen analysis of prior infected patients raising concerns about it being transmitted through sexual route.
This virus causes severe human hemorrhagic fever (Marburg Haemorrhagic Fever, MHF). The disease progresses through three distinctive phases: the generalization phase, the early organ phase, and the late organ/convalescence phase.9 Incubation period of the disease ranges from 2 days to 2 weeks with the usual duration being five to nine days in humans.8,10 In most of the cases, the disease is characterized by high fever and severe headache with malaise. Some cases also see watery diarrhea by the time the first week ends. When the first week ends, the second week of the illness is marked with separate features with hemmorhagic manifestations like spontaneous bleeding from venipuncture sites, hematemesis, and hematochezia.11 Mortality rates depend on the strain’s virulence, host susceptibility, physical status, and medical management. The average MVD mortality rate is around 50%, with significant variations up to 88% in some cases.
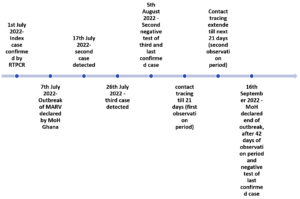
Most of the outbreaks of the disease have happened in Africa, with the Democratic Republic of Congo (DCR) (128 deaths in the year 2000) and Angola (227 deaths in the year 2004) being the most severely affected countries.12,13 The most recent outbreak was seen in August 2021 in Guinea, where one male was infected and died.14 A year later, on July 17th 2022, Ghana declared its first-ever outbreak of MARVD when three cases were discovered in the southern Ashanti region, known for its gold and cocoa production. The index case was a young male in 20s, reported on the 4th of July whereas the second was a 14-month-old child. The third and last case seen was a 24-year-old female reported on the 21st of July (Figure). All three cases were related to each other and were from the same household. They had alike symptoms like diarrhoea, nausea, and vomiting. Unfortunately, the first two cases died within one-two days of admission to the same hospital. The third one was the only survivor of the three confirmed cases and has been discharged and has reunited with the family. The government acted swiftly and employed all the resources in order to stop the spread of the outbreak.
A national coordination mechanism and response activities were initiated by WHO and key partners i.e., Centre for Disease Control, UNICEF and United Kingdom. Technical experts from WHO guided in consolidating Infection Prevention and Control (IPC), harmonization among all tiers of health care, surveillance, risk assessment and testing capacity. An integrated disease surveillance and response (IDSR) system was established to track and test cases at rapid rate. Contact tracing was done, and a total of 198 contacts, including HCWs and other members were recognized and continuously monitored for symptoms for a period of 21 days, which was further extended by 21 more days by the health authorities.15 The WHO has also contacted and alerted neighbouring high-risk countries, such as Guinea, Ivory Coast, and Nigeria. Ivory Coast shares a 719-kilometre border with Ghana that has not been under surveillance, putting it at high risk of importing the disease. Given Ghana’s proximity to Nigeria and the massive daily movement between the two countries, the Nigeria Centre for Disease Control and Prevention (NCDC) has issued a red alert for the country’s borders. The NCDC conducted a risk assessment and concluded that Nigeria is at moderate risk of contracting the disease. There no information on how the two patients contracted the infection. The most concerning fact are that with no history of MARVD in the community, determining the exact transmission route becomes a significant concern. Although exposure to bats from caves is a risk factor, it has yet to be reported in this country.
On 16 September 2022, 42 days after second negative test of the last confirmed case on 5 August 2022, Ghana declared the end of the outbreak in accordance with WHO recommendations. The successful effort by Ministry of health, Ghana in curbing the outbreak is praiseworthy.15 Despite the propensity of MARV to cause large outbreaks with high mortality, this current outbreak was contained efficaciously because of well organised structure of track, test and treat in place. The collaborative efforts by all the international partners played a key role in achieving his milestone, which served as a lesson for the all the African countries. This achievement illustrates that a country’s continued efforts in preparedness for outbreak threats and inclination to act earliest can principally change trajectory of outbreak or epidemic and save many lives.15,16
On the contrary, in Uganda death toll from the Sudan variant Ebola virus (SUDV) outbreak has reached ginormous number of 48, whereas 131 cases have been confirmed by testing. SUDV is on rampant spread and still outbreak end cannot be predicted despite using all IPC measures like contact-tracing, testing, risk communication, and appropriate treatment and burials, reinforcement of points of entry and assessment and reinforcement of case management capacities. WHO assessed the risk of this event as high at the country level and low at global levels.16
Amidst the current outbreaks, in developing countries, genomic surveillance, which came to the forefront during the COVID-19 pandemic, is still unavailable. There is a significant risk of cases being imported into neighbouring countries, which must be avoided at all costs. At all entry points, vigilance and surveillance should be increased. All symptomatic individuals travelling from affected countries must provide a detailed travel history, and appropriate infection prevention and control measures must be implemented. As in previous outbreaks, investments in outbreak preparedness and early detection are urgently needed, including developing rapid diagnostics. It is not always possible to send samples to a WHO-accredited reference laboratory. More research in therapeutics and vaccines is required to control the spread of disease.
