Heavy metals (HMs) are widespread and vital to life, but their accumulation in organisms can be hazardous. Lead (Pb) has lately attracted considerable attention due to its devastating impacts on the environment and human health, such as its ability to produce neurodevelopmental disorders in children even at low exposure levels. Cardiovascular, renal, digestive, hematological, and reproductive consequences exist. The current review sheds light on the familiar sources of the HMs, their ecological hazards, the most common types with particular reference to Pb, its natural and artificial sources, physical and chemical characteristics, environmental and human health hazards, and control strategies using different approaches as remediation through (physical, chemical and biological strategies), microorganism-assisted bacteria with particular reference to the advantages and limitation of each approach. Through this review, we introduce a solution to eliminating the problem of Pb toxicity & accumulation in the food chain through endophytes bacteria, as it has high efficiency in treating lead toxicity. The presentation will show the mechanism of these microbes in treating lead toxicity.
Lead, Toxicity, Organisms, Mechanisms, Bioremediation
The environment’s suitability for human life, including the air, water, and soil, is a devastating global problem. Among them, soils are important parts of the domain. In addition to producing food and fibre, soil supports the global environment. Any changes to the soil can potentially affect the pedosphere, atmosphere, hydrosphere, & biosphere.1 Lately, soil pollution with heavy metals (HMs) has been a critical worldwide ecological trouble due to the anthropogenic impacts of the absence of adequate handling. This issue has detrimental repercussions on the ecosystem and adverse implications on the species’ health, economy, and community.
Normally, Essential and non-essential HMs are two categories of HMs.2 Biomes require essential elements in minimal amounts for necessary biochemical and physiological actions.3 Molybdenum, cobalt, selenium, copper, zinc, magnesium, manganese, nickel, and chromium are among their elements. An inadequate supply and an abundance of these micronutrients reveal many deficiency illnesses.4 Biomes do not require HMs that are toxic or non-essential for physiologic and metabolic activities.3
They consist of arsenic, beryllium, gallium, bismuth, germanium, cadmium, barium, gold, indium, antinomy, lead, lithium, aluminum, silver, mercury, uranium, strontium, platinum, tellurium, titanium, thallium, vanadium, and tin.5
Pb is a severe problem in addition to the HMs polluted in the soils for several reasons:
- Humans have extensively used Pb for a long time, becoming the greatest pollutant on earth.
- Pb is poisonous to every biota, even at small levels.6
- Pb does not naturally degrade biologically or over an extended period (150–5000 years) in soils.5
Pb compounds remain essential to modern human existence.7 It’s important to take the soil’s lead pollution seriously. These encouraged us to look for a viable Pb remediation technique. Once Pb has contaminated the soil, it is hard to remove.1,7 Although physiochemical techniques can clean up soils, almost are expensive, laborious, and difficult to operate. As a result, valuable soil components are degraded, the properties, structure, and fertility of the soils are altered, local soil microorganisms’ biological processes are disturbed, and secondary pollution hazards are created.8
Attention has been drawn to using plants connected with microorganisms as an environmentally beneficial method.9 Since bacterial metabolites greatly affect ambient metal speciation and transport and are biodegradable and less harmful.10 Plant-promoting bacteria (PGPB) are microbes that can assist plants in removing heavy metals from contaminated environments.11 Plant-promoting rhizospheric bacteria (PGPR) and plant growth-promoting endophytic bacteria are two subgroups of PGPB (PGPE). While PGPE is found inside plant cells, PGPR surrounds the roots of herbs.5 PGPR has been widely researched in phytoremediation studies for a long time. PGPE has drawn more and more attention.12 Since PGPE has more benefits than PGPR, including eliminating competitive issues,13 methods for enhancing phytoremediation are mostly comparable. They can, for instance, withstand extremely high HM concentrations. In addition to making siderophore and inorganic phosphate, it solubilizes and mobilizes HMs.14
Therefore, PGPE might be preferable for this study’s estimation of Pb phytoremediation. The selection of the host plant affects endophyte metabolic outputs & the isolation of endophytic bacteria.15 The roots of metallophytes with the maximum Pb level are believed to contain the beneficial Pb-resistant endophytic bacteria.16 In general, metallophytes that accumulate a significant amount of HMs can provide endophytes with a particular niche (HMs-stressed ecology) where they can exist strategies to withstand the toxicity of HMs.17 Pb metallophytes are typically detected in Pb-polluted environments.
Song Tho was picked as one of these Pb mines because little was recognized about the endophytic microbes produced by the nearby Pb-storing herbs. As indicated, increasing the Pb phytoremediation capability is difficult by inoculating the effective PGPE into fast-growing trees as its recent host. PGPE must colonize plant tissues successfully. The ability of Pb phytoremediation in trees connected with PGPE must be assessed using hydroponic and pot studies. A hydroponic examination is typically a quick and affordable operation.1
Since it lowers the phase of plant development and period of the subject, it also lowers the area required for studies and differences because of the ecological effect.18 However, their absorption ability varies from those in soil because of photo availability. These influences are not found in the hydroponic exam. Thus, the hopeful studies correlated with effective PGPE require being cultivated in a polluted Pb environment to have the proper knowledge on Pb absorption impacted by soil chemical and microbial reactions.
Lead (Pb)
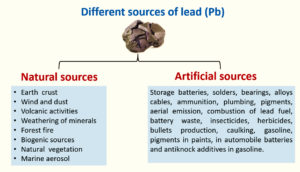
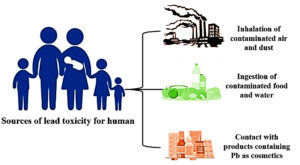
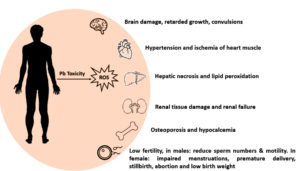
Pb is a non-essential HMs that are tremendously toxic and has adverse effects on most human and animal organ systems, leading to multisystem illness (Table 1). It is seen as a potential environmental threat on a global scale. Within the food chain, it experiences biomagnification. Humans exposed to lead in the environment or at work may experience long-term health impacts such as musculoskeletal, neurological, cardiovascular, hepatic, renal, pulmonary, and reproductive dysfunctions. The International Agency for Research on Cancer (IARC) recognized lead as a human carcinogen. Although lead toxicity and its reduction have been extensively studied and published, full control and avoidance of lead exposure still seem to be a long way off. The various sources of Pb are seen in Figures 1, 2, and 3.
Table (1):
Hazard effect of lead toxicity among different living creatures.
| Species | Permissible doses | Most affected tissues (Higher Pb accumulation) | Hazard effects | References |
|---|---|---|---|---|
| Human | 0.36 to 1.24, up to 2.43 µg/kg body weight per day in European high consumers.
|
Renal, reproductive, and nervous systems. | Acute exposure led to behavioral changes. | 87 |
| Chronic renal failure, carcinogenic effect. | 88-89 | |||
| Affect fertility, sperm motility, and count. | 88, 90 | |||
| Induce miscarriages, prematurity, reduced birth weight, and growth troubles during childhood. | 88, 91 | |||
| Lead interferes with the growth of neurochemicals, involving neurotransmitters, and organization of ion channels, and lower neuronal development. | 92 | |||
| Prenatal and early childhood Pb exposure was related to violent crimes in adulthood. | 91 | |||
| Fish | The maximum permissible lead limit in fish and shellfish should not exceed 0.30 mg/kg. | Gills > intestinal > liver > muscle. | Induce oxidative stress and alter immunity in Channa argus. | 92-93 |
| Gills>muscle>heart>kidney. | Induce oxidative stress and alter the immune response in Hydrocynus forskahlii. | 92, 94 | ||
| Kidney>liver>gills>intestine>muscle. | Induce oxidative stress and alter the immune response in Pelteobagrus fulvidraco. | 92, 95-96 | ||
| Gills>liver>kidney>muscle. | Induce oxidative stress and alter the immune response in Coregonus lavaretus. | 92, 97 | ||
| Plant | Pb-contaminated soils contains about 400-800 mg Kg-1 soil, while in industrial area mar reach 1000 Pb Kg-1 soil (Angelone and Bini, 2017). | Root, shoot, and different plant parts. | Stunted development, chlorosis, blackening of the root system, inhibits photosynthesis, upsets mineral nutrition and water balance, variations hormonal condition, and affects membrane composition and permeability. | 7 |
| Cow and buffalo | 6 mg/kg body weight cause chronic toxicity and intakes more than 10 mg/kg BW may cause acute Pb poisoning. | Blood, brain. | Central nervous system damage alter blood parameters. | 98-99 |
| Dairy | Blood, milk. | Lead is excreted in the milk of Pb-exposed cow and alters its blood parameters. | 99-100 | |
| Sheep | lambs only show signs if intake more then 4.5 mg/kg BW. | Low detection in tissue. | In the plumbiferous area of Derbyshire, sheep consume a large amount of Pb annually, which is more in the winter than in the summer. Pb is relatively non-toxic due to its ruminant stomach nature. | 99, 101 |
| Pigs | Pigs, goats, and rabbits are more resistant than sheep or cows, and only mild symptoms of poisoning takes place at intakes of 60 mg/kg BW. | Blood. | Alter blood parameters. | 99, 102 |
| Goat | Milk. | Pb is present in higher levels than the maximum permissible limits in goat milk. | 99, 103 | |
| Broiler chickens | Birds can withstand feed intakes of 100 mg/kg diet without signs but at a level of 500 mg/kg made serious poisoning. | Kidneys>livers> ovarian > egg yolk > muscle | Alter feed conversion rate, reduce average body weights at the end of 6th week old. | 99, 104 |
| Layer chickens | As blood Pb level increase, its levels in the yolk of eggs parallels increase. | 99, 105 | ||
| Wild birds and waterfowl | Blood | Body weight loss changes in blood parameters and delay in development in ducks. | 99, 106 | |
| Muscles of guns hunted wild birds. | Contamination of bird tissues and surrounding environment with Pb in unacceptable limits in most cases. | 99, 107 |
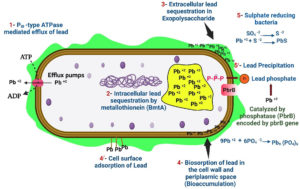
Figure 5. Lead resistant mechanisms operational in bacteria, (1) PIB-type ATPase mediated efflux of lead, (2) Lead sequestration by metallothionein (BmtA), (3) Lead sequestration in exopolysaccharide, (4) Cell surface adsorption of lead, (4/) Biosorption of lead in the cell wall and periplasmic space (bioaccumulation), (5) Lead precipitation by sulfate-reducing bacteria, (5/) Lead precipitation catalyzed by Phosphatase enzyme (PbrB).
