ISSN: 0973-7510
E-ISSN: 2581-690X
Pakoba fruit (Syzygium sp.) is one of the medicinal plants of Minahasan folks and it is an endemic species in North Sulawesi. Pakoba can only be found in Minahasa areas and is very popular among people in Minahasa. The taste is sour, making this fruit as the main ingredient to be processed into rojak, sweets, dodol, jam, or syrup. Pakoba fruit is widely used for treatment in the simplest way because this fruit contains many bioactive compounds. Pakoba fruit can act as a source of probiotics because it contains good microorganisms, namely Lactic Acid Bacteria (LAB). Some types of LAB are able to synthesize exopolysaccharides (EPS), which are polysaccharide polymers considered essential for health. Therefore this research aims to determine the presence of lactic acid bacteria (LAB) in Pakoba fruit and their potential to produce exopolysaccharides. The presence of lactic acid bacteria in Pakoba fruit was detected through the pour plate method on MRSA media (de Man, Rogosa and Sharpe Agar) + CaCO3 1%. The culture was incubated at 37°C for 48 hours. Growing colonies are observed morphological features of colonies, cells, biochemical and physiological properties. The isolates believed to be LAB, were then tested for its potential to produce EPS. From the total of 35 producing-acid bacteria, 17 isolates were tested as lactic acid bacteria group that had characteristics of bacilli cells, gram-positive bacteria, catalase-negative, non-motile, non-spore-forming, gas production, mesophilic, aciduric, can ferment carbohydrates. Based on Bergey’s Manual of Systematic Bacteriology, the seventeen isolates were identified as members of the Lactobacillus genus. The seventeen isolates also showed the ability to produce exopolysaccharides in the range of 102-1570 mg/L.
Exopolysaccharides, Lactic Acid Bacteria, Pakoba, Fruit, Endemic Species
Lactic acid bacteria (LAB) are beneficial microorganisms and still need to be explored and some lactic acid bacteria are probiotic candidates with certain conditions. Probiotic bacteria have several requirements, namely resistant to low pH conditions (acidic), resistant to bile acids and salts, can produce antimicrobials so that they can develop in the human digestive tract and can balance the microflora in the digestive tract, and are safe for use.1,2
Lactic acid bacteria (LAB) are bacteria that have a major contribution in the fields of food, agriculture and medicine (pharmacology). These bacteria have many benefits, including as probiotics, increasing the organoleptic value of food and beverages, and biopreservatives (natural preservatives) of food and processed products.3 Lactic acid bacteria are known as food grade microorganisms, which is a group of bacteria that do not produce toxins harmful to health but on the contrary, their presence in food provides a good function for health because it has the ability to inhibit pathogenic microbes naturally.4 Some bioactive compounds produced by LAB are lactic acid, ethanol, hydrogen peroxide and bacteriocin, and these bioactive compounds are agents that can be used to kill pathogenic microbes.3,4 The good and functional character possessed by LAB, makes the group of bacteria widely used in various fields, especially in the food sector and pharmacology. Therefore, exploration of LAB isolated from various substrates to obtain functional BAL, until now is still need to carried out. One of the potential substrates used to obtain BAL is fruits.
LAB exploration from various sources is currently increasing. LAB can be isolated from various foodstuffs for use as probiotics and functional foods such as fruit,5,6,7 vegetables8; and fermented foods,9 Pakoba fruit (Syzygium sp.) as an endemic species of North Sulawesi is known to have the potential to increase endurance because it contains palmitic acid, oleic acid, and linoleic acid and also produces antioxidants.10 Pakoba fruit is found in the North Sulawesi area with a high content of simple sugars and organic acids in ripe fruit, so it is very potential as a substrate for bacterial growth. Lactic acid bacteria are one of the bacteria that are resistant to organic acids contained in the fruit.
Lactic acid bacteria can also produce and secrete exopolysaccharides (EPS) that are beneficial to health.10 Some health functions include immunostimulation, antioxidant activity antitumor, and lowering of blood cholesterol.11 In addition, EPS plays a role in protecting LAB cells from harmful environments and supports adhesion and biofilms. EPS is also reported to play a role in food technology such as texture formation and rheology, producing important polymers and food grade so that they are safe to use in food products for example to increase the viscosity of processed food products.6,12 Therefore, EPS production becomes an interesting property to consider in the selection of probiotic strains.
