ISSN: 0973-7510
E-ISSN: 2581-690X
Kyllinga nemoralis also known as, whitehead spike sedge is a perennial herb which contains antiviral, antibacterial, antioxidant and anti-bleeding properties. This study was designed to determine the biological activity of K. nemoralis methanolic roots extract on herpes simplex virus type 1 (HSV-1) replication cycle. The effect on HSV-1 replication phases was observed by performing time-of-addition and time removal assays. Meanwhile, the level of HSV-1 selected genes expression was analysed by quantitative Real Time-Polymerase Chain Reaction (qRT-PCR). In the time addition assay, K. nemoralis extract anti-HSV-1 activity was found to be most optimum when given at 2 hpi (>45% plaque reduction). The time removal assay showed that >80% plaque reduction was achieved when treatment of K. nemoralis extract was given until 24 hpi. K. nemoralis extract suppressed immediate early, early and late phases of HSV-1 replication cycle by altering the expression of UL54, UL27 and UL30 genes during the infection. This study showed that K. nemoralis methanolic roots extract has potential as anti HSV-1 by reducing the expression of HSV-1 genes at different phases of viral replication.
Kyllinga nemoralis, Antiviral, Herpes Simplex Virus, Plaque Reduction Assay, Replication Cycle
Herpes Simplex Virus (HSV) is more commonly known as herpes. They are categorized in the Herpesviridae family. Nowadays, HSV infection cases have raised public health issues worldwide. The infection of HSV-1 is the most common compared to infection of HSV-2, and it has become an endemic throughout the world, and it is a highly contagious infection that can reach the brain and cause neurodegenerative disorders.1 HSV-1 has become the health problem as a new variant found recently. Non-cytopathic HSV-1 isolated from acyclovir-treated patients with recurrent infections.2 Acyclovir (ACV) had been considered the gold standard as an antiviral agent for both the prevention and treatment of HSV-1 infection. However, long-term usage of ACV has resulted in treatment resistance, especially in immunocompromised people, and it causes the side effects such as malaise, nausea, vomiting, rash and others.3 Therefore, K. nemoralis is considered to be used as a new antiviral agent against HSV-1 as it has been found to have the ability to treat a variety of disorders, including pathogenic fungal and bacterial infections.4,5 This plant contains higher flavonoids and phenolic, the active compound of antivirus agents that will inhibit viral replication.6 As no one has yet reported on the antiviral activity of K. nemoralis root methanolic extract against HSV-1, this will be the first study on the antiviral activity of K. nemoralis root methanolic extract against HSV-1 infection. Thus, it can help in developing new agents for the prevention and control of HSV-1 infection.
Natural plants are being used to treat diseases and disorders because they contain important medicinal properties or chemical sources. Based on a previous study, secondary metabolites such as tannin, flavonoid, saponins, cardiac glycosides, and terpenoids have been found in Cynometra cauliflora leaf extract that contributes to anti-HSV-1 properties. The methanolic extracts of C. cauliflora could protect cells before or after HSV-1 infection because they can work directly against HSV-1 virion particle that was carried out on Vero cells.7 Besides that, styrylpyrone derivatives from Goniothalamus sp. has shown potential activity against HSV-1 after the evaluation of the SPD in the antiviral test, in vivo, in vitro, and in silico studies was successful conducted. SPD shows a good pharmacokinetic profile by addressing on the exact binding site of the viral protein, SPD can prevent HSV-1 infection. in silico study discovered that SPD exhibited potent inhibitory activity against target viral proteins of HSV-1.8 Apart from that, Polygonum minus had been reviewed that it contains antioxidants, flavonoid and phenolic compounds, which essentially contributes to the antiviral property and possibly acts through the antioxidant potentials. However, the stem extract of P. minus showed mild antiviral activity with possible cell protective effect towards the virus infection or alteration towards virus particle.9 The purpose of this work was to determine the efficacy of K. nemoralis root methanol extract treatments on HSV-1 replication cycle.