Occurrence in nature
Pb is generally found in the soil with a luxury of 14.8 mg/kg.19 Pb levels in surface soils range from 10 to 67 mg/kg globally, with the median value being 32 mg/kg .20 Pb content ranges from 10 to 25 mg/kg in igneous rocks and 14 to 40 mg/kg in argillaceous sediments. It rarely occurs in 0.1-8.0 and 3.0-10 mg/kg rates in calcareous sediments and ultramafic rocks.21 Pb could be detected in sedimentary rocks such as sandstones and shales (22 mg/kg) (10 mg/kg).17 Galena is the Pb mineral that is most common (PbS). Anglesite, mimetite, minium, pyromorphite, and cerussite are some of their minerals.21
Pb contamination and behavior in soils
HMs can be found in five different states: fraction 1 is a soluble fraction, consisting of HMs in the soil solution as free ions & complexes; fraction 2 is exchangeable, consisting of HMs uptake on inorganic soil components & ion-exchange sites; fraction 3 is organic, consisting of HMs bound to organic matter; fraction 4 is insoluble, consisting primarily of HMs concentrated as oxides, carbonates.22 These can be further broken down into overall and reachable HM levels.
The total concentration is unavailable to plants for rapid uptake.19 The concentration in fractions 1 or 2 is available for plant absorption. However, different soil amendments can liberate fractions 3 and 4, but metals in fraction five may not be available.22 Pb can generally be found in soils in different forms: Pb can exist as a free metal ion and form complexes with inorganic substances, including HCO3–, CO32–, SO42–, and Cl–. (4) Pb may be adsorbed onto particle surfaces like Fe-oxides, organic matter, biological material, & clay particles. (3) Pb may be present as organic ligands like amino acids, humic acids, & fulvic acids.17 Pb is considered to have the lowest bioavailability.17
Pb harmfulness to microorganisms
Pb is lethal to bacterial cells, even at small levels.5 For instance, at three mg/L of Pb (II) chloride, no discernible Desulfovibrio desulfuricans G20 growth was found (PbCl2).5 Pb enters microbial cells through the absorption pathways for necessary divalent metals like Mn2+ and Zn2+. Pb poisoning results through changes in protein and nucleic acid structure, inactivation of enzyme action, disturbance of membrane functions, oxidative phosphorylation, and modifications in the osmotic balance, among other factors. Additionally, Pb2+ exhibits a higher affinity for thiol & oxygen groups than other necessary metals like Ca and Zn.5
Pb also interferes with bacterial survival & multiplication by destroying DNA, proteins, and lipids and replacing vital metal ions like Zn, Ca, and Fe in enzymes.23 Thermus thermophilus strain Samu-SA1 was recently affected by Pb2+ at 200 and 300 mg/L. Pb2+ decreases protein content and -glucosidase and -maltosidase enzyme activity at 100 mg/L. Additionally, Pb influences the secretion of various proteins from T. thermophilus that range in molecular weight from 15 to 236 kDa.24
Remediation strategies
HMs must be remedied to protect the ecosystem from their harmful effects and preserve its wonderful condition for future human generations.25 There are typically three methods, which are separated into physical, chemical, and biological mechanisms:
Physical Approach
This strategy involves excavation and subsequent disposal at a landfill site or on-site management.26 Thermal desorption techniques and soil substitution comprise on-site management. To lower pollution, pure soil is substituted for contaminated soil. The second method is soil spading. In a nutshell, dilution and normal degradation are achieved by deeply excavating the contaminated soil, which causes the toxins to disseminate into deep places.
The new soil is supplied and then covered at the surface using soil capping. This method is effective for soil with small regions and severe pollution but is also quite expensive and labor-intensive. The only HMs for which the thermal desorption method is best are volatile HMs. In a nutshell, volatile pollutants are produced by heating contaminated soil with steam, microwaves, or infrared radiation. As a result, they are collected using carrier gas or negative vacuum pressure. The ease of usage and reuse of the remedied soil are two benefits of this approach. However, the price of this approach is high, and the desorption period is lengthy.27 In the case of soil removal and landfill placement, this moves the pollution problem elsewhere and creates additional risks due to the transit & migration of the pollutants to surrounding biological components.26
Chemical method
This strategy includes knowledge vitrification, electro-kinetic remediation, chemical leaching, and chemical fixing. By rinsing the contaminated soil with freshwater, chemicals, or gas, chemical leaching can remove the contaminant from the soil by ion exchange, concentration, adsorption, & chelation. As a result, HMs in soil were moved from the liquid stage to the leachate, where they were collected.17
This method is expensive, results in metal-rich byproducts that need future processing, & typically renders the land unfit for the development of plants because it eliminates any biological effects.26 To create insoluble with HMs, chemical fixing adds the reagents to the polluted soil. This procedure achieves soil remediation while reducing the transport of HMs to water, plants, and other ecological media. However, this method can clean up the soil with low pollution levels. Additionally, the bioavailability of fixed HMs could change due to ecological change.
The impact of the soil’s microbes and the makeup of the soil could be changed by the use of chemical agents to some extent. Electrokinetic remediation is a novel method. It primarily applies voltage to the two sides of the soil, creating an electric field gradient. By electromigration, electroosmotic flow, or electrophoresis, the contaminant is transported to the two-pole treatment room, where it will subsequently be handled.27 Additionally, using chemicals increases expenses, the possibility of secondary contamination, and the volume of sludge produced.17
Biological method
This strategy is more appropriate because it is a typical ecological process with no negative environmental effects.26 It includes phytoremediation and bioremediation, utilizing microbiomes like bacteria, algae, yeast, fungi, and plants).28 Various techniques are available for bioremediation, including composting, land formation, venting, bioreactors, biofilters, bio-stimulation, and bioaugmentation.25 Microbes can affect migration & transformation even though they cannot break down and destroy HMs because they change their physical and chemical properties.
Precipitation, extracellular complexation, oxidation-reduction reaction, and intracellular precipitation are all steps in the repair process.27 Because they maintain the typical soil characteristics, are inexpensive, and enjoy widespread acceptability, the techniques used in this methodology are advantageous over chemical & physical processes.25 Earthworms, a lower animal, exhibit the qualities of adsorbing, decomposing, and migrating the HMs, eliminating and inactivating HMs toxicity at this time.27 Using plants to clean up a contaminated environment is known as phytoremediation.29 In this instance, naturally occurring and genetically modified plants are possible.30 Additionally, phytoremediation refers to using herbs & related soil bacteria to lower or eliminate the toxic impacts of toxins in the ecosystem’s soil, water, & air.17
Microbial remediation of lead
Engineering repair, physical and chemical restoration, or a mix of these procedures are common conventional heavy metal pollutant remediation techniques; however, no one technique guarantees total heavy metal degradation. Many of these techniques are economically and environmentally unsustainable.31 Bioremediation uses living organisms, mainly plants and bacteria, to clean up contaminated soils and water.32 These bacteria create and use various detoxifying methods, including biomineralization, biotransformation, biosorption, and bioaccumulation. It is a widely used remediation technique since it happens spontaneously and is reasonably priced.33
Using bacteria or enzymes, bioremediation involves changing hazardous heavy metals and other chemicals into less damaging forms.20, 34-35 This method for revitalizing the environment is economical and kind to the environment.36-37 The nature of the cell wall constituents and functional groups of microorganisms involved in the cleanup of heavy metals is the primary cell component.37
Bacteria have developed defenses to withstand metal ion uptake in stressful settings. These mechanisms include the accumulation of metal ions in a less toxic state, biosorption to cell walls and entrapment in the extracellular capsule, precipitation of the efflux of metal ions outside the cell, and chemisorption of metal ions inside the cell.36-37 It has been discovered that numerous bacteria, fungi, and algae can bind lead in different quantities. The function of bacteria in lead removal and recovery by biosorption is covered in detail in this review.