The production of EPS by probiotic bacteria is related to genes encoding enzymes that play a role in the reaction of EPS formation, including the fructosyltransferase (ftf) gene which encodes the fructosyltransferase enzyme, and the glucosyltransferase (gtf) gene which encodes the glucosyltransferase enzyme. The polymorphism of the ftf gene and gtf gene is influenced by the origin and species of the LAB strain which has not been studied, especially from local food sources. Genetic diversity refers to differences in genes carried and is closely related to the metabolic processes carried out and the number of metabolites produced. Each gene is responsible for overseeing the formation, function, and ability of an enzyme to work.13,14 The identification and screening of FTF genes and GTF genes in an isolate will have an impact on enzyme variations that play a role in EPS biosynthesis so that the impact will enrich the variations of EPS polymers with unique structures and new functions that have the potential to be developed in use in the pharmaceutical, health, and food fields.12,15,16
Based on research data that has been mentioned that LAB can be isolated from various types of fruits. LAB in fruits has a considerable likelihood of being discovered and can also produce EPS. EPS has extensive benefits for human life, especially in the fields of health and pharmaceuticals, so this study is important to know the presence of lactic acid bacteria in Pakoba fruit (Syzygium sp.) and know its potential in producing exopolysaccharides. This LAB-EPS isolates are expected to be used as a source of genes encoding new sucrase enzymes, both glucansiltransferase (GTF) and fructosyltransferase (FTF).
Isolation lactic acid bacteria
Pakoba Fruit (Syzygium sp.) was taken from the North Minahasa Regency, North Sulawesi, Indonesia. The initial growth of LAB from Pakoba fruit is carried out through the enrichment stage. A total of 10 g of fruit flesh samples were cut into small pieces mashed, and put in an Erlenmeyer flask (100 ml) containing MRS broth (Man Ragosa Sharpe)(Merk) pH medium (5.5), then incubated at room temperature for 2 x 24 hours.
LAB isolation is carried out by the pour plate method. One ml of Pakoba sample resulting from enrichment was pipetted and suspended into 9 ml of PBS (dilution 10-2) and made a dilution series of up to 10-7. One ml of suspension of dilution 10-5, 10-6, and 10-7 was inoculated in sterile petri dishes and MRSA + CaCO3 1% + Sodium acid 1 ppm media was added. The Petri dishes were then incubated at 37°C for 48 hours in an incubator in a micro-aerophilic atmosphere. Isolated bacterial colonies are bacteria that show clear zones around their colony. Purification of isolated bacterial colonies is carried out by streaking them again on MRSA media until a uniform bacteria colony is obtained. Pure LAB culture is stored in colonies in a test tube with oblique MRSA media at 4°C for further characterization.
Phenotypic characterization of lactic acid bacteria (LAB)
Macroscopic observations (seen directly) in the form of visible colony morphology. The parameters observed are color, shape, elevation, and edge of the colony. While microscopic observations are carried out using a microscope to see the shape, arrangement, color, and presence, or absence of endospores from bacterial cells. Cell morphology observations were carried out on gram stain to distinguish gram-positive bacteria (purple) from gram-negative bacteria (red). Staining was carried out on a 24-hour-old bacterial culture grown on the MRS Agar oblique medium. Staining consists of gram A (Crystal violet) paint. gram B (Lugol solution), gram C (Alcohol), and gram D (Safranin). The staining results were observed under a 1000x magnification microscope. Staining such grams can see the shape, arrangement, and color.
In endospore staining using the main stain, namely Malachite green 5%, and safranin. Bacterial isolates are etched on the surface of glass objects using ose needles, after which 1-2 drops of Malachite green are then fixated. Next drip 1-2 drops of safranin and dried aerated. The results obtained were given immersion oil and observed under a 1000x magnification microscope. A positive result is the presence of green spores. Lactic acid bacteria are bacteria that do not produce endospores.
Catalase test by dripping H2O2 solution 3 % on a glass object, then taking a smear of bacteria using ose and applying it to the solution on a glass object, seen the reaction formed if no bubbles form indicates a positive reaction.17
The motility test is carried out by inserting 1 straight ose bacterial culture into SIM media (Sulfite Indol Motility) by separating 1/4 part of the butt then incubated 48 h, 37°C and observed the presence or absence of propagation around the puncture.18
Crude exopolysaccharide test
In crude exopolysaccharide testing is carried out by gravimetric method. 1 ose isolate was inoculated into 10 mL MRSB which had 5% sucrose added and incubated for 48 hours at 35°C. Separation of bacterial cells is carried out through a centrifugation process at 4500 rpm at 4°C for 45 minutes, the supernatant precipitated by the addition of 96% cold ethanol as much as 10 mL (2x the sample volume) and allowed to stand for overnight at cold temperatures. Furthermore, centrifuged at a temperature of 4°C at a speed of 4500 rpm for 45 minutes, then the pellets were dried in an oven at a temperature of 100°C for 15 minutes and weighed the dry weight of EPS.19
Pakoba fruit samples used in this study were obtained from North Minahasa Regency. Occurrence of lactic acid bacteria (LAB) performed on Pakoba Fruit (Syzygium sp.) has succeeded in obtaining 35 colonies of acid-producing bacteria indicated by the formation of clear zones around the colonies grown on MRS Agar selective media containing calcium carbonate (CaCO3) 1% and sodium azide 1 ppm (Figure 1). MRS is a growth medium for acid-producing bacteria because in MRS media there is glucose which is a source of carbon and energy for the growth of these bacteria. Calcium carbonate (CaCO3) suspension that appears cloudy in the medium so that it will be dissolved by acids produced by acid-producing bacteria including LAB to form a clear zone around the colony.
