Mansi Srivastava¹, Sushma Ahlawat² and Manoj Kumawat3
Department of Biochemistry and Biochemical Engineering, SamHigginbottom
Institute of Agriculture, Technology and Sciences, Allahabad, India.
ABSTRACT
Extraction, isolation, and purification of an antimicrobial peptide was carried out from the flowers of Catharanthus roseus (white flower) and (pink flower) varieties and their antimicrobial activity was monitored against bacteria Escherichia coli( Gram-negative)and Staphylococcus aureus (Gram-positive).Cathranthusroseus is well known for its medicinal properties. This study illustrates the antibacterial property of this plant against pathogenic and opportunistic organisms like Escherichia coli and Staphylococcus aureus.The results showed that the Catharanthusroseus flowers (white)and (pink) purified extracts have antibacterial activity against Escherichia coli and Staphylococcusaureus. Therefore providing protection to the flowers against pathogen invasion. This study also shows that an antibacterial compound has been isolated and detected.
Keywords: Catharanthus roseus; Peptide; Antimicrobial; Purification.
INTRODUCION
Living organisms undergo a constant threat of infection from different microbes but the living organisms are not defenseless against these various microbes, they produce a wide variety of molecules which protect them from various types of infections. One such molecule is antimicrobial peptide. The antimicrobial peptides are quite ubiquitous among all the eukaryotes including mammals, insects, and plants (Gabay, 1994). These act as the first line of defense against various microbes. Plants also produce these antimicrobial peptides against various infections. Catharanthus roseus is an important medicinal plant of family Apocynaceae. It is cultivated mainly for its alkaloids, which are having anticancer effects (Jaleel et al., 2009).Catharanthus roseus, a perennial tropical tree-plant of associates Apocynaceae, produces a high number of monoterpenoidindole alkaloids of which two dimeric alkaloids, vincristine, and vinblastine are clinically useful oncolytic drugs (Cordell 1980). The antibacterial potential in crude extracts of different parts (viz., leaves, stem, root, and flower) of Catharanthus roseus against clinically significant bacterial strains reported. (Raza, et al.,2009). Of these defensins were first isolated from wheat and barley. They have structural and functional similarities with insect and mammalian defensins. Plant defensins are able to inhibit a wide variety of bacterial species, especially those that are phytopathogenic and these molecules contribute greatly to the innate immunity of plants (Thomma et al., 2002) and (Lay et al., 2005).Global attention has been shifted towards finding supplementary chemicals, specific to function as future drugs. These natural products can manage to pay for unique elements of molecular diversity and biological functionality, which is vital for novel drug discovery. In this paper, the isolation and biological properties of a flower derived defensin from Catharanthus roseus (white flower) and (pink flower) have been described.
MATERIALS AND METHODS
Biological materials
The plants were purchased from the nursery of Sam Higginbottom Institute of Agriculture, Technology and Sciences, (ICAR), Allahabad, India. The bacterial strains were provided by Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology, IMTECH, Chandigarh, India and the monitoring of the antimicrobial activity of the Catharanthus roseus(white flower) and (pink flower) extracts was carried out.
Extraction of floral defensins
Defensins were extracted from flowers of Catharanthus roseus(white flower) and (pink flower) by using a modification of the procedure for extraction of thionins from barley (Hordeumvulgare) flour (Ozaki et al., 1980) and further modification of the procedure used for isolation of floral defensins from ornamental tobacco and petunia(Lay et al., 2003). Whole of Catharanthus roseus(white flower) and (pink flowers)flowers up to petal colouration stage of flower development were grounded to a fine powder with liquid nitrogen by using a mortar and pestle and were further processed in a homogenizer in 50mM sulphuric acid. After stirring for 1 hr at 4°C, the insoluble material was removed by filtration through Whatman No. 4 filter paper, followed by centrifugation (12,000 rpm, 15 min, 4°C).The slurry was adjusted to pH 7.8 by slow addition of 10 M NaOH and was stirred for 1 h at 4°C before removal of precipitated material by centrifugation (12,000 rpm, 15 min 4°C). Solid ammonium sulphate 80 % (w/v) saturation was added and the mixture was stirred for 3 to 4 h at 4°C to precipitate the defensin protein. The precipitate was collected and was dissolved in 50 ml of gel filtration buffer (150mM KCl and 10 mMTris-HCl, pH 8.0) before heating at 90°C for 10 minutes. The supernatant was loaded onto a glass column containing Sephadex G-50 (Sigma) media and gel filtration chromatography was carried out.