Bioremediation of lead by bacterial strains
The pb-tolerant bacterial strains from various sources are included in Table 2 and their mode of action for pb cleanup. Many bacterial strains have been identified and studied for their capacity to decrease lead in soil and liquid medium. The most effective way for bacterial strains to remove metals from an aqueous medium is by bioaccumulation, which depends on the metabolic activity of the bacterial cell.36 The numerous methods established by bacteria in response to metal toxicity have been depicted using bacterial strains in several studies for lead breakdown.35 Novel catabolic enzymes enable bacterial strains to thrive in ecological settings.37
Table (2):
Pb-tolerant bacteria, sources and their mechanisms.
Bacterial strains |
Sources |
Mechanism |
References |
|---|---|---|---|
Geobacillus thermodenitrificans |
It was isolated from the Damodar River, India. |
G. thermodenitrificans reduced 36.86% of Pb (II) from synthetic metal solutions and 18.22% of Pb (II) for industrial wastewater. |
108 |
Bacillus sp. |
It was isolated from metal-polluted soil. |
The maximum biosorption capacity of Bacillus sp. for Pb (II) ions was reported as 92.27 ± 1.17 mg/g at 250 mg/L initial concentration of Pb. |
109 |
Pseudomonas aeruginosa ASU6a |
The bacterial strain was isolated from El-Malah Canal, Assiut, Egypt. |
Biosorption capacities for lead using non-living and living cells were reported as 123 mg/g and 113.6 mg/g, respectively. |
110 |
Bacillus sp., Pseudomonas sp., and Micrococcus sp. |
It was isolated from samples of an electroplating industry in Tiruchchirappalli district, Tamil Nadu, India. |
The biosorption potential of immobilized bacterial cells were reported as 84.27%. |
40 |
Micrococcus luteus DE2008 |
Isolated from a consortium of microorganisms living in the microbial mats of the Ebro Delta, Catalonia (Spain) |
M. luteus DE2008 removed 1965 mg/g lead from an aqueous solution. |
111 |
Pseudomonas sp. CY63 |
It was isolated from hypersphere-contaminated soils. |
This strain showed a Pb accumulation capacity of 158.9%. |
87 |
Bacillus thuringiensis strain OSM29 |
Isolated from the Rhizosphere, cauliflower is grown in soil irrigated consistently with industrial effluents from Aligarh, North India. |
90.6% of lead removal was reported at the low initial concentration (25 mg/ L) of lead. |
91 |
Stenotrophomonas maltophilia (SS1), Aeromonas veronii (SS2) and Bacillus barbaricus (SS3) |
Isolated from Taptapani Hot Spring Odisha, India. |
At optimum adsorption conditions, the adsorption of lead was found to be 93%. |
112 |
Enterobacter cloacae B1 |
It was isolated from polluted soil in Ghaziabad, India. |
The minimum inhibitory concentration of the strain was reported as 1100 PPM, and bioaccumulation was extremely high at 95.25%. |
113 |
Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus plantrium and Streptococcus thermophiles |
It was isolated from commercial probiotics and sour milk products. |
Heavy metal ion binding by LAB was reported as a metabolism-independent surface process. |
38 |
Bacillus species AS2 |
Isolated from water and sediment samples collected from different sites of Tarik Darreh Pb Ore in North-eastern, Iran. |
Lead removal capacity was 74.5 mg/g (99.5% of initial Pb) at pH 4.5, temperature 30 C, inoculum size 1.0% (v/v), and an initial Pb concentration of 500 PPM after 24h. |
46 |
Arthrobacter sp. 25. |
– |
An adsorption capacity of 9.6 mg/g was achieved under optimum conditions. |
53 |
Alcaligenes sp. BAPb.1 |
It is isolated from the lead mining area. |
Under optimum conditions, there is a maximum biosorption rate of 85.2% and a maximum capacity of 56.8 mg/g. |
42 |
Pseudomonas sp. W6 |
Isolated from the extreme habitat of hot water springs in North–East India |
These strains showed the minimum inhibitory concentration of up to 1.0 mM of lead |
47 |
Leuconostoc mesenteroides |
Isolated from fermented Kimchi (Korean fermented food) and purchased from the local market, Iksan, South Korea. |
This strain’s heavy metal Pb removal potential was reported as 60.6 mg Pb/g. |
45 |
Bacillus subtilis X3 |
Isolated from lead-contaminated soil of a lead mine in Nanjing, Jiangsu Province, China. |
The adsorption capacity of adsorbent for lead increased with the increase in pH, eventually reaching the maximum value of 192.05 mg/g at pH 4.0 |
114 |
Lactobacillus acidophilus ATCC4356 |
Bacterial strains were purchased from the Iranian Research Organization for Science and Technology. |
At optimum condition: pH, metal, and bacterial concentration 6.76, 391 mg L-1, and 4.60 g/L, respectively, for 73.9% lead removal. |
115 |
Oceanobacillus profundus |
Isolated from an abandoned mine |
The maximum removal percentage for Pb (II) was 97% at an initial 50 mg/L concentration. |
48 |
Lactobacillus acidophilus ATCC 4356 |
Bacterial strains commonly used in the dairy industry were selected and purchased from Tak Gene Zist Company, Tehran (Iran). |
80% removal of Pb was observed, respectively, at 1 × 1012 CFU of L. acidophilus in milk on the 4th day and the initial ion concentration of 100 µg/L. |
41 |
Stenotrophomonas rhizophila, Variovorax boronicumulans and Stenotrophomonas rhizophila |
Isolated from Iranian Mine Calcareous Soils |
96.25% of lead was reduced by Stenotrophomonas rhizophila, 95.93% of lead by Variovorax boronicumulans and 98.71% of lead within 72h reduced by Stenotrophomonas rhizophila. |
51 |
According to Murali et al.,32 after five days, bacteria recovered from soil samples contaminated with tannery effluent reduced by 3 mg/L Pb. These bacteria included Bacillus subtilis, Bacillus megaterium, Aspergillus niger, and Penicillium sp. Heavy metals are absorbed by bacteria by a variety of mechanisms, including biosorption, encapsulation in extracellular capsules, complexation, oxidation-reduction processes, precipitation, and transport through cell membranes. According to Dai et al.,36 Lactobacillus brevis biosorbed Pb2+ (53.632 mg/g), and the ideal circumstances for Pb2+ ions adsorption were pH 6.0, 120 rpm/min, 3 g/L of bacterial concentration, 40°C, and a 12-hour contact period. The authors of this study also said that temperature had a significant impact on the biosorption of Pb2+ ions and that as the temperature rose, so did the biosorption capacity. The biosorption capacity reached a maximum of 42.35 mg/g at 40°C.
Similar to how pH dramatically altered biosorption capacity, biosorption was exceedingly poor when pH was raised to 3, and 3.6 mg/g of biosorption was attained at pH 2. The biosorption rose with pH and reached its maximum lead binding (44.4 mg/g) at pH 6. However, the biosorption amount fell as pH levels rose above 6 and peaked at 7.
Another Lactobacillus species was isolated and identified by Yi et al.,37 and after 30 minutes of incubation, this strain’s maximum adsorption capacity was determined to be 60.6 mg Pb/g. 52 of the 96 LAB strains isolated for this study from kimchi bought at a local market in Iksan, South Korea, showed Pb resistance. Pre-cultured lactic acid bacteria (LAB) were incubated in 160 g of Man Rogosa Sharp broth media (MRS) broth medium at 37°C for 18 hours to remove Pb using the chosen lactic acid bacteria (LAB).
SEM-EDS analysis extensively studied the surface morphology of strain L-96 before and after Pb biosorption. The findings showed that Pb was mostly associated with bacterial cell surfaces and that EPS secretion was responsible for alterations in surface shape. Elsanhoty et al.,38 subcultured various Lactobacillus species utilizing Man Rogosa Sharp broth media, including Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus plantrium, and Streptococcus thermophiles (MRS broth). This study assessed the impact of bacterial concentration and pH on the ability to remove lead. The data demonstrated that removal increased approximately linearly with pH, with pH 7 producing the maximum removal of Pb (71.1%). Pb cations’ interaction with protons could result from pH effects for negatively charged binding sites.
Increased bacterial population helped with Pb elimination, especially when L. plantarum was used alone or in conjunction with L. acidophilus. Maximum Pb elimination was seen at pH 7 and high bacterial concentration. According to George et al.,39 Bifidobacterium longum 46, Lactobacillus fermentum ME3, and Bifidobacterium lactis Bb12 all removed lead, with B. longum 46 having the highest capacity of 175.7 mg/g dry biomass. In this investigation, the effects of pH (2–6), bacterial content (0.5–1.5 g/l), and temperature (4, 22, and 37°C) were also examined. When the starting metal concentration was 1000 g/L, pH was 6, and the temperature was 37°C, 97 % of the lead was removed from the solutions.