Figure 1. The Appearance of Pakoba fruit (Syzygium sp.) and Colonies of acid-producing bacteria : (a) Pakoba fruit; (b) Bacterial colonies that produce clear zone on MRSA + CaCO3
The presence of calcium carbonate (CaCO3) is an indication that the bacteria growing on MRS media are acid-producing bacteria which include lactic acid bacteria (LAB). Before the isolation process, enrichment is carried out to obtain isolates of acid-producing bacteria which were incubated for 2 x 24 hours at room temperature.5,6 Furthermore, purification was carried out on 35 isolates obtained as much as 2x with the quadran streak technique on MRSA+ CaCO3 media.
Early detection of LAB colonies is based on the presence of clear zones formed around the colony on MRSA+CaCO3 1% isolation media. Furthermore, screening of LAB was carried out by conducting a confirmatory test on isolates suspected of being LAB by conducting a Gram reaction test, catalase reaction test, endospore test, and motility test.
LAB screening of 35 colonies of acid-producing bacteria that have a circular shape with white and yellowish-white colony colors, gram positive, catalase negative, non-motile, without spores showed that seventeen colonies of acid-producing bacteria were confirmed as members of the lactic acid bacteria group and eighteen colonies of acid-producing bacteria were not included in the lactic acid bacteria group because they showed characteristics gram negative, catalase positif, motile and has spores (Table 1). This indicates that LAB isolates have been successfully obtained from samples of Pakoba fruit (Syzygium sp.). In addition to LAB, bacteria members of the genus Micrococcus, Bacillus, Pseudomonas, Staphylococcus, Salmonella, Shigella, Vibrio, Klepsiella are usually found in fruits and fruit products such as fresh fruit juice (Mango fruit and Avocado fruit), fresh guava fruit juice, banana fruit, papaya fruit, Jujube fruit, fruit, Plum fruit, Apple fruit, Cantaloupe fruit, Watermelon fruit, Dragon fruit.20-23
Table (1):
Screening of LAB and Non LAB Isolates Growing on MRSA-CaCO3 Medium Obtained from Buah Pakoba (Syzygium sp.)
| No | Isolate Code | Color Colony | Acid Producer (Clear Zone) | Gram | Catalase | Formation sporeMotilityLAB / NON LAB | ||
|---|---|---|---|---|---|---|---|---|
| 1 | PM5.2 | Yellowish-white | + | + | – | – | – | LAB |
| 2 | PM5.2 | Yellowish-white | + | + | – | – | – | LAB |
| 3 | PM5.3 | Yellowish-white | + | + | – | – | – | LAB |
| 4 | PM5.4 | Yellowish-white | + | + | – | – | – | LAB |
| 5 | PM5.5 | Yellowish-white | + | – | + | – | – | Non LAB |
| 6 | PM5.6 | Milk white | + | + | + | – | – | Non LAB |
| 7 | PM5.7 | Milk white | + | – | + | – | nd | Non LAB |
| 8 | PM5.8 | Yellowish-white | + | + | + | + | nd | Non LAB |
| 9 | PM5.9 | Yellowish-white | + | + | + | – | – | Non LAB |
| 10 | PM5.10 | Yellowish-white | + | + | + | + | nd | Non LAB |
| 11 | PM5.11 | Milk white | + | – | + | – | nd | Non LAB |
| 12 | PM5.12 | Milk white | + | + | – | + | nd | Non LAB |
| 13 | PM6.1 | Yellowish-white | + | + | + | – | – | Non LAB |
| 14 | PM6.2 | Yellowish-white | + | – | + | – | nd | Non LAB |
| 15 | PM6.3 | Yellowish-white | + | + | – | – | – | LAB |
| 16 | PM6.4 | Yellowish-white | + | + | – | – | – | LAB |
| 17 | PM6.5 | Yellowish-white | + | + | – | – | – | LAB |
| 18 | PM6.6 | Yellowish-white | + | + | – | – | – | LAB |
| 19 | PM6.7 | Milk white | + | + | + | + | nd | Non LAB |
| 20 | PM6.8 | Yellowish-white | + | + | + | – | – | Non LAB |
| 21 | PM6.9 | Milk white | + | + | + | – | – | Non LAB |
| 22 | PM6.10 | Yellowish-white | + | + | – | – | – | LAB |
| 23 | PM6.11 | Yellowish-white | + | + | – | – | – | LAB |
| 24 | PM7.1 | Yellowish-white | + | + | – | – | – | LAB |
| 25 | PM7.2 | Yellowish-white | + | + | – | – | – | LAB |
| 26 | PM7.3 | Yellowish-white | + | + | – | – | – | LAB |
| 27 | PM7.4 | Yellowish-white | + | + | – | – | – | LAB |
| 28 | PM7.5 | Yellowish-white | + | – | + | – | nd | Non LAB |