Plant Material
K. nemoralis plants were collected, washed, and dried at 60°C. The plant authentication was performed by a competent botanist. Dried roots of K. nemoralis plants were cut into smaller segments and ground into powder. About 200g powder was soaked in 500 mL of methanol for three days. The extract was filtered and the solvent was evaporated under reduced pressure using a rotary vacuum evaporator. Finally, the extract was blow-dried to give 25 g of extract, and then stored in a refrigerator at 4°C until further use.10
Cells and Virus
African green monkey kidney cell (Vero) from American Type Culture Collection (ATCC) was used in this study. The cell line was propagated and maintained in DMEM supplemented with 10% fetal bovine serum (FBS) (Gibco, NY, USA). Vero cells were incubated at 37°C. Clinical strain of HSV-1 was propagated in Vero cells and harvested until cytophatic effects progressed. Virus stock titer was determined by plaque forming assay (PFA) and then aliquoted and stored at -80°C.
Time-of-Addition Assay
Confluent monolayers of Vero cells were infected with 50 PFU of HSV-1 for 2 h at 37°C. K. nemoralis root methanol extract (0.125 mg/mL) was added to the infected cells at 2, 4, 6, 8, and 10 hpi, followed by the addition of 1% methyl cellulose. Cells were then incubated at 37°C until 24 hpi.11 Plaques were stained with crystal violet solution (0.4%, w/v) and the percentage of plaques inhibition was calculated using the following formula:
The percentage of antiviral activity = [ Average of control plaque – Average of tested plaque / Average of control plaque ] × 100%
With, Average of control plaque = Non-treated infected cells
Average of tested plaque = K. nemoralis treated infected cell
Time Removal Assay
Confluent monolayers of Vero cells were infected with 50 PFU of HSV-1 for 2 h at 37°C. K. nemoralis root methanol extract (0.125 mg/mL) was added to the infected cells directly after treatment. The medium of plant extract was discarded at 2, 4, 6, 8, 16, 18, 20, 22 and 24 hpi. Upon removal of plant extract, cells were overlaid with 400 µL of 1% MCS medium and 2% DMEM. The cells were incubated at 37°C in 5% CO2 for 48 hours. Following the incubation period, cells were stained with 500 µL of crystal violet solution and incubated at room temperature for 30 minutes.12 The plaques were counted and percentage of plaque inhibition (%) was determined using the following formula:
The percentage of antiviral activity = [ Average of control plaque – Average of tested plaque / Average of control plaque ] × 100%
With, Average of control plaque = Non-treated infected cells
Average of tested plaque = K. nemoralis treated infected cell
qRT-PCR
HSV-1 RNA was harvested from the HSV-1 infected Vero cells. Viral RNA was extracted using RNA extraction kits (GENEzol TM TriRNA kit). The real-time RT-PCR assay was carried out by adding 3 μL of extracted HSV-1 RNA to the qPCRBIO SyGreen mixture (qPCRBio SyGreen One-Step Go Hi-ROX) which consisted of 2X qPCRBIO SyGreen 1-Step Mix (10 μL), RNAse Inhibitor (20 units), ddH2O (4.4 μL), forward and reverse primers (0.8 mL). The specific primers for qRT-PCR are listed in Table, which represented all three phases of HSV-1 life cycle: Immediate early phase (UL54), early phase (UL30), late phase (UL27); and housekeeping genes (RPL- 32). The amplification was carried out using the Step One Plus Real-time PCR System (Applied Biosystems) with the following thermal cycling conditions: reverse transcription at 45°C for 10 minutes, polymerase enzyme activation at 95°C for 2 minutes followed by 40 cycles of denaturation at 95°C for 5 seconds and annealing at 65°C for 30 seconds followed by a final melt curve analysis using instrument default settings.
Table :
List of forward and reverse primers.
Gene |
Forward Primer (5’ to 3’) |
Reverse Primer (3’ to 5’) |
|---|---|---|
UL54 |
GAC GGG TCT CCT GGG AAA C |
ATA ATG GGG TCC TGG GGG C |
UL30 |
CGC CCC GCT CTG TTT TAC |
CCA GCC GAA GGT GAC GAA C |
UL27 |
CGG TGG TTC GTC GTA TGG G |
GGC GGC GTT GGG TTT TTC |
RPL-32 |
AAC ATT CCA TCT CCT CCT CGG |
TTG ACA TAC CGG TCT GAC TGG TGC |
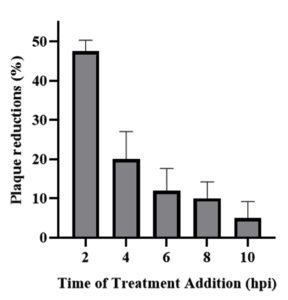
Time dependent studies were carried out to determine the impact of postponed treatment and also the impact of dissimilar time exposure to K. nemoralis root methanol extract against its HSV-1 antiviral activity. In the time addition study, K. nemoralis root methanol extract treatment was detected to be most effectual when added at 2 hpi (Figure 1). More than 40% reduction was detected when K. nemoralis root methanol extract was administered at 2 hpi and the reduction percentage gradually dropped at later time points.