In Tamil Nadu, India’s Tiruchchirappalli District, an effluent sample from the electroplating sector revealed that Micrococcus sp. had removed 84.27 percent of Pb. Bacterial isolates that perform best at various temperatures, pH levels, biomass levels, and Pb concentrations do so because these variables impact biosorption.40
Tak Gene Zist Company sold Lactobacillus acidophilus ATCC 4356. The bacteria were injected into 10 ml MRS broth and then cultivated for 48 hours at 37°C. The isotherm models developed by Langmuir and Freundlich were used to investigate the capacity of the bacterial cells to absorb Pb at a particular time. The efficiency of Pb bio elimination was optimized across the selected contact times (1-4 days) using L. acidophilus concentrations (between 1010 and 1013 CFU), which the authors additionally looked at about contact time.41
Jin et al.,42 recovered Alcaligenes sp., BApb.1 from a mining site in Heilongjiang Province, China. For the maximum biosorption rate of 85.2 % and the maximum capacity of 56.8 mg/g, the ideal pH range, adsorbent dosage, initial Pb2+ concentration, contact duration, and temperature were reported to be 5, 1.5 mg/g, 100 mg/L, and 30 min.
Ten lactic acid bacteria strains to see how well they could tolerate and absorb lead. With a rate of lead absorption as high as 99.9% and a minimum inhibitory concentration of lead on L. plantarum reported as being higher than 1000 mg/L, Lactobacillus plantarum YW11 was discovered to have the strongest ability of lead absorbing and tolerance.43 According to George et al.,44 firmicutes—mostly lactic acid bacteria like Lactobacillus spp., with some Lactococcus, Pediococcus, and Carnobacterium representatives—actinobacteria, and protozoa—were effective at removing the potentially dangerous trace element lead. Between 50 and 90 % of the 99 distinct LAB strains tested for their capacity to remove Pb2+ salts at 25 PPM were able to immobilize the metal in the solution. According to the authors, the majority of the strains of Gram-negative bacilli were found to have Pb2+ removal capabilities between 45 and 65 %. In contrast, the ability of the Enterobacterales strains to eliminate Pb was consistently and somewhat low (54.14 6.7 %). Only two E. coli strains and one Hafnia alvei strain could absorb more than 75% of the lead in a solution with a 25 PPM concentration.
Numerous other researchers have also reported that certain bacterial strains, including Leuconostoc, mesenteroides,45 and Bacillus sp, can degrade lead. AS2 46, Pseudomonas sp. W6.47 Pardo et al., 49 evaluated the biosorption potential of inactive freeze-dried Pseudomonas Putida biomass, and they found that 80 percent of the Pb was removed within 10 minutes of contact. The pH significantly impacts metal biosorption, and a range of 6.0 to 6.5 was the ideal pH. Another bacterial isolate, Serratia marcescens, could live at a lead concentration of 0.025 mg/L and reduce lead to a level between 0.0133 and 0.213 g/g in under 120 minutes. This strain’s pigment biosynthesis was also examined, and results showed that pigment production significantly decreased up to 80 minutes before ceasing.50
In a different study, Jalilvand et al.,51 evaluated the biosorption potential of ureolytic bacteria isolated from calcareous mine soils in Iran. They found that Stenotrophomonas rhizophila, Variovorax boronicumulans, and Stenotrophomonas rhizophila could remove lead from the environment in under 72 hours. According to the authors of this study, these only bacterial strains could biomineralize. Based on morphological, biochemical, and 16S rDNA sequencing analysis, Ren et al.,52 determined that Bacillus sp. PZ-1 is a Pb2+-resistant bacterium. The researchers found that the biosorption rate was 93.01 percent, the adsorption capacity was 9.30 mg/g, and the ideal values for the initial Pb (II) concentration, pH, contact time, biomass concentration, and temperature were 400 mg/L, 5.0, 20 min, 40 g/L, and 15°C, respectively.
In a related experiment, a team of scientists isolated the lead-tolerant bacteria Arthrobacter sp. 25. In this study, the theoretical anticipated value (9.6 mg/g) of lead absorption was obtained using the response surface methodology (RSM). The maximum adsorption capacity (9.6 mg/g) was achieved under ideal conditions with initial lead ion concentration, pH value, and biosorbent dosage as 108.79 mg/L, 5.75 g/L, and 9.9 g/L, respectively.
SEM, energy-dispersive X-ray spectroscopy, atomic force microscopy (AFM), X-ray diffraction, and Fourier transform infrared spectroscopy (FTIR) were used to establish the biosorption mechanisms.53 Bacillus gibsonii S-2 waste biomass was utilized as an inexpensive biosorbent to remove Pb2+ from an aqueous solution.54 This study examined the maximal capacity of a particular bacterial strain to remove lead under ideal pH and temperature circumstances. The results revealed that the biosorption was best at a pH of 4.0 at three different temperatures (20, 30, and 40°C). The scientists found that Bacillus gibsonii S-2 may lower 333.3 PPM of lead. The mechanisms of Pb2+ biosorption, ion exchange, and complexation with the functional groups involved in Pb2+ adsorption have been studied using Fourier-transform infrared spectroscopy (FTIR) and Energy-dispersive X-ray spectroscopy (EDX).
Geobacillus thermodenitrificans, isolated from the Damodar River in India, was tested for its biosorption capacity using two different kinds of aqueous solutions by Chatterjee et al.55 After being seeded in nutritious broth, the samples for this investigation were incubated at 65°C for 48 hours (Hi-media, Mumbai, India). Based on morphological traits, 30 colonies were divided and kept in slant cultures at 4°C. To establish the optimal pH for maximum Pb removal from an aqueous media, the pH range of 3.0-9.0 was examined. According to the data, pH was a factor in the metal adsorption. Dead biomass from G. thermodenitrificans is particularly sensitive to pH, and at pH 4.5, Pb2+ adsorption is at its highest level. The dead biomass of G. thermodenitrificans was reportedly reduced by 18.22 % and 36.86 %, respectively, of Pb2+ in industrial wastewater and synthetic metal solutions.
The biosorption capability of isolated industrial wastewater containing Staphylococcus saprophyticus was examined by Ilhan et al. in 2004. The research team also investigated the ideal biosorption conditions to attain maximal values. It looked into the biosorption process at various pH, temperature, and metal ion starting concentrations. A pH of 4.5 was found to be ideal, while a metal content of 100 mg/L was shown to be ideal for maximal biosorption. According to the authors, Staphylococcus saprophyticus may eliminate Pb2+ under these circumstances. Qiao et al. discovered a lead-resistant bacteria from the lead-contaminated soil of a lead mine in Nanjing, Jiangsu Province, China (2019). The biosorbent (Bacillus subtilis capacity)’s for lead adsorption grew as the pH rose, eventually reaching a maximum of 192.05 mg/g at pH 4.0. Bacillus subtilis X3’s adsorption rate rose with the number of contact hours and was measured at 260 mg/g after 10 minutes.
Endophytic bacteria-assisted phytoremediation
Definition & classification
The PGPE can support plant development.17 Therefore, endophytic bacteria could be identified as microorganisms that colonize interior plant cells without showing symptoms of infection or harming the plant.56 Additionally, they may be collected from inside herbs or retrieved from surface-disinfected plants.57 Because of the variety of hosts’ living environments and the intricate relationships between endophytes and their hosts, it is still unclear where endophytes came from. There are two theories, though. First, endophytes share the same genetic ancestry as the host because they originated from mitochondria & chloroplast in plant cells. Second, endophytes enter the host from outside the plant through the surface, a wound in the root, or stimulated channels.58
Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Firmicutes Actinobacteria, and Bacteroidetes were split into 82 genera to represent endophytic bacteria. Most fall under the third first group.59 They represent a wide spectrum of Gram-negative & Gram-positive microbes. The most cultivatable endophytic spp., found on various hosts, including woody trees, herbaceous plants, and grass spp., are Pseudomonaceae, Burkholderiaceae, and Enterobacteriaceae.60
Procedures for altering heavy metal phytotoxicity
Plants produce ethylene in response to heavy metal stress, but excess ethylene harms the herbs. Endophytic microbe could create 1- aminocyclopropane-1-carboxylic (ACC) deaminase, which can control ethylene concentrations, enhance plant development, & ultimately lower poisonousness.61 Additionally, endophytic microbe could lower the poisonousness of Cd by elevating the plant’s absorption of a trace element like Zn or Fe.
Additionally, endophytic microbes create siderophores that promote plant growth in iron-deficient environments, lower the toxicity of HMs in HM-contaminated environments, & promote plant development in iron-deficient environments.17 In other words, endophytic bacteria diminish phytotoxicity induced by HMs levels, resulting in a healthy plant that can withstand HMs and extend the contract period. These factors raise the possibility of phytoremediation (Figure 4).