| 29 | PM7.6 | Yellowish-white | + | + | + | – | – | Non LAB |
| 30 | PM7.7 | Yellowish-white | + | + | + | + | nd | Non LAB |
| 31 | PM7.8 | Yellowish-white | + | + | + | – | – | Non LAB |
| 32 | PM7.9 | Yellowish-white | + | + | – | – | – | LAB |
| 33 | PM7.10 | Yellowish-white | + | + | – | – | – | LAB |
| 35 | PM7.11 | Yellowish-white | + | + | – | – | – | LAB |
Based on Table 2, it is known that the morphology of the seventeen colony of LAB isolates has almost the same character. The morphological observations of the colonies of the seventeen isolates show that the seventeen isolates have a circular colony shape, yellowish-white color, entire edge (flat) and opaque inner structure (not translucent). The morphological character of LAB colonies from Pakoba fruit obtained in this study is the same as the results of research by24 who reported that lactic acid bacteria isolates from Sweet Arum mangoes have colony characteristics, namely yellowish-white color, round shape, entire edge, smooth surface and convex elevation, and opaque inner structure.
Table (2):
Morphological characteristics of BAL colonies of Pakoba Fruit (Syzygium sp.)
No. |
Isolate Code (LAB) |
Colony form |
Color |
Elevation |
Edge |
Inner structure |
|---|---|---|---|---|---|---|
1 |
PM5.2 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
2 |
PM5.2 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
3 |
PM5.3 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
4 |
PM5.4 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
5 |
PM6.3 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
6. |
PM6.4 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
7. |
PM6.5 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
8. |
PM6.6 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
9. |
PM6.10 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
10. |
PM6.11 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
11. |
PM7.1 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
12. |
PM7.2 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
13. |
PM7.3 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
14. |
PM7.4 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
15. |
PM7.9 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
16. |
PM7.10 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
17. |
PM7.11 |
Circular |
Yellowish-white |
Convex |
Entire |
Opaque |
Based on the results of Gram stain, it is known that the seventeen LAB isolates from Pakoba fruit are bacil (stem) shaped and purple cells. This shows that all seventeen bacteria are Gram positive. Gram positive bacteria have the ability to retain the main crystal violet paint after washing with alcohol, so the cells are purple
(Figure 2). According to,17 bacteria that can withstand purple paint (crystal violet) even though they have been denatured with alcohol or acetone and still give purple color to bacterial cells are called Gram positive bacteria. Conversely, bacteria that cannot withstand crystal violet dye after decolorization with alcohol and will absorb safranin paint with red characteristics are Gram negative bacteria. The ability of Gram positive bacteria to maintain crystal violet paint is due to the fact that the cell wall of Gram positive bacteria has a lower lipid content than Gram negative bacteria, so that the bacterial cell wall will be more easily degraded due to treatment with alcohol. The degraded cell wall causes the size of the cell pores to be small and the permeability is reduced so that the violet crystal dye cannot leave the cell and the cell will remain purple. While Gram negative bacteria look red because bacteria lose crystal violet dye when rinsing with alcohol but can absorb the last dye, safranin. Gram-negative bacteria contain lipids, or substances such as fat in a higher percentage than those contained in Gram-positive bacteria.25
Figure 2. Cell shape of isolate LAB obtained from Pakoba fruit (Syzygium sp.)