Figure 1. Time addition assay. Activity of K. nemoralis root methanol extract when treatment was added at different time after infection
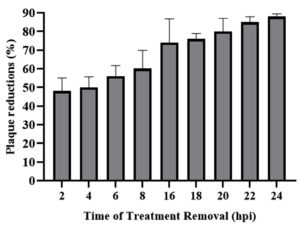
Time removal assay was done to determine the minimum time of K. nemoralis root methanol extract treatment that confers optimum anti-HSV-1 activity. Treatment of K. nemoralis root methanol extract began at 2 hpi and removal of K. nemoralis root methanol extract continued until 24 hpi. Results showed that low plaque reduction was detected as early as 2 hpi (Figure 2). 50% plaque reduction was detected as early as 4 hpi and plaque reduction reached its peak after exposure of 15 hpi.
Figure 2. Time removal assay. Treatment was removed at designated time point to evaluate the effect of different time of exposure of K. nemoralis root methanol extract towards anti-HSV-1 activity of the extract
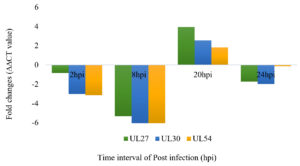
Figure 3. shows the expression levels of the virus gene relative to the expression in non-treated samples at four different time points within 24 hours of replication in Vero cells
Figure 3. The expression levels of the virus gene (UL27, UL30 And UL54) relative to the expression in non-treated samples at four different time points within 24 hours replication in Vero cells. During the immediate-early phase of virus replication (2 hpi), K. nemoralis methanolic extract was downregulated of the UL27 gene transcription due to the adjustment and interruption of cell receptor and viral glycoproteins during attachment and entry of the virus. During the early phase, 8 h, all three genes, including UL27, UL30 and UL54 were downregulated by the K. nemoralis methanolic. All genes were not affected by the K. nemoralis treatments in late phase (20 hpi) as these genes were transcribed and not affected by treatment at the late phase. At 20 hpi, all three genes were up-regulated, and which are estimated that the viral progeny was released. However, all genes were downregulated at 24 hpi.
Plant-based or herbal medicine has been used traditionally to treat various infections, including those caused by viruses. Recently, styrylpyrone derivative extracted from Goniothalamus umbrosus was shown to exhibit antiviral activity against herpes simplex virus13 and dengue virus.14 In this study, the potential use of this plant to inhibit in vitro HSV-1 replication was investigated. The time addition assay was carried out to observe the effect of K. nemoralis methanolic extract when added at different times, beginning from the time of infection and at 2 h intervals until 10 hpi. The percentages of plaque inhibition were started to decrease when both parts of K. nemoralis methanolic extract were added from 2 hpi until 10 hpi because the K. nemoralis methanolic extract prevented the attachment and penetration of HSV-1 that was present in the early stage of the viral replication cycle of HSV-1. About 47.5% of virus plaque reduction occurs at 2 hpi and starts to decrease to 5% at 10 hpi after added the treatment of the root of K. nemoralis methanolic extract at 2 h intervals (Figure 1). Since SPD optimal activity is when added at 2 hpi, the most probable target for K. nemoralis methanolic extract is by inhibiting one of the stages in the viral early replication cycle. HSV-1 immediate early protein (IE) consists of 5 proteins i.e ICP0, ICP4, ICP22, ICP27 and ICP47.15 Immediate early protein ICP27 for example was expressed as early as 2 hpi and production will be at its peak at 3-4 hpi.16 Subsequently, early HSV-1 proteins will be at a peak after 4 hpi until 7 hpi.17 The anti-HSV-1 activity of K. nemoralis methanolic extract was at its peak, within the same time frame of these immediate early/early genes, and antiviral efficiency dropped when administered at the later stage of infection. Our evidences so far substantially support the thought of these immediate early/early proteins as targeted proteins by K. nemoralis methanolic extract.