Procedures for modifying HMs bioavailability
The presence of HMs in the non-soluble form in the soil, particularly Pb, makes it difficult for plants to absorb them. By creating and releasing the bacterial siderophore and organic acids, PGPE can convert the form of heavy metal to a bioavailable form. Bacterial siderophore functions as a solubilizing factor by creating a combination with other metals such as Al, Cu, Cd, Pb, Ga, In, & Zn. As a result, HMs are released and dissolved from soil particles, boosting their bioavailability and absorption.17 Significantly, siderophores are seen to regulate and lower HMs absorption.62 To elevate the solubility of HMs, PGPE can also generate & produce low-molecular-mass organic acids like 5-ketogluconic acid, formic acid, citric acid, & oxalic acid.63
Procedures of Pb resistance in bacteria
According to Lenart and Wolny-Koladka,64 heavy metals severely impair microbial activity, soil fertility, and plant growth. In fact, significant alterations in a genetic and physiological trait of the soil microbial population may be seen at high Pb concentrations.65 As a result, heavy metal-tolerant species persist while sensitive species with no beneficial effects are eliminated, leading to decreased functionality and species variety.66 The microbial population was greatly impacted by how the bacteria altered root secretions, food cycles, and carbon transport from autotrophic plants. Associated bacteria reduce root development, respiration, and carbon transfer to the root. This carbon flux is the primary and crucial regulator of the soil’s microbial community.67
The bacteria may withstand metal stress in various ways, including forming an extracellular barrier, actively transporting metal ions (efflux), sequestering metal ions inside and outside cells, and reducing metal ions.68 Different strains of bacteria belonging to the genus “Pseudomonas” are disinfectants resistant to heavy metals, antibiotics, and detergents. Pseudomonas could be environmental pollution indicators.69-70 Pseudomonas can withstand heavy metal stress in various ways, including P. aeruginosa-specific active extrusion of metal ions from cells and cellular mechanisms that reduce metal ions to less harmful forms.71 In general, the rehabilitation of Pb-polluted soils can benefit from appropriate bacterial inoculation.
By altering the synthesis of chlorophyll contents, enzymatic activities, and soil respiration, as well as lowering the Pb concentration in plants, bacterial inoculation may reduce Pb toxicity and its accumulation in dill plants. Compared to other strains and the control, strain P159 had the greatest influence on the parameters under study. This makes Pseudomonas strain P159 possibly ideal for plant development in Pb-affected soils and cleaner dill production, which can be further grown in contaminated soils without any potential dangers of metal toxicity, especially in low concentrations of Pb. Pb intrusion into other environmental compartments can be minimized, and Pb-contaminated soils can be covered.
Benefits and limitations of applying endophytic bacteria
Applying endophytic bacteria in phytoremediation has numerous advantages. The efficiency of the remediation techniques can be assessed by quantitative gene expression of the catabolic genes of contaminating bacteria. As harmful contaminants ingested by plants may be destroyed within the plant by endophytic degraders, genetic engineering of bacterial catabolic pathways is simpler to modify than that of plant catabolic pathways. This minimizes the toxicity of pollutants in ecological soil on flora & fauna.17 Additionally, there are a lot of drawbacks, including the possibility that the plant’s selection will limit its potency to certain seasons. Pollutant effects on phytotoxicity go together with it. If pollutants are not fully detoxified, and the local wildlife consumes the plants, pollutants or their metabolites may also enter the food chain.72
Investigation on bacteria-assisted phytoremediation
A wide spectrum of PGPE is now known to successfully support phytoremediation by promoting plant growth, lowering phytotoxicity, accumulating metals, improving plants’ ability to withstand metals, altering metal bioavailability in soil, and translocating metal in plants.12 It has been discovered that some endophytic bacteria promote plant development. Depending on the initial Cd content, S. nigrum, a Cd hyperaccumulator, PGPE extracted from it, boosted plant growth. Enterobacter sp on soil with a low Cd contamination level (12.1 mg/kg). LSE04 increased S. nigrum shoot length (13.7%), fresh weight (28.2%), & dry weight (41.4%).5 Briefed the lead bioremediation by bacteria (Figure 5).
Chen et al., 73 found that Acinetobacter sp. LSE06 was demonstrated as the most significant development influence at the intermediate Cd concentration (about 63.7 %), with increases of up to 10.9 % for shoot length, 15.7 % for fresh weight, & 23.1 % for dry weight. Serratia nematodiphila LRE07 was the most effective at high Cd content (116.5 %). The plant’s shoot length, fresh weight, and dry weight rose by 18.9%, 23.1%, and 19.8%, respectively, compared to uninjected plants. When plants were injected with the growth-stimulating endophytic bacterium Pseudomonas sp A3R3, the fresh & dry weights of B. juncea were significantly increased compared to plants that weren’t injected, by 50% and 45%, respectively. Additionally, Psychrobacter sp. SRS8 may promote the growth of crops used for energy.74
Ricinus communis has a percentage increase of 32% for fresh weight & 38% for dry weight, while Helianthus annuus has a percentage increase of 39% and 41%, respectively. Compared to the uninjected control, the endophytic Bacillus sp. MN3-4 boosted the root elongation of B. napus seedlings by 46.25 %.75 From a metal-tolerant plant (C. communis) cultivated on Pb/Zn mine tailing, Pb-resistant with ACC deaminase-producing endophytic bacteria (Acinetobacter sp. Q2BJ2) Bacillus sp. Q2BG1) were identified. Compared to the uninjected controls, they can enhance the dry weight of B. napus aboveground cells raised on quartz sand, including 100 mg/kg of Pb (39 to 71 %) and roots (35 to 123 %).76
In comparison to uninjected controls, which were 10, 27, 26, 38, and 52 % in soils containing Cd, 22 %, 17 %, 27 %, 31 %, and 34 % in soils containing Pb, and 24 %, 28 %, 19 %, 26 %, and 41 % in soil containing Zn, B. napus injected with the Rahnella sp. JN6 showed a marked elevation in chlorophyll content, plant height, and root length.77 By altering the solubility and availability of HMs, PGPE could effectively increase phytoremediation. In contrast to uninjected controls (381 g/L), Pb-resistant endophytic bacteria P. fluorescens G10 & Microbacterium sp. G16 can considerably improve the water solubility of Pb post 60 hours of injection.78
Different Zn resources, like zinc carbonate, zinc phosphate, or zinc oxide, were digested by Gluconacetobacter diazotrophicus, generating 5-ketogluconic acid and forming Zn available for plant absorption. By producing metal-mobilizing metabolites, the endophyte actinobacterium could mobilize zinc and copper.63 Cd, Pb, & Zn solubilization in the metal-amended soil was significantly (p 0.05) increased by Rahnella sp. JN6 was 1.46, 1.25, and 1.30 times greater than that in the uninjected soil.77 Metal precipitation is the major factor affecting how effective phytoremediation is. Some endophytic bacteria that are HMs resistant and promote plant growth can increase metal uptake and precipitation in plants.17 In contrast to uninjected plants, Psuedomonas sp. A3R3 considerably enhanced the Ni content in the shoot of A. serpyllifolium & B. napus by 10% & 15%, respectively, when cultivated in soil treated with 450 mg/kg of Ni.74 P. fluorescens G10 and Microbacterium sp. G16 injection into B. napus increased Pb absorption in a shoot from 76 to 131 % by P. fluorescens and 59 to 80 percent by Microbacterium sp., contrasted to the uninjected control group.78
Additionally, compared to the uninjected control, Acinetobacter sp. Q2BJ2 & Bacillus sp. Q2BG1 markedly (p 0.05) increased total Pb concentration in B. napus shoots (3.4-fold to 5.6-fold) and roots (2.1-fold to 3.5-fold).76 Rahnella sp. JN6, a strain isolated from an Mn hyperaccumulator (P. pubescens), considerably elevated the metal levels in B. napus cells. Metal (Cd, Pb, & Zn) levels were 49 %, 47 %, and 28 % in aboveground tissues of uninjected control plants, compared to 106 %, 97 %, and 62 % in injected plants.
The concentrations of those metals in the root tissues of the uninjected control plants were 58 %, 46 %, and 33 %. In comparison, they were 140 %, 95 %, and 89 %, respectively, in the injected plants.77 Similar to this, compared to uninjected plants, Bacillus pumilus E2S2 dramatically increased plant Cd absorption, root, & shoot length, and fresh & dry biomass. Several endophytic bacteria, like Herbaspiillum seropedicae, & Bulkholderia capacia may increase nickel precipitation in the yellow lupin roots, increasing the plant’s capacity for phytoremediation.61 Lately, Pseudomonas sp. LK9 dramatically boosted metal absorption when introduced into Cd hyperaccumulator (S. nigrum) growing in multi-HMs polluted soil.79 Similar to this, the multi-metal resistant endophytic bacterium Bacillus sp. L14 recovered from S. nigrum improved the absorption of Cd2+, Pb2+, and Cu2+, respectively, 76%, 80%, and 21%.80
On the contrary, metal-resistant endophytic microbes reduced HM absorption by plants, increasing plant biomass.81-82 discovered that the rice tissues-derived endophytic bacteria Methylobacterium oryzae and Burkholderia sp. reduced the levels of Ni & Cd in the roots & shoots of Lycopersicon esculentum Mill. The translocation factor was computed to evaluate the effectiveness of PGPB injection on the translocation of HMs from roots to shoots.