a. Cell shape of isolate LAB PM6.10; b. Cell shape of isolate LAB PM6.4; c. Cell shape of isolate LAB PM7.2; d. Cell shape of isolate LAB PM5.1
The characteristics of the seventeen LAB isolates obtained from Pakoba fruit as shown in Table 3 and Table 4, correspond to the key characters of lactic acid bacteria.4 In general, lactic acid bacteria have featured characters, namely including the group of Gram positive bacteria, non-spore, bacil or cocci shaped, catalase negative, single or pair structure cell (two-two)non-motile and produce lactic acid as the main end product during carbohydrate fermentation.3 The four genera most commonly found as the BAL group are Lactobacillus, Leuconostoc, Pediococcus and Streptococcus.3,26 The genus Lactobacillus is a group of Gram positive bacteria in the form of bacil (rods), while the genera Leuconostoc, Pediococcus and Streptococcus are Gram positive bacteria in the form of coccus (round). Based on the data in Table 4 which shows that the seventeen LAB isolates from Pakoba fruit are Gram positive bacteria in the form of bacil, so it is indicated that the seventeen LAB isolates belong to the genus Lactobacillus. The main characteristics for differentiating LAB isolates to the genus level are cell shape, cell structure, and gas from glucose. Previous research also found LAB genus Lactobacillus from fruits, including guava rice, tome-tome, mangosteen, honey pineapple, salak, soursop and banana goods.6,27-29
Table (3):
Characterization (Confirmation Test) Cell of LAB Isolates obtained from Pakoba Fruit (Syzygium sp.)
| No. | Isolate Code | Cell Morphology | Cell arrangement | Gram reaction | Catalase | Formation spore | Motility test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PM5.1 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 2 | PM5.2 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 3 | PM5.3 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 4 | PM5.4 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 5 | PM6.3 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 6. | PM6.4 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 7. | PM6.5 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 8. | PM6.6 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 9. | PM6.10 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 10. | PM6.11 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 11. | PM7.1 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 12. | PM7.2 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 13. | PM7.3 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 14. | PM7.4 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 15. | PM7.9 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 16. | PM7.10 | Rod | Single/pair/chain | + | – | – | – | ||||||
| 17. | PM7.11 | Rod | Single/pair/chain | + | – | – | – | ||||||
Table (4):
Morphological, Biochemical, and Physiological Characterization Cell of LAB from Pakoba (Syzygium sp.)
Key Character* |
Leuconostoc |
Pediococcus |
Lactobacillus |
Ia |
|---|---|---|---|---|
Number of Strain |
17 |
|||
Shape |
Coccus |
Coccus |
Rods |
Rods |
Cell structure |
Pair/chain |
Tetrad |
Single/pair/chain |
Single/pair/chain |
Spore formation |
– |
– |
– |
– |
Catalase |
– |
– |
– |
– |
Gas from glucose |
+/- |
– |
– |
+/- |
Motility test |
– |
– |
– |
– |
Mode of glucose fermentation |
Heterofer-mentatif |
Homofermentatif |
Heterofermen tatif/ Homofermentatif |
Heterofermentatif/ Homofermentatif |
Visible Growth : |
||||
at 100C |
+/- |
+/- |
+/- |
+ |
at 400C |
+/- |
+/- |
+/- |
+ |
at 500C |
– |
+/- |
+/- |
– |
at pH 3.5 |
– |
+/- |
+/- |
+ |
at pH 7.5 |
+ |
+ |
+ |
– |
at pH 9.6 |
– |
– |
– |
– |
at NaCl 6.5% |
+/- |
+/- |
+/- |
+ |
at NaCl 18% |
– |
– |
– |
– |
*Key character description genera Lactobacillus, Leuconostoc and Pediococcus based on Ia Lactobacillus
Fruit is one of the natural habitats for bacteria, including LAB. This is because the fruit contains various chemical compounds such as water, carbohydrates, fats, vitamins, organic acids, and various minerals needed as nutrients for LAB. Some types of fruit that are thought to be the habitat of LAB include Langsat fruit (Lactobacillus sp.),5 Salak fruit, banana, soursop fruit, mangosteen fruit, guava rice-rice fruit (Lactobacillus, Pediococcus, Lactococcus and Leuconostoc),28 Kersen fruit (Lactobacillus),28 and sweet arum mango fruit (Lactobacillus).24
Crude production of exopolysaccharides (EPS) LAB in Pakoba fruit (Syzygium sp.)
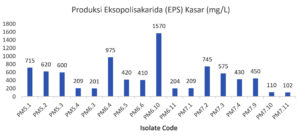
In this study, crude production of exopolysaccharides (EPS) produced by each LAB isolate obtained from Pakoba fruit was carried out. The Seventeen isolates of LAB from Pakoba fruit (Syzygium sp.) It has the ability to produce various amounts of exopolysaccharides, ranging from 102 mg / L to 1570 mg / L.
Figure 3. Production of Exopolysaccharides (EPS) Crude LAB Isolates from Pakoba Fruit (Syzygium sp.)