In addition to the outbreak of MARV, Ghana has been affected by the recent multi-country spread of the monkeypox virus (MPV). MPV is a DNA virus from the family Poxviridae, subfamily Chordopoxvirinae, and genus Orthopoxvirus. It was named so as it was first discovered in monkeys in a Danish laboratory in 1958.17 However, the authentic MPV reservoir remains unknown.18 The first case of MPV was identified in the month of August in the year 1970 in the village of Bukenda in Zaire’s Equatorial region, in a 9-year-old child with smallpox-like vesicular skin lesions (now DRC).19 This case was discovered during a period of increased smallpox surveillance conducted nine months after the WHO certified the eradication of smallpox in the DRC.20 Then it spread to Central and West Africa and outside Africa, with imported animals from Ghana reaching the United States in 2003.21,22 MPV has two genetic clades: West African and Central African (Congo Basin).23
A systematic review found that the number of confirmed MPV cases was highest in the Congo, with 38 confirmed cases during the year 1970-1979, 520 during the year 1990-1999, and 85 in the year 2010-2019, respectively. MPV was found in 47 cases outside of Africa, primarily in the United States, between 2000 and 2009.22 MPV began resurfacing in 2022, affecting many regions worldwide, with no known connection between cases. As of Nov 16, 2022, MPV was confirmed in 79682 cases across 79 non-endemic countries, with the highest burden in Spain, the USA, Germany, the United Kingdom, the Netherlands, and France, respectively.24 Same day, WHO declared monkeypox a Public Health Emergency of International Concern (PHEIC). On June 8, 2022, Ghana confirmed five cases with MPV. However, 12 suspected cases have been investigated since May 24, 2022.25 As of Nov 16, 2022 total 107 cases have been confirmed to be infected with MPV with 4 deaths within Ghana.24 MPV has similar presentation, severity, and mortality but is less likely to be transmitted from human to human.20 The incubation period has been estimated to be ranging from 5 to 21 days, with symptoms lasting 2 weeks to one month. The illness starts with nonspecific flu-like symptoms such as fever with chills, headaches, lethargy, asthenia, lymph node swelling, and generalised myalgia before rashes appear.26,27 Rashes of varying sizes appear 1 to 5 days after the onset of fever, on the face, hands, legs, and feet. The rash progresses through different phases like macules, papules, vesicles, pustules, crusting and falling of scabs during recovery. Different stages of the rash can be observed at the same time. Erythema and hyperpigmentation are regularly observed around discrete lesions. There may also be inflammation of the pharyngeal, conjunctival, and genital mucosa.27,28
Given the current burden on Ghana’s healthcare system, in the light of current MPX and MARV outbreaks, available resources should be directed towards the containment of both diseases through implementing proper measures. That can be done through the establishment of a task force whose sole goal is to ensure timely and appropriate implementation of preventive measures, increasing awareness of the public regarding the current situation and the recommended steps in seeking health care. Finally, efforts should be directed towards proper surveillance, travel restrictions, case identification, isolation, management, and prevention of disease spread. Support from the WHO and international organization might required to contained the virus. One way to asses how well a country responds to an infectious disease outbreak is by measuring a country’s response through timelines; a start-to-end assesement of the spread of the speed of case detection, notification to public health authorities and response to suspected health threats.29,30
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Kuhn JH, Durrwald R, Bao Y, et al. Taxonomic reorganization of the family Bornaviridae. Arch Virol. 2015;160(2):621-632.
Crossref - Feldmann H, Slenczka W, Klenk H-D. Emerging and reemerging of filoviruses. Arch Virol Suppl. 1996;11:77-100.