Ma et al.,75 PGPB Sanguibacter sp., reduced the TF of Zn in Nicotiana tabacum L., a plant cultivated in soil enriched in Cd & Zn. Ma et al.,12 found that Cu-resistant endophytic bacteria increased the amount of Cu that B. napus transported from its roots to its shoots, increasing its capacity for phytoextraction. Ma et al.,75 observed that when the amount of multi-metal polluted serpentine soil increased, the injection of PGPB strain (Pseudomonas sp. A3R3) marginally elevated the TF of Ni in B. juncea & R. communis. This shows that the PGPB injection significantly impacts Ni precipitation in plant shoots. The TF of Zn in both herb sp. was thereby drastically reduced by Pseudomonas sp. A3R3. Phytotoxicity can prevent phytoremediation from occurring.
Various endophytic microbes can lessen the toxicity of HMs. For example, by raising the protein and chlorophyll concentrations in leaf tissue, Psychrobacter sp. SRS8 could shield the plants (H. annuus & R. communis) from the suppressing impact of Ni.74 Methylobacterium oryzae & Burkholderia sp. recovered from rice lowered the poisonousness of Ni & Cd in tomatoes and promoted plant growth in pot testing. Additionally, Shin et al.,83 found that Bacillus sp. recovered from the hyperaccumulator A. firma’s roots can significantly lessen HMs phytotoxicity & improve Pb precipitation in A. firma. As demonstrated by the situations above, phyto-bacterial procedures are substantially more effective than individual phytoremediation methods for eliminating HMs. As a result, PGPB plays a crucial role in boosting HMs phytoremediation.
According to numerous studies, lead is a persistent environmental contaminant that slowly builds up, causing biomagnifications at various trophic levels in food chains and having serious negative impacts on people. Waste from factories, power plants, and incineration facilities should be removed from the area where pollution is produced since it contains significant amounts of dangerous lead. Bioremediation technologies are more economical, environmentally benign, and highly effective than physicochemical techniques for removing heavy metals from damaged environmental sites. Utilizing microorganisms for lead bioremediation has received a lot of interest during the past few decades. Potential biotechnological agents for the bioremediation of lead-contaminated sites include lead-resistant bacterial strains that have the various lead-resistant mechanisms discussed in this review, including the efflux mechanism, extracellular sequestration, biosorption, precipitation, alteration in cell morphology, and enhanced siderophore production. Bacterial bioreporters created by fusing lux genes and lead-specific regulatory genes (PbrR) are relatively affordable, simple to use, and lead-specific. They can detect up to nanomoles of lead in contrast to expensive and time-consuming chemical analysis approaches. Since bacteria grow quickly, the sample doesn’t need to be pre-treated before the biosensing assay.
Future perspectives
Using conventional genetic engineering techniques, advantageous traits already present in some bacterial strains can be combined or enhanced. High levels of bacterial metallothionein (BmtA) expressed by genetically modified bacteria (GMB) can be used to bioremediate, heavily lead-contaminated environmental locations. By lowering metal bioavailability, these hyper-metal accumulating bacteria preserve metal homeostasis. The ability of bacteria to bioaccumulate substances can be increased by metallothioneins being expressed on the cell surface through fusion with cell surface proteins. For the biosorption of large levels of hazardous lead either on the cell surface or microbial products such as EPS and biosurfactants, bacterial strains may be genetically modified to express high levels of metal-binding ligands, such as carboxyl, hydroxyl, sulfate, phosphate, and amine. In tests using field samples, genetically modified Caulobacter crescentus that overexpresses the hexahistidine peptide on the surface of the bacterial cells effectively sequestered several folds more cadmium than the control strain.84 This strain is presently used as a whole-cell cadmium adsorbent. Recombinant microorganisms can boost heavy metal bioremediation performance by three to six times.85 Therefore, a good technique for the bioremediation of lead from industrial effluents will be to genetically modify potential lead-resistant natural bacterial isolates to overproduce EPS, metallothionein protein, and biosurfactants. The release of chemicals can be harmful to natural biota (calcium phosphate, ferric sulfate, etc.), and physical-chemical methods like chemical precipitation, activated carbon adsorption, ion exchange, reverse osmosis, and foam flotation techniques used for bioremediation of heavy metals are found to be expensive. They are also inappropriate for large effluents containing complex organic matter.85,86,116 However, the treatment of large volumes of effluents with low concentrations of heavy metals, high uptake capacity, environmentally friendly technological solution, and extremely cost-effective since bacteria grow very quickly on simple media/organic wastes are major advantages of bioremediation of heavy metal-polluted environmental sites using bacteria.86, 87, 117-121
ACKNOWLEDGMENTS
None.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Abdelbary S, Elgamal MS, Farrag A. Trends in heavy metals tolerance and uptake by Pseudomonas aeruginosa (Chapter). Pseudomonas aeruginosa-An Armory Within (Book) 2019:1-12.
Crossref - Akhtar FZ, Archana KM, Krishnaswamy VG, Rajagopal R. Remediation of heavy metals (Cr, Zn) using physical, chemical and biological methods: a novel approach. Appl Sci. 2020;2(2):267.
Crossref - Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals-concepts and applications. Chemosphere. 2013;91(7):869-881.
Crossref - Aliasgharzad N, Molaei A, Oustan S. Pollution induced community tolerance (PICT) of microorganisms in soil incubated with different levels of Pb. Int J Environ Ecol Eng. 2011;5:838-842.
- Allcroft R, Laxter K. Lead as a nutritional hazard to farm livestock: V. The toxicity of lead to cattle and sheep and an evaluation of the lead hazard under farm conditions. J Comp Pathol Ther. 1950;60:209-218.
Crossref - Alloway BJ. Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer Science & Business Media. 2012;22.
Crossref - Almוs ֵR, Bakken LR, Mulder J. Changes in tolerance of soil microbial communities in Zn and Cd contaminated soils. Soil Biol Biochem. 2004;36(5):805-813.
Crossref - Banerjee G, Pandey S, Ray AK, Kumar R. Bioremediation of heavy metals by a novel bacterial strain Enterobacter cloacae and its antioxidant enzyme activity, flocculant production, and protein expression in presence of lead, cadmium, and nickel. Water Air Soil Pollut. 2015;226(4):91-99.
Crossref - Bhatnagar S, Kumari R. Bioremediation: a sustainable tool for environmental management-a review. Annu Res Rev Biol. 2013:974-993.
- Bono GD, Braca G. Lead poisoning in domestic and wild ducks. Avian Pathol. 1973;2(3):195-209.
Crossref - Cephidian A, Makhdoumi A, Mashreghi M, Mahmudy Gharaie MH. Removal of anthropogenic lead pollutions by a potent Bacillus species AS2 isolated from geogenic contaminated site. Int J Environ Sci Technol. 2016;13(9):2135-2142.
Crossref - Chain EPoCitF. Scientific opinion on lead in food. EFSA J. 2010;8(4):1570.
Crossref - Chatterjee S, Bhattacharjee I, Chandra G. Biosorption of heavy metals from industrial waste water by Geobacillus thermodenitrificans. J Hazard Mater. 2010;175(1-3):117-125.
Crossref - Chen L, Luo S, Xiao X, et al. Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl Soil Ecol. 2010;46(3):383-389.
Crossref - Chen L, Luo S, Li X, Wan Y, Chen J, Liu C. Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol Biochem. 2014;68:300-308.
Crossref - Chen W-M, Tang Y-Q, Mori K, Wu X-L. Distribution of culturable endophytic bacteria in aquatic plants and their potential for bioremediation in polluted waters. Aquat Biol. 2012;15(2):99-110.
Crossref - Cherian S, Weyens N, Lindberg S, Vangronsveld J. Phytoremediation of trace element-contaminated environments and the potential of endophytic bacteria for improving this process. Crit Rev Environ Sci Technol. 2012;42(21):2215-2260.
Crossref - Cristani M, Naccari C, Nostro A, Pizzimenti A, Trombetta D, Pizzimenti F. Possible use of Serratia marcescens in toxic metal biosorption (removal). Environ Sci Pollut Res. 2012;19(1):161-168.
Crossref - da Conceiחדo Gomes MA, Hauser-Davis RA, de Souza AN, Vitoria AP. Metal phytoremediation: General strategies, genetically modified plants and applications in metal nanoparticle contamination. Ecotoxicol Environ Saf. 2016;134(1):133-147.
Crossref - Dai Q, Bian X, Li R, Jiang C, Ge J, Li B et al. Biosorption of lead (II) from aqueous solution by lactic acid bacteria. Water Sci Technol. 2019;79(4):627-634.
Crossref - DalCorso G, Fasani E, Manara A, Visioli G, Furini A. Heavy metal pollutions: state of the art and innovation in phytoremediation. Int J Mol Sci. 2019;20(14):3412.
Crossref - Doty SL. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytologist. 2008;179(2):318-333.
Crossref - Dwivedi S, Swarup D, Dey S, Patra R. Lead poisoning in cattle and buffalo near primary lead-zinc smelter in India. Vet Hum Toxicol. 2001;43(2):93-94.