Fractions with highest antibacterial activity were selected and were purified by Reverse Phase (RP-HPLC), (Waters) on a C18silica column using a pump (model 515, Waters) and photodiode array detector (UV:Waters). Samples were eluted with a linear gradient of 0.1 % (v/v) trifluoroacetic acid to 60% (v/v) acetonitrile for 20 min at a flow rate of 2 ml/min. The absorbance was measured till 700nm.
Antimicrobial screening
The purified protein extracts of Catharanthus roseus(white flower) and (pink flower)were screened against two bacterial strains. The test organisms were E. coli (MTCC 1687) and S.aureus (MTCC 7443).
Preparation of inoculums
Slopes of nutrient agar were prepared in which stock culture was maintained at 4p C.The active cultures of bacteria were prepared for experiments by transferring a loopful of bacteria from stock cultures to test tubes of Mueller-Hinton Broth (MHB). A turbidity standard for inoculum preparation was used, a BaSO4turbidity standard, equivalent to a 0.5 McFarland standard.
Antimicrobial Susceptibility test
Kirby-Bauer disc diffusion method was used to screen the antimicrobial activity of the peptide extracts. In this test the antimicrobial activity was screened by using Mueller Hinton Agar (MHA) as media, obtained from (Himedia, Mumbai).The MHA plates were prepared by pouring 15 ml of molten media into the sterile petri plates. The plates were allowed to solidify for 5 minutes and inoculum suspension was swabbed uniformly and the suspension was allowed to dry for 5 minutes. Sterile discs (6mm) were loaded with 50 µl of peptide extract. The loaded disc was placed on the surface of media and the compound was allowed to diffuse for 5 minutes and then the plates were kept for incubation at 37°C for 24 hrs. At the end of incubation, inhibition zones were formed around the disc, they were measured with a transparent ruler (Himedia) in millimeter (Goyalet al., 2008).
RESULTS AND DISCUSSION
As can be seen from the literature survey that this plant has been mostly studied once idolize to its antimicrobial properties and its anti-fungal properties. The protein was extracted from flowers of Catharanthus roseus(white flower) and (pink flower)in 50mM sulphuric acid and purified using ammonium sulphate precipitation, heat treatment, and gel filtration. Proteins in the fractions were resolved further by Reverse Phase RP-HPLC. The highest peak was identified as a floral defensin(Fig. 1 and 2) for Catharanthus roseus (white flower) and (Fig. 3 and 4) for Catharanthus roseus (pink flower).The highest peak was observed at a retention time of2.425minutes for Catharanthus roseus (white flower) extract.The highest peak was observed at a retention time of 2.478 minutes for Catharanthus roseus (pink flower) extract.
observed at a retention time of 2.478 minutes for Catharanthusroseus (pink flower) extract.
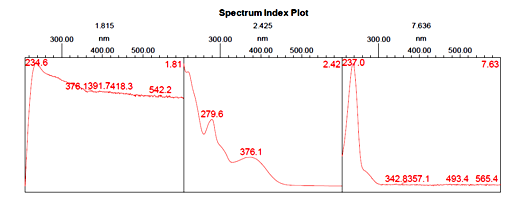
Fig. 1: Spectrum Index Plot of Catharanthusroseus (white flower) extract.
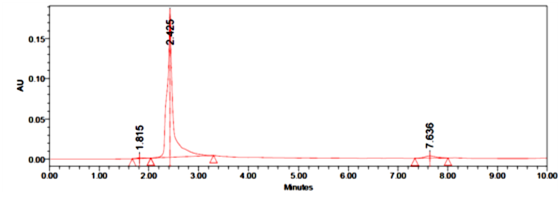
Fig. 2: Retention time in minutes of Catharanthusroseus (white flower) extract.
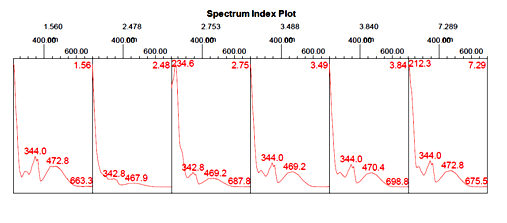
Fig.3: Spectrum Index Plot of Catharantusroseus(pink flower) extract.
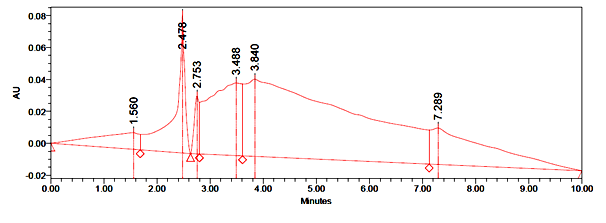
Fig. 4: Retention time in minutes of Catharantusroseus (pink flower) extract.