Based on Figure 3, the lowest EPS value produced by PM7.11 isolate was 102 mg/L while the highest EPS value was produced by PM6.10 isolate was 1570 mg / L. EPS value of LAB PM6.10 isolate from pakoba fruit was higher when compared to commercial LAB isolate Lactobacillus casei with the same treatment of 1340 mg / L.16 The results of previous studies that isolated EPS-BAL from various types of products, such as from fermented carp (Dekke Na Niura) obtained EPS levels of 2490-3490 mg / L,19 Kersen Fruit obtained EPS levels of 870-1910 mg / L,12 and fermented Kefir Kolostrum obtained EPS levels of 1600-4840 ml / L,16 compared to the results of EPS dry weight obtained from this study was quite large.
EPS production by seventeen LAB isolates from Pakoba fruit showed varying amounts although the seventeen isolates were identified in the same genus Lactobacillus. This is due to the influence of its genetic diversity. Species differences refer to differences in genes carried so that they are closely related to the metabolic processes carried out and the number of metabolites produced. Each gene is responsible for overseeing the formation, function, and ability of an enzyme to work, so that when there are different genes even though they are still classified as the same species but with different strains, the final results of metabolism can be different.19 Lactic acid bacteria produce secondary metabolite products in the form of exopolysaccharides that are secreted outside the cell when under unfavorable conditions. Differences in the ability to produce EPS due to differences in species or strains.16
The difference in the amount of EPS obtained in this study with other studies is thought to be caused by several factors, one of which is the incubation time used. in this study EPS production by LAB was harvested at the 48th hour after being grown on 10 mL of liquid media.16 The maximum production of EPS produced by a culture will be influenced by the growth factors of the culture, where the optimum EPS production occurs during maximum cell production, namely in the stationary phase or the final phase of LAB.30 The stationary phase of LAB isolates begins in the range of 20-24 hours and in the next stage of growth there will be degradation of EPS. This happens because EPS produced by microbes in the stationary phase can be reused as a source of carbon in the near-death phase because microbes have enzymes that can degrade the EPS. As a result, the extension of incubation time will actually decrease EPS production.19
Other factors that can affect the amount of EPS produced by LAB in addition to incubation time are excess carbohydrates in the media, the type of media used, fermentation conditions such as temperature, pH, and oxygen levels, and the physiology of each bacterium,31 the optimum temperature of LAB is known to be 37°C, so in this study, to obtain maximum polysaccharides, the incubation temperature was lowered to 35°C.
EPS also tends to be influenced by the bacterial environment such as pH, growth phase, availability of nutrients such as carbon and nitrogen sources, temperature, and fermentation conditions.14 This test used MRS Broth medium which added 5% Sucrose as a growing medium from lactic acid bacteria because this media contains many nutrients favored by LAB. The added sucrose is an excellent substrate for EPS synthesis with abundant yields.
EPS production increases with increasing sucrose concentrations and involves extracellular sucrase-type enzymes used by LAB isolates to polymerize EPS, namely fructansucrase and glucansucrase.13 In this test LAB isolates aged 24 hours. The use of 24-hour age isolates because the logarithmic phase ends at that time and will enter the stationary phase where in this phase bacteria produce EPS optimally. Bacteria begin to experience extreme conditions such as reduced growth nutrients resulting in secondary metabolite products in the form of exopolysaccharides that are released outside the cell when conditions are unfavorable. The principle of this test is to separate exopolysaccharides from bacterial cells using centrifugation at 4°C to prevent protein denaturation. The pellets obtained from the results of the process are dried and weighed until they reach a constant weight. The results obtained are then analyzed quantitatively.
The filtrate obtained was added with 96% cold ethanol by twice the volume of the sample used to precipitate EPS. The use of ethanol with double the sample aims to facilitate the rate of diffusion so that the distribution of particles will be greater with the greater surface area. 96% ethanol is used as a precipitator for polysaccharides and has a relatively small ability to dissolve polysaccharides, although the ability to dissolve other substances is relatively large.32
Ethanol has a much lower dielectric constant than water so it has lower polarity than water, whereas polysaccharides contain many hydroxyl groups that give their polar characteristics, as a result of which ethanol concentrations increase in solution and cause a decrease in polysaccharide solubility or cause precipitation.32
Exopolysaccharides produced by microbes have great potential for the future, the use of microbes to produce polysaccharides is very profitable in economic terms compared to plant polysaccharides. Producing microbial polysaccharides can be done continuously on a large scale and does not require large areas of land such as plants.32
35 isolates of acid-producing bacteria were obtained from Pakoba fruit (Syzygium sp.). Seventeen isolates were confirmed as lactic acid bacteria namely PM5.1, PM5.2, PM5.3, PM5.4, PM6.3, PM6.4, PM6.5, PM6.6, PM6.10, PM6.11, PM7.1, PM7.2, PM7.3, PM7.4, PM7.9, PM7.10 and PM7.11. Based on phenotypic characterization, seventeen LAB isolates belong to the genus Lactobacillus and capable of producing exopolysaccharides (EPS) as much as 102 mg/L – 1570 mg/L.