- El-Badry S, Raslan A. Estimation of lead and copper residues in sheep, goat milks and Karish cheese. Benha Vet Med J. 2016;30(2):1-5.
Crossref - Elsanhoty RM, Al-Turki I, Ramadan MF. Application of lactic acid bacteria in removing heavy metals and aflatoxin B1 from contaminated water. Water Sci Technol. 2016;74(3):625-638.
Crossref - Ezzouhri L, Castro E, Moya M, Espinola F, Lairini K. Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier, Morocco. Afr J Microbiol Res. 2009;3:35-48.
- Fahr M, Laplaze L, Bendaou N, et al. Effect of lead on root growth. Front Plant Sci. 2013;4:175.
Crossref - Gabr R, Hassan S, Shoreit A. Biosorption of lead and nickel by living and non-living cells of Pseudomonas aeruginosa ASU 6a. Int Biodeterior Biodegr. 2008;62(2):195-203.
Crossref - Garg SK, Tripathi M, Srinath T. Strategies for chromium bioremediation of tannery effluent. Rev Environ Contam Toxicol. 2012;217:75-140.
Crossref - Gashkina NA, Moiseenko TI, Kudryavtseva LP. Fish response of metal bioaccumulation to reduced toxic load on long-term contaminated Lake Imandra. Ecotoxicol Environ Saf. 2020;191:110205.
Crossref - Gavrilescu M. Removal of heavy metals from the environment by biosorption. Eng Life Sci. 2004;4(3):219-232.
Crossref - George F, Mahieux S, Daniel C, et al. Assessment of Pb (II), Cd (II), and Al (III) removal capacity of bacteria from food and gut ecological niches: Insights into biodiversity to limit intestinal biodisponibility of toxic metals. Microorganisms. 2021;9(2):456.
Crossref - Girma G. Microbial bioremediation of some heavy metals in soils: an updated review. Egypt Acad J Biol Sci G Microbiol. 2015;7:29-45.
Crossref - Goudarzi L, Kermanshahi RK, Khaniki GJ. Response surface design for removal of lead by different lactic acid bacteria. Health Scope. 2020;9:e101049
Crossref - Halttunen T, Salminen S, Tahvonen R. Rapid removal of lead and cadmium from water by specific lactic acid bacteria. Int J Food Microbiol. 2007;114(1):30-35.
Crossref - Hassan S, Abskharon R, Gad El Rab S, Shoreit A. Isolation, characterization of heavy metal resistant strain of Pseudomonas aeruginosa isolated from polluted sites in Assiut city, Egypt. J Basic Microbiol. 2008;48(3):168-176.
Crossref - He H, Ye Z, Yang D, et al. Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere. 2013;90(6):1960-1965.
Crossref - He L-Y, Chen Z-J, Ren G-D, Zhang Y-F, Qian M, Sheng X-F. Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria. Ecotoxicol Environ Saf. 2009;72(5):1343-1348.
Crossref - Helmy NA, Maarouf AA, Hassan MA, Hassanien FS. Detection of heavy metals residues in fish and shellfish. Benha Vet Med J. 2018;34(2):255-264.
Crossref - Hrynkiewicz K, Baum C. Application of microorganisms in bioremediation of environment from heavy metals. Environmental Deterioration and Human Health. 2014;7:215-227.
Crossref - Ilhan S, Nourbakhsh MN, Kilicarslan S, Ozdag H. Removal of chromium, lead and copper ions from industrial waste waters by Staphylococcus saprophyticus. Turk Elec J Biotechnol. 2004;2:50-57.
- Jalilvand N, Akhgar A, Alikhani HA, Rahmani HA, Rejali F. Removal of heavy metals zinc, lead, and cadmium by biomineralization of urease-producing bacteria isolated from Iranian mine calcareous soils. J Soil Sci Plant Nut. 2020;20(1):206-219.
Crossref - Jin Y, Wang X, Zang T, et al. Biosorption of lead (II) by Arthrobacter sp. 25: process optimization and mechanism. J Microbiol Biotechnol. 2016;26(8):1428-1438.
Crossref - Jin Y, Yu S, Teng C, et al. Biosorption characteristic of Alcaligenes sp. BAPb. 1 for removal of lead (II) from aqueous solution. 3 Biotech. 2017;7(2):1-12.
Crossref - Kabata-Pendias A, Pendias H. Trace elements in soils and plants, Florida: CRC. Boca Raton. 2011:37-45.
Crossref - Kalita D, Joshi S. Study on bioremediation of Lead by exopolysaccharide producing metallophilic bacterium isolated from extreme habitat. Biotechnol Rep. 2017;16:48-57.
Crossref - Karami A, Shamsuddin ZH. Phytoremediation of heavy metals with several efficiency enhancer methods. Afr J Biotechnol. 2010;9(25):3689-3698.
- Khan Z, Doty S. Endophyte-assisted phytoremediation. Curr Opin Plant Biol. 2011;11:97-105.
- Kumar K, Singh D. Toxicity and bioremediation of the lead: a critical review. Int J Environ Health Res. 2023;8:1-31.
Crossref - Lassen E, Buck W. Experimental lead toxicosis in swine. Am J Vet Res. 1979;40(10):1359-1364.
- Lenart A, Wolny-Koladka K. The effect of heavy metal concentration and soil pH on the abundance of selected microbial groups within ArcelorMittal Poland steelworks in Cracow. Bull Environ Contam Toxicol. 2013;90(1):85-90.
Crossref - Li H-Y, Wei D-Q, Shen M, Zhou Z-P. Endophytes and their role in phytoremediation. Fungal Divers. 2012;54:11-18.
Crossref - Liu S, Zheng Y, Ma Y, et al. Evaluation and proteomic analysis of lead adsorption by lactic acid bacteria. Int J Mol Sci. 2019;20(22):5540.
Crossref - Luo S-l, Chen L, Chen J-l, et al. Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere. 2011;85(7):1130-1138.
Crossref - Ma Y, Rajkumar M, Luo Y, Freitas H. Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. J Hazard Mater. 2011;195:230-237.
Crossref - Ma Y, Rajkumar M, Rocha I, Oliveira RS, Freitas H. Serpentine bacteria influence metal translocation and bioconcentration of Brassica juncea and Ricinus communis grown in multi-metal polluted soils. Front Plant Sci. 2015;5:757.
Crossref - Ma Y, Rajkumar M, Zhang C, Freitas H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manage. 2016;174:14-25.
Crossref - Madhaiyan M, Poonguzhali S, Sa T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere. 2007;69(2):220-228.
Crossref - Mahmood T. Phytoextraction of heavy metals-the process and scope for remediation of contaminated soils. Soil Environ. 2010;29(2):91-109.
- Massoud R, Khosravi Darani K, Sharifan A, Asadi G, Zoghi A. Lead and cadmium biosorption from milk by Lactobacillus acidophilus ATCC 4356. Food Sci Nutr. 2020;8(10):5284-5291.
Crossref - Mateo R. Lead poisoning in wild birds in Europe and the regulations adopted by different countries. Ingestion of lead from spent ammunition: implications for wildlife and humans. 2009:71-98.
Crossref - Mihdhir A, Assaeedi A, Abulreesh H, Osman G. Detection, identification and characterization of some heavy metals tolerant bacteria. J Microb Biochem Technol. 2016;8(3):226-230.
Crossref - Murali O, Mehar SK. Bioremediation of heavy metals using Spirulina. Int J Geol Earth Environ Sci. 2014;4:244-249.
- Murtala BA, Abdul WO, Akinyemi AA. Bioaccumulation of heavy metals in fish (Hydrocynus forskahlii, Hyperopisus bebe occidentalis and Clarias gariepinus) organs in downstream Ogun coastal water, Nigeria. Int J Sci Technol. 2012;4(11):119-133.
Crossref - Mwandira W, Nakashima K, Kawasaki S, et al. Biosorption of Pb (II) and Zn (II) from aqueous solution by Oceanobacillus profundus isolated from an abandoned mine. Sci Rep. 2020;10(1):21189.
Crossref - Naik MM, Dubey SK. Lead resistant bacteria: lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotoxicol Environ Saf. 2013;98:1-7.
Crossref - Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease-a systematic review. Environ Health Perspect. 2007;115(3):472-482.
Crossref - Ndeddy Aka RJ, Babalola OO. Identification and characterization of Cr-, Cd-, and Ni-tolerant bacteria isolated from mine tailings. Bioremediat J. 2017;21(1):1-19.
Crossref - Nicolaus B, Poli A, Di-Donato P, et al. Pb2+ effects on growth, lipids, and protein and DNA profiles of the thermophilic bacterium Thermus thermophilus. Microorganisms. 2016;4(4):45.
Crossref - Okoduwa SIR, Igiri B, Udeh CB, Edenta C, Gauje B. Tannery effluent treatment by yeast species isolates from watermelon. Toxics. 2017;5(1):6.
Crossref - Pandey VC, Bajpai O, Singh N. Energy crops in sustainable phytoremediation. Renew Sust Energ Rev. 2016;54:58-73.