The time removal assay was carried out to determine the plaque inhibition from the early stage of viral replication, 2 hpi, to the late phase of viral replication, 24 hpi. The percentages of plaque inhibition started to increase from 2 hpi because the K. nemoralis methanolic extract inhibited the attachment, penetration and early stage of viral replication. About 48% of virus plaque reduction occurs at 2 hpi and start to increase to 88% at 24 hpi after removed the treatment of the root of K. nemoralis methanolic extract at 2 hpi intervals (Figure 2). However, the treatment able to affect at the late phase of viral replication, 24 hpi as the progeny was released from the infected cells.
Based on the chronological order of gene transcription and translation, the HSV-1 gene can be separated into immediate early genes, early genes, and late genes during virus replication. There were three expressions of genes involved, including UL27 (immediate early gene), UL30 (early gene), UL54 (late gene), and the housekeeping gene, Ribosomal Protein L32 (RPL32). UL27 is an important gene that encodes Glycoprotein B for virus entry.18 Glycoprotein B is one of the essential viral proteins necessary for the attachment and entry of the virus into the cell.19 UL30 is an essential gene that codes for DNA polymerase and involves viral replication. Besides that, the UL54 gene affects the functions of other early and late genes, including gC for viral attachment.20 It encodes DNA polymerase catalytic enzyme that important proteins during the viral lytic cycle and increasing the transcription of the late genes.21,22
Based on the findings, at the immediate early replication phase (2 hpi), the K. nemoralis methanolic extract caused all three genes to be down-regulated because of the alteration and disordering of cell receptors and viral glycoproteins during virus attachment and entry. The down-regulation of UL27 genes causes inhibition of gB expression that is responsible for viral attachment and entry. According to the finding, as for UL54 gene affects the gC expression, this K. nemoralis methanolic extract causes the down-regulation of UL54 gene. This effect will inhibit gC essential for viral attachment. This indicates that the K. nemoralis methanolic extract targeted the UL27 and UL54 genes to prevent HSV-1 attachment and penetration into the cells.
At the early replication phase (8 hpi), the K. nemoralis methanolic extract was down-regulated to all genes. This extract will inhibit the expression of the UL30 gene that is essential during DNA replication, and its action occurs in the nucleus and is associated with the transcription of UL54. During the late replication phase (20 hpi and 24 hpi), all the genes were up-regulated according to the genes that were transcribed and not to be influenced by the treatment at 20 hpi. However, all genes were down-regulated at 24 hpi even in this phase, when the virus progeny was expected to be released. Overall findings in the RT-qPCR analysis showed that the transcription level of these genes in infected cells treated with K. nemoralis root methanol extract was decreased compared to infected cells without treatment. This is parallel to the results, which are the percentage of plaque reduction was increased at early replication (2 hpi) of time addition assay and increased at late replication (24 hpi) of time removal assay.
Based on the findings, it is suggested that further studies on the phytochemical composition in each part of the K. nemoralis plant should be done to identify other phytochemical components that can be a source of new antiviral agents. In addition, it is suggested that in vivo studies also need to be done to evaluate the safety, toxicity and efficacy of K. nemoralis in animal models before it can be used to treat human infections.
The methanol extract of K. nemoralis root has the potential to modify HSV-1 replication cycle at almost all stages. It is proposed that K. nemoralis root methanol extract be further appraised in in vivo for its therapeutic capability as an anti-HSV agent.
ACKNOWLEDGMENTS
The authors would like to thank the University Sultan Zainal Abidin, Malaysia for the facilities and laboratory instruments.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was funded by Universiti Sultan Zainal Abidin, Malaysia with research grant number UniSZA/2020/LABMAT/06.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Laval K, Enquist LW. The potential role of herpes simplex virus type 1 and neuroinflammation in the pathogenesis of Alzheimer’s disease. Front Neurol. 2021;12:658695.
Crossref - Roy S, Sukla S, De A, Biswas S. Non-cytopathic herpes simplex virus type-1 isolated from acyclovir-treated patients with recurrent infections. Sci Rep. 2022;12(1):1345.
Crossref - Bacon TH, Levin MJ, Leary JJ, Sarisky RT, Sutton D. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin Microbiol Rev. 2003;16(1):114-128.