Crossref - Gear JS, Cassel GA, Gear AJ, et al. Outbreak of Marburg virus disease in Johannesburg. Br Med J. 1975;4(5995):489-493.
Crossref - Bharat TA, Riches JD, Kolesnikova L, et al. Cryo-electron tomography of Marburg virus particles and their morphogenesis within infected cells. PLoS Biol. 2011;9(11):e1001196.
Crossref - Towner JS, Pourrut X, Albarino CG, et al. Marburg virus infection detected in a common African bat. PloS One. 2007;2(8):e764.
Crossref - Amman BR, Schuh AJ, Albarino CG, Towner JS. Marburg virus persistence on fruit as a plausible route of bat to primate filovirus transmission. Viruses. 2021;13(12):2394.
Crossref - Schuh AJ, Amman BR, Jones MEB, et al. Modelling filovirus maintenance in nature by experimental transmission of Marburg virus between Egyptian rousette bats. Nat Commun. 2017;8:14446.
Crossref - Kortepeter MG, Dierberg K, Shenoy ES, et al. Marburg virus disease: A summary for clinicians. Int J Infect Dis. 2020;99:233-242.
Crossref - Stille W, Bohle E. Clinical course and prognosis of Marburg virus (“Green-Monkey”) disease, in Marburg virus disease. Springer. 1971:10-18.
Crossref - Slenczka W. The Marburg virus outbreak of 1967 and subsequent episodes. Curr Top Microbiol Immunol. 1999(235):49-75.
Crossref - Granoff A, Webster RG. Encyclopedia of virology. Elsevier. 1999.
- Colebunders R, Tshomba A, Van Kerkhove MD, et al. Marburg hemorrhagic fever in Durba and Watsa, Democratic Republic of the Congo: clinical documentation, features of illness, and treatment. J Infect Dis. 2007;196(Supplement_2):S148-S153.
Crossref - Towner JS, Khristova ML, Sealy TK, et al. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J Virol. 2006;80(13):6497-6516.
Crossref - World Health Organization. Marburg virus disease – Guinea. https://www.who.int/emergencies/disease-outbreak-news/item/2021-DON331. Accessed on July 24, 2022.
- Marburg Virus Disease – Ghana (who.int). Available from: www.who.int/emergencies/disease-outbreak-news/item/2022-DON409. Accessed on November 8, 2022
- Marburg virus disease – Ghana (who.int) Available from : www.who.int/emergencies/disease-outbreak-news/item/2022-DON402. Accessed on Novevember 08, 2022.
- Von Krempelhuber A, Vollmar J, Pokorny R, et al. A randomized, double-blind, dose-finding phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE®. Vaccine. 2010;28(5):1209-1216.
Crossref - Reynolds MG, Guagliardo SAJ, Nakazawa YJ, Doty JB, Mauldin MR. Understanding orthopoxvirus host range and evolution: from the enigmatic to the usual suspects. Curr Opin Virol. 2018;28:108-115.
Crossref - Marennikova S, Seluhina EM, Mal’ceva NN, Cimiskjan KL, Macevic GR. isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. 1972;46(5):599. PMCID: PMC2480798
- Breman JG, Ruti K, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970-79. Bulletin of the World Health Organization. 1980;58(2):165.
- Centers for Disease Control and Prevention. Monkeypox: Vaccine Guidance. https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html. Accessed on July 23, 2022.
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141.
Crossref - Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(10):2661-2672.
Crossref - Global Health. Monkeypox cases worldwide: Key statistics. www.global.health. Accessed July 23, 2022.
- Ghana confirm five cases of monkeypox. https://www.bbc.com/pidgin/articles/cl5dygq2z3qo. Accessed on July 24, 2022.
- World Health Organization. Monkeypox: Key facts. https://www.who.int/news-room/fact-sheets/detail/monkeypox. Accessed July 23, 2022.
- Di Giulio DB, PB Eckburg. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15-25.
Crossref - Jezek Z, Szczeniowski M, Paluku KM, Mutombo M, Grab B. Human monkeypox: confusion with chickenpox. Acta Tropica. 1988;45(4):297-307. PMID: 2907258
- Ebola Disease caused by Sudan virus – Uganda (who.int) www.who.int/emergencies/disease-outbreak-news/item/2022-DON410. Accessed on November 08, 2022.
- Epidemic Preparedness – Prevent Epidemics. https;//preventepidemics.org/preparedness/. Accessed on November 10, 2022
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.