Further research will be carried out characterizing the genes encoding Glucosyltransferase (gtf) and Fructosyltransferase (ftf) from selected EPS-producing lactic acid bacteria isolates from Pakoba fruit to be further developed as a source of genes encoding new sucrase enzymes, both glucansiltransferase (GTF) and fructosyltransferase (FTF).
ACKNOWLEDGMENTS
The authors would like to thank Indonesian Ministry of Education, Culture, Research, and Technology through Fundamental Research Grant (Regular) for funding this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by Indonesian Ministry of Education, Culture, Research, and Technology through a Fundamental Research Grant (Regular).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Melia S, Aritonang SN, Juliyarsi I, Kurnia YF, Rusdimansyah, Hernita VO. The Screening of Probiotic Lactic Acid Bacteria drom Honey of Stingless bee from Wesr Sumatra, Indonesia and Using as Starter Culture. Biodiversitas, 2022;23(12): 6379-6385.
- Seveline S. Kajian Pustaka Teknik Pengeringan Semprot (Spray Drying) Untuk Pengawetan Dan Produksi Probiotik. [Literature Review of Spray Drying Techniques for Probiotic Preservation and Production]. Jurnal Agroindustri Halal, 2018;3(1), 80-86.
Crossref - Bintsis T. Lactic Acid Bacteria: their applications in foods. J Bacteriol Mycol Open Access. 2018;6(2):89–94.
- Zapasnik A, Sokolowska B, Bryla M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods, 2022; 11(4):1283.
Crossref - Lawalata HJ, Rengkuan M, Satiman U. Antibacterial Activities of LAB from Langsat Fruit (Lansium domesticum) against Phatogenic Bacteria and Spoilage Bacteria, International Journal of Environment, Agriculture and Biotechnology, 2019; 4(6):1806-1810.
- Walean M, Rumondor R, Maliangkay HP, dan Melpin R. Effect of Pakoba Bark Ethanol Extract (Syzygium sp.) Against ethylene glycol-induced renal hystopalogy features of white rats. Chem. Prog. 2018;11 (1).
- Bawole KV, Umboh SD, dan, Tallei TE. Uji Ketahanan Bakteri Asam Laktat Hasil Fermentasi Kubis Merah ( Brassica oleracea L.) Pada pH 3 [Resistance Test of Lactic Acid Bacteria from Red Cabbage Fermentation ( Brassica oleracea L.) at pH 3]. Jurnal Mipa Unsrat, 2018;7(2):20-23.
Crossref - Lawalata HJ, Suriani W. Antagonistic activity of Pediococcus isolated from bakasang againts Pseudomonas fluorescens (producing-histamine bacteria). International Journal of Advanced Research in Biological Sciences, 2017;4(10):221-225.
- Sangkal A. Identification of Bioactive Compounds of Red Pakoba Fruit Ethanol Extract (Syzygium sp.) As an antidiabetic with peroral glucose tolerance test method. Chem Prog. 2021;14(2):108-115.
Crossref - Korcz E, Varga L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends in Food Science & Technology, 2021; 110: 375-384
- Ma’unatin A, Harijono H, Zubaidah E, Rifa’i M. The Isolation of Exopolysaccharide-producing Lactic Acid Bacteria from Lontar (Borassus flabellifer L.) sap. Iranian Hournal of Microbiology., 2020;12(5): 437-444.
- Korcz E, Ker´enyi Z, Varga L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food & Function, 2018; 9:3057–3068.
Crossref - Anindita NS. Identifikasi Glukosiltransferase (gtf) Penyandi Eksopolisakarida Pada Strain Weisella Confusa Probiotik Asal Air Susu Ibu (ASI) [Identification Glycosiltransferase (gtf) as Exopolysaccharides Producer in Strains Weisella confusa Probiotic from Human Breastmilk]. Jurnal Pangan dan Agroindustri, 2020;8(2):75-85.
Crossref - Guérin M, Da Silva CR, Garcia C, Remize F. Lactic Acid Bacterial Production of Exopolysaccharides from Fruit and Vegetables and Associated Benefits. Review. Fermentation, 2020;6(4): 115.
Crossref - Alfaruqi HQD, Anindita NS, Bimantara A. Kajian Molekuler Pada Probiotik Asal Air Susu Ibu Dalam Sintesis Eksopolisakarida (EPS) [Molecular Studies on Probiotic of Human Breast Milk in the Synthesis of Exopolysaccharide (EPS)]. Jurnal Bioteknologi & Biosains Indonesia. 2021;8(1): 114-122.