Crossref - Raju S, Kavimani S, Uma MV, Sreeramulu RK. Kyllinga nemoralis (Hutch & Dalz) (Cyperaceae): Ethnobotany, Phytochemistry and Pharmacology. Pharmacogn J. 2011;3(24),7-10.
Crossref - Abd Wahab NZ, Abd Rahman AHA. Phytochemical analysis and antibacterial activities of Kyllinga nemoralis extracts against the growth of some pathogenic bacteria. J Pure Appl Microbiol. 2022;16(4):2568-2575.
Crossref - Sindhu T, Rajamanikandan S, Srinivasan P. In vitro antioxidant and antibacterial activities of methanol extract of Kyllinga nemoralis. Indian J Pharm Sci. 2014;76(2):170-174.
- Abd Wahab NZ, Azizul A, Badya N, Ibrahim N. Antiviral activity of an extract from leaves of the tropical plant Cynometra cauliflora. Pharmacogn J. 2021;13(3):752-757.
Crossref - Fouzi SS, Abd Wahab NZ, Yan LC, Ibrahim N. Styrylpyrone derivative from Goniothalamus sp.: a powerful drug for fighting against herpes simplex virus type 1. Pharmacogn J. 2021;13(6)Suppl:1598-1606.
Crossref - Abd Wahab NZ, Bunawan H, Ibrahim N. Cytotoxicity and antiviral activity of methanol extract from Polygonum minus. AIP Conference Proceedings. 2015;1678:030036.
Crossref - Abd Wahab NZ, Azizul A, Muhamad N, Ibrahim N. Cytotoxic and antiviral activities of Clinacanthus nutans crude extract. Malays J Chem. 2021;23(4):131-136.
- Cheng H, Yang CM, Lin TC, Shieh DE, Lin CC. ent-Epiafzelechin-(4a–>8)-epiafzelechin extracted from Cassia javanica inhibits herpes simplex virus type 2 replication. J Med Microbiol. 2006;55(1):201-206.
Crossref - Zhen H, Fang F, Ye DY, et al. Experimental study on the action of allitridin against human cytomegalovirus in vitro: Inhibitory effects on immediate-early genes. Antivir Res. 2006;72(1):68-74.
Crossref - Ibrahim N, Shahar S, Abd Wahab NZ, Md. Nor NS. Effect of styrylpyrone derivative (SPD) and SPD/foscarnet combination towards virus infected cell. AIP Conference Proceedings. 2019;2111, 040002.
Crossref - Abd Wahab NZ, Ibrahim N. Styrylpyrone derivative (SPD) extracted from Goniothalamus umbrosus binds to dengue virus serotype-2 envelope protein and inhibits early stage of virus replication. Molecules. 2022;27(14),4566.
Crossref - Lilley CE, Groutsi F, Han Z, et al. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J Virol. 2001;75(9):4343-4356.
Crossref - Guan X, Zhang M, Fu M, Luo S, Hu Q. Herpes simplex virus type 2 immediate early protein ICP27 inhibits IFN-β production in mucosal epithelial cells by antagonizing IRF3 activation. Front Immunol. 2019;10:290.
Crossref - Isler JA, Schaffer PA. Phosphorylation of the herpes simplex virus type 1 origin binding protein. J Virol. 2001;75(2),628-637.
Crossref - Laing KJ, Magaret AS, Mueller DE, et al. Diversity in CD8+ T cell function and epitope breadth among persons with genital herpes. J Clin Immunol. 2010;30,703-722.
Crossref - Spear PG, Longnecker R. Herpes virus entry: An update. J Virol. 2003;77:10179-10185.
Crossref - Csabai Z, Takacs IF, Snyder M, Boldogkoi Z, Tombacz D. Evaluation of the impact of ul54 gene-deletion on the global transcription and DNA replication of pseudorabies virus. Arch Virol. 2017;162(9):2679-2694.
Crossref - Zarrouk K, Piret J, Boivin G. Herpesvirus DNA polymerases: structures, functions and inhibitors. Virus Res. 2017;234,177-192.
Crossref - Harkness JM, Kader M, DeLuca NA. Transcription of the herpes simplex virus 1 genome during productive and quiescent infection of neuronal and nonneuronal cells. J Virol. 2014;88(12),6847-6861.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.