Antimicrobial activity of the purified extracts
The purified peptide extracts of Catharanthus roseus (white flower) showed a zone of clearance against both the bacteria E.coli and S.aureus. A zone of clearance of 11 mm was observed against E.coliand a zone of clearance of 12 mm was observed against S.aureus(Fig. 5A,B and C) respectively.
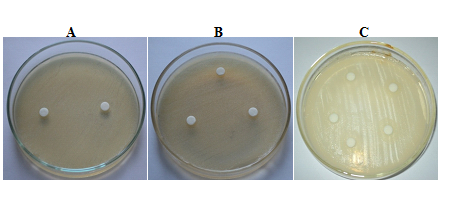
Fig. 5: Antimicrobial activity of Catharanthusroseus (white flower) extract against E.coli.
The purified peptide extracts of Catharanthus roseus(pink flower) showed the marked zone of clearance against both the bacteria E.coli and S.aureus. A zone of clearance of 12mm was observed against E.coliand a zone of clearance of 14mm was observed against S.aureus(Fig.6A, B and C) respectively.Earlier, reports on antimicrobial effect of its plant parts against Vibrio cholerae and Mycobacterium pyrogeneaus (Virmaniet al.,1978). Chopra and coauhersreported the same against spinach mosaic virus in their in vitrostudies (Chopra et al., 1980).
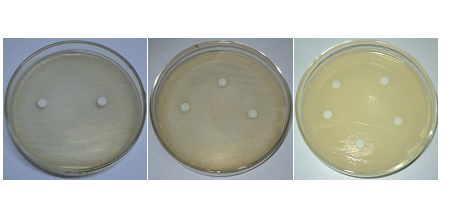
Fig.6 The antimicrobial activity of Cathranthusroseus (pink flower) extracts against E.coli.
Conclusion
This observation has lead towards the isolation of an antimicrobial peptide and the detection of the antimicrobial activity of this compound from the flowers of Catharanthus roseus(white flower) and (pink flower). The extracted compound shows significant antimicrobial activity. This is a promising compound which can have various applications in agriculture as well as medicine. Plants prove to be a significant source of antimicrobial compounds of peptide nature. Further investigation is required for screening of this compound in other parts of the plant like seeds and leaves.
Acknowledgements
The authors express their gratitude to Dr.Ashokkumar (Principal Scientist), Central Institute for Research on Goats (CIRG), Mathura, India for lending his support and providing facilities for above study. Authors also thank Sam Higginbottom Institute of Agriculture, Technology, and Sciences, Allahabad, India for the support of this study.
References
- Gabay, J.E., Ubiquitous natural antibiotics. Science, 1994; 264(5157): 373-374.
- Thomma, B.P., Cammue, B.P. and Thevissen, K., Plant defensins.Planta, 2002; 216(2): pp.193-202.
- Lay, F.T. and Anderson, M.A., Defensins-components of the innate immune system in plants. Current Protein and Peptide Science, 2005; 6(1): 85-101.
- Ozaki, Y., Keishiro, w.a.d.a., matsubara, h., nakanishi, t. and yoshizumi, h., Amino acid sequence of a purothionin homolog from barley flour. Journal of biochemistry, 1980; 87(2): 549-555.
- Lay, F.T., Brugliera, F. and Anderson, M.A., Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiology, 2003; 131(3): 1283-1293.
- Cordell, G.A., Information: Abstracts of International Symposium on Recent Advances in Natural Products Research; The Botanical, chemical, Biosynthetic and Pharmacologic Aspects of Catharanthus roseus (L). G. Don (Apocynaceae). 1980; 11(1): 48-49.
- Jaleel, C.A., Gopi, R. and Panneerselvam, R., Alterations in non-enzymatic antioxidant components of Catharanthus roseus exposed to paclobutrazol, gibberellic acid and Pseudomonas fluorescens. Plant Omics, 2009; 2(1): 30.
- Goyal, P., Khanna, A., Chauhan, A., Chauhan, G. and Kaushik, P., In vitro evaluation of crude extracts of Catharanthus roseus for potential antibacterial activity. International Journal of Green Pharmacy (IJGP), 2008; 2(3).
- Virmani, O.P., Srivastava, G.N. and Singh, P., Catharanthus roseusthe tropical periwinkle. Indian Drugs,1978.
- Chopra RN, Badhawar RL, Ghosh S., Poisonous Plants of India. Kolkata: Govt of India Press, 1980.