Crossref - Pardo R, Herguedas M, Barrado E, Vega M. Biosorption of cadmium, copper, lead and zinc by inactive biomass of Pseudomonas putida. Anal Bioanal Chem. 2003;376(1):26-32.
Crossref - Park SK, O’Neill MS, Vokonas PS, et al. Air pollution and heart rate variability: effect modification by chronic lead exposure. Epidemiol. 2008;19(1):111-120.
Crossref - Puyen ZM, Villagrasa E, Maldonado J, Diestra E, Esteve I, Sole A. Biosorption of lead and copper by heavy-metal tolerant Micrococcus luteus DE2008. Bioresour Technol. 2012;126:233-237.
Crossref - Qiao W, Zhang Y, Xia H, et al. Bioimmobilization of lead by Bacillus subtilis X3 biomass isolated from lead mine soil under promotion of multiple adsorption mechanisms. R Soc Open Sci. 2019;6(2):181701.
Crossref - Rahman S, Joshi M. Effect of lead toxicity on growth and performance of broilers. Tamilnadu J Vet Anim Sci. 2009;5(2):59-62.
- Rajeshkumar S, Li X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep. 2018;5:288-295.
Crossref - Rajeshkumar S, Liu Y, Zhang X, Ravikumar B, Bai G, Li X. Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere. 2018;191:626-638.
Crossref - Rajkumar M, Ae N, Freitas H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere. 2009;77(2):153-160.
Crossref - Rajkumar M, Sandhya S, Prasad M, Freitas H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv. 2012;30(6):1562-1574.
Crossref - Rani MJ, Hemambika B, Hemapriya J, Kannan VR. Comparative assessment of heavy metal removal by immobilized and dead bacterial cells: a biosorption approach. Afr J Environ Sci Technol. 2010;4(2):077-083
- Ren G, Jin Y, Zhang C, Gu H, Qu J. Characteristics of Bacillus sp. PZ-1 and its biosorption to Pb (II). Ecotoxicol Environ Saf. 2015;117:141-148.
Crossref - Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92-99.
Crossref - Sarwar N, Imran M, Shaheen MR, et al. Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere. 2017;171:710-721.
Crossref - Segura A, Rodrםguez Conde S, Ramos C, Ramos JL. Bacterial responses and interactions with plants during rhizoremediation. Microbial Biotechnol. 2009;2(4):452-464.
Crossref - Sen SK, Raut S, Dora TK, Mohapatra PKD. Contribution of hot spring bacterial consortium in cadmium and lead bioremediation through quadratic programming model. J Hazard Mater. 2014;265:47-60.
Crossref - Sessitsch A, Kuffner M, Kidd P, et al. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem. 2013;60(100):182-194.
Crossref - Sharma P, Dubey RS. Lead toxicity in plants. Braz J Plant Physiol. 2005;17:(1)35-52.
Crossref - Sheng X-F, Xia J-J, Jiang C-Y, He L-Y, Qian M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut. 2008;156(3):1164-1170.
Crossref - Shin M-N, Shim J, You Y, et al. Characterization of lead resistant endophytic Bacillus sp. MN3-4 and its potential for promoting lead accumulation in metal hyperaccumulator Alnus firma. J Hazard Mater. 2012;199:314-320.
Crossref - Siddiqui M, Rajurkar GR. Lead-An Emerging threat to livestock. Vet World. 2008;1(7):213-216.
- Srivastava N, Majumder C. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J Hazard Mater. 2008;151(1):1-8.
Crossref - Srivastava S, Paul A. Associated microflora of medicinal ferns: biotechnological potentials and possible applications. Int J Bioassays. 2016;5(3):4927-4943.
Crossref - Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Molecular, clinical and environmental toxicology. Exp Suppl. 2012;101:133-164.
Crossref - Trampel DW, Imerman PM, Carson TL, Kinker JA, Ensley SM. Lead contamination of chicken eggs and tissues from a small farm flock. J Vet Diagn Invest. 2003;15(5):418-422.
Crossref - Tunali S, Cabuk A, Akar T. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem Eng J. 2006;115(3):203-211.
Crossref - Ullah A, Heng S, Munis MFH, Fahad S, Yang X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot. 2015;117:28-40.
Crossref - Vamerali T, Bandiera M, Mosca G. Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett. 2010;8:1-17.
Crossref - Verma VC, Prakash S, Singh RG, Gange AC. Host-mimetic metabolomics of endophytes: looking back into the future. Adv Endophytic Res. 2014:203-218.
Crossref - Waldner C, Checkley S, Blakley B, Pollock C, Mitchell B. Managing lead exposure and toxicity in cow-calf herds to minimize the potential for food residues. J Vet Diagn Investig. 2002;14(6):481-486.
Crossref - Wang Z, Wang H, Nie Q, et al. Pb (II) bioremediation using fresh algal-bacterial aerobic granular sludge and its underlying mechanisms highlighting the role of extracellular polymeric substances. J Hazard Mater. 2023;444(Part B):130452.
Crossref - Weyens N, van der Lelie D, Taghavi S, Vangronsveld J. Phytoremediation: plant-endophyte partnerships take the challenge. Curr Opin Biotechnol. 2009;20(2):248-254.
Crossref - Wuana RA, Okieimen FE. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int Sch Res Notices. 2011;402647.
Crossref - Xu Z, Lei Y, Patel J. Bioremediation of soluble heavy metals with recombinant Caulobacter crescentus. Bioeng. Bugs. 2010;1(3):207-212.
Crossref - Yao Z, Li J, Xie H, Yu C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ Sci. 2012;16:722-729.
Crossref - Yi Y-J, Lim J-M, Gu S, et al. Potential use of lactic acid bacteria Leuconostoc mesenteroides as a probiotic for the removal of Pb (II) toxicity. J Microbiol. 2017;55(4):296-303.
Crossref - Yu C-C, Lin J-L, Lin-Tan D-T. Environmental exposure to lead and progression of chronic renal diseases: a four-year prospective longitudinal study. J Am Soc Nephrol. 2004;15(4):1016-1022.
Crossref - Zacchini M, Pietrini F, Scarascia Mugnozza G, Iori V, Pietrosanti L, Massacci A. Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut. 2009;197(1):23-34.
Crossref - Zaidi A, Wani PA, Khan MS. Toxicity of heavy metals to legumes and bioremediation. Springer, 2012.
Crossref - Zaynab M, Al-Yahyai R, Ameen A, et al. Health and environmental effects of heavy metals. J King Saud Univ Sci. 2022;34(1):101653.
Crossref - Zhang B, Fan R, Bai Z, Wang S, Wang L, Shi J. Biosorption characteristics of Bacillus gibsonii S-2 waste biomass for removal of lead (II) from aqueous solution. Environ Sci Pollut Res. 2013;20(3):1367-1373.
Crossref - Zhang Y-f, He L-y, Chen Z-j, et al. Characterization of lead-resistant and ACC deaminase-producing endophytic bacteria and their potential in promoting lead accumulation of rape. J Hazard Mater. 2011;186(2-3):1720-1725.
Crossref - Zhao L, Zheng Y-G, Feng Y-H, Li M-Y, Wang G-Q, Ma Y-F. Toxic effects of waterborne lead (Pb) on bioaccumulation, serum biochemistry, oxidative stress and heat shock protein-related genes expression in Channa argus. Chemosphere. 2020;261:127714.
Crossref - Zhenhua X, Dongmei G, Xiuli S, Ying X. A review of endophyte and its use and function. Adv Biomed Eng. 2012;8:124.
- Zhu Y-G, Miller RM. Carbon cycling by arbuscular mycorrhizal fungi in soil-plant systems. Trends Plant Sci. 2003;8(9):407-409.
Crossref - AbuQamar SF, Abd El-Fattah HI, Nader MM, et al. Exploiting fungi in bioremediation for cleaning-up emerging pollutants in aquatic ecosystems. Mar Environ Res. 2023;190:106068.
Crossref - El-Saadony MT, Saad AM, El-Wafai NA, et al. Hazardous wastes and management strategies of landfill leachates: A comprehensive review. Environ Technol Innov. 2023;31:103150.
Crossref - Saad AM, Sitohy MZ, Sultan-Alolama MI, El-Tarabily KA, El-Saadony MT. Green nanotechnology for controlling bacterial load and heavy metal accumulation in Nile tilapia fish using biological selenium nanoparticles biosynthesized by Bacillus subtilis AS12. Front Microbiol. 2022;13:1015613.
Crossref - El-Saadony MT, Saad AM, Soliman SM, eta l. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front Plant Sci. 2022;13:923880.
Crossref - Ashry NM, Alaidaroos BA, Mohamed SA, Badr OA, El-Saadony MT, Esmael A. Utilization of drought-tolerant bacterial strains isolated from harsh soils as a plant growth-promoting rhizobacteria (PGPR). Saudi J Biol Sci. 2022;29(3):1760-1769.
Crossref - Desoky ESM, Merwad ARM, Semida WM, Ibrahim SA, El-Saadony MT, Rady MM. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol Environ Saf. 2020;198:110685.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.