Crossref - Nurhasanah, Intan TF, Heri S, Suripto DY. Analysis of Exopolysaccharides From Lactic Acid Bacteria Results Of Colostrum Kefir Fermentation. Analytical and Environmental Chemistry, 2020;5(01):65-73.
Crossref - Putri AL, Kusdiyantini E. Isolasi dan identifikasi bakteri asam laktat dari pangan fermentasi berbasis ikan (Inasua) yang diperjualbelikan di Maluku-Indonesia [Isolation and Identification of Lactic Acid Bacteria from Fish-Based Fermented Food (Inasua) Traded in Maluku-Indonesia]. Jurnal Biologi Tropika. 2018;1(2):6-12.
Crossref - Detha A, Datta FU, Beribe E, Foeh N, Ndaong N. Karakteristik Bakteri Asam Laktat Yang Diisolasi Dari Susu Kuda Sumba. [Characteristics of Lactic Acid Bacteria Isolated from Sumba Horse Milk]. Jurnal Kajian Veteriner, 2019;7(1), 85-92.
Crossref - Nasution AY, Rasyidah, Mayasari U. Potensi Bakteri Asam Laktat Sebagai Penghasil Eksopolisakarida Dari Dekke Na Niura [The potential of lactic acid bacteria as producers of exopolysaccharides from Decke na Niura]. Jurnal Al-Azhar Indonesia Seri Sains Dan Teknologi. 2022;7(3):214-220.
Crossref - Lawalata HJ, Rungkat JA, Nangoy WMS, Tengker ACC, Gress NGH. Bacteriocin Activity of Lactic Acid Bacteria From Ripe Tome-Tome Fruit (Flacoutia inermis) Material. Advances in Science and Technology, 2023; 128: 157-162.
- Babiye B. Isolation and Identification of Bacteria From Fresh Fruit Juice Prepared in Cafeterias and Restaurants, Axum Town, Ethiopia, Biosciences Biotechnology Research Asia, 2017; 14(1): 307-313.
- Hasan NA, Zulkahar IM. Isolation and Identification of Bacteria from Spoiled Fruits. AIP Conference Prosidding; 2018.
Crossref - Herwin F, Ayyub HN. Isolation of cellulose-producing bacteria from fruits in Makassar Traditional Market. As-Syifaa Jurnal Farmasi, 2020;12(1):47-50.
Crossref - Sarker ARM, Mazedul HM, Rafia AR, Fateha AE, Ariful IM, Minara K. Isolation and identification of bacteria from fresh guava (Psidium guajava) sold at local markets in Mymensingh and their antibiogram profile. Veterinary World; 2018;11(8):1145-1149.
- Yanti NA, Ardiansyah, Yati K. Lactic Acid Bacteria from Sweet Arum Mango (Mangifera Indica L. Var. Arum Sweet). Jurnal Bionature, 2022;3(2):132-137.
Crossref - Agustina L, Oktirianti Y, dan Jumiyati. Identifikasi Total Bakteri Asam Laktat (BAL) pada Yoghurt dengan Variasi Sukrosa dan Susu Skim [Total Identification Of Laktat Acid Bacteria (BAL) in Yoghurt with Various Sukrosa and Skim Milk]. Journal of The World of Nutrition, 2018;1(2):1-5.
Crossref - Ayivi RD, Gyawali R, Krastanov A, et al. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy, 2020;1(3):202–232.
Crossref - Biswas B, Azad MAK, Absar N, Islam S, Amin S. Isolation and Identification of Pathogenic Bacteria from Fresh Fruits and Vegetables in Chittagong, Bangladesh. Journal of Microbiology Research, 2018;10(2):55-58.
- Giyatno DC, Retnaningrum E. Isolation and characterization of exopolysaccharide-producing lactic acid bacteria from cherry fruit (Muntingia calabura L.). Journal Sains Dasar, 9(2):42-49.
Crossref - Panthavee W, Noda M, Danshiitsoodol N, Kumagai T, Sugiyama M. Characterization of Exopolysaccharides Produced by Thermophilic Lactic Acid Bacteria Isolated from Tropical Fruits of Thailand. Biol Pharm. Bull. 2017; 40(5):621-629.
- Eko F, Lestari YNA, Susilo MT, Rachmawati L. Potensi Eksopolisakarida Bakteriasamlaktat Untuk Mencegah Danmengendalikan Diabetes Mellitus Tipe 2 (BAB III). Book Chapter Kesehatan Masyarakat Jilid 2.
Crossref - Klinchongkon K, Bunyakiat T, Khuwijitjaru P, Adachi S. Ethanol Precipitation of Mannoligosaccharides from Subcritical Water-Treated Coconut Meal Hydrolysate. Food and Bioprocess Technology. 2019;12:1197-1204.
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.