Bulti Nayak1, Satarupa Roy1, Abhijit Mitra2 and Madhumita Roy1*
1Department of Biotechnology; Techno India University; Sector V; Salt Lake; Kolkata-91, India.
2Department of Marine Science. University of Calcutta, 35, B.C road; Kolkata-19, India.
ABSTRACT
During the screening of heavy metal resistant plant growth promoting rhizobacteria of the mangrove associate species Ipomoea pes-caprae of Indian Sundarbans, one multiple heavy metal resistant bacterium was isolated. The strain was identified as Stenotrophomonas maltophila strain BN1at 16S rDNA level. The strain was found to be resistant to 11 different antibiotics including ampicillin, streptomycin, cefuroxime, netillin, penicillin, gentamycin, nitrofurantoin, chloramphenicol, amikacin, amoxyclav, imipenem and could tolerate multiple heavy metals. The scanning electron micrography revealed small rod shaped bacteria and the cell surface forms microspheres. This bacterium was not only positive for various plant growth promoting features like IAA, siderophore and ammonia production but also showed phosphate solubilization and protease production ability. Therefore, dual expressions of antibiotics and heavy-metal resistance along with the plant growth promoting properties would make the isolate Stenotrophomonas maltophila strain BN1 ideal candidate strain for bioremediation of heavy metal contaminated sites effected with salinity.
Keywords: Ipomoea pes-caprae; Stenotrophomonas maltophila; heavy metal resistant,multiple drug resistant, protease, plant growth promoting rhizobacteria (PGPR).
INTRODUCTION
Ipomoea pes-caprae or goat’s foot (named after its bi-lobed leaf structure) or beach morning glory, is a pantropical, perennial, coastal sand dune plant and belongs to the family Convolvulaceae. It has been studied widely due to its ecological importance for soil and sand binding and preventing soil erosion and protecting sand dunes of beaches against storms, tidal and wave actions (Devall et al. 1992) and possession of various medicinal properties like inflammation and pain (Bragadeeswaran et al. 2010; Souza et al. 2000). It is one of the most widely distributed beach plants throughout tropical and subtropical areas around the world successfully occupying the supra littoral regions of sandy beaches forming large mats that assist in stabilizing sands. In India, it can be found in the east coast and west coast and in the Indian Sundarbans delta region, located at the Bay of Bengal. Mangroves and mangrove associates are responsible for protecting and shielding of coastal environment against tidal actions, storms, cyclones etc. The highly dynamic and productive microbial community inhabiting the halophytic vegetation is involved in various ecosystem services like photosynthesis, nitrogen fixation, methanogenesis, production of antibiotics and enzymes and transformation of nutrients from detritus rich mangrove vegetation into the sources of nitrogen, phosphorus etc. In recent times, Indian Sundarbans mangrove ecosystem is facing a major threat from heavy metal contamination. Due to its close location with metropolis Kolkata it is receiving increased quantity of agricultural runoff, domestic sewage, clinical and biomedical wastes, and industrial effluent through many channels of Hooghly. Different industries (tanneries, jute mills, pulp and paper mills, pesticide plants, thermal power plants, fertilizer, soap factories) located on two sides of river Hoogly and the oil refineries of Haldia are contributing to the trace metal load (Gopal and Chauhan 2006). These industries are considered to be the major sources of metals like Cu, Zn, Cr, Co, Ni, Pb, Cd, Fe, and Mn to the system. During the investigation of heavy metal resistant plant growth promoting rhizomicrobes from rhizosphere of Ipomoea pescaprae, one multiple drug resistant Stenotrophomonas maltophila strain BN1 was isolated. The present study characterized the halotolerant strain Stenotrophomonas maltophilia in terms of its multiple heavy metal tolerance, multiple antibiotic tolerance and plant growth promoting properties. Although S.maltophila is a well known rhizobacteria (Ryan et al. 2009; Mukherjee and Roy 2016) but its occurrence as rhizobacteria of a halophyte from the highly saline environment of Sundarbans has been reported here for the first time.
MATERIALS AND METHODS
Sample collection
Rhizosphere soil sample was collected from 6-8cm depth under the root surface of Ipomoea pes-caprae located at Bonnie camp of Sundarbans ((21o49′ 47.87″ N, 88o 37′ 22.33″ E). The collected sample was aseptically transferred into the laboratory by using sterile polyethylene bag and stored at 4ºC until use.
Isolation of the strain
For isolation of heavy metal resistant rhizobacteria, serial dilution of the rhizosphere soil sample was done in metal amended nutrient agar plate. 5 gram rhizosphere soil sample was added and mixed well into 90 ml of distilled water. Bacteria were isolated from the mixture using the spread plate technique on nutrient agar plates supplemented with 50 mgl-1 concentration of each metal (Zn, Cu, As (V), Cr, Fe, Pb, Mn). Plates were incubated at 300C for 48 h and the colonies were transferred to the higher metal concentration. Growing colonies differed in morphological characteristics were selected and streak plated containing increasing concentration of metal. After few rounds of plating, finally one bacterium was finally selected for its highest tolerance towards all the metals tested.
Table 1: Results of biochemical characterization of Stenotrophomonas maltophilia strain BN1
Biochemical tests |
Result |
Pigmentation |
Yellowish white and transparent colonies |
Colony morphology |
Watery, muciloaginous yellow, button shaped |
Spores, capsules |
Negative |
Gram reaction, cell shape |
Gram negative rods |
Catalase test |
Positive |
Starch hydrolysis |
Negative |
Methyl red |
Negative |
Aerobic |
Positive |
Urease |
Negative |
Triple sugar iron test |
Positive |
Citrate test |
Negative |
Indole production |
Negative |
Catalase test |
Positive |
Motility |
Positive |
Growth at 30ºC |
Positive |
Growth at 4ºC |
Negative |
Hydrolysis of gelatin |
Positive |
NaCl range (>5%) |
Negative |
MR-VP test |
Negative |
Identification of the strain
The strain was identified by morphological, biochemical and molecular methods. Morphological study was done by gram staining. Various biochemical assays were done by routine methods as present in Bergey’s manual for systematic bacteriology (Krieg and Holt 1984). For molecular characterization the 16S rRNA gene from the strain was amplified using universal primers (27F; 5-AGA GTT TGA TCC TGG CTC AG-3 and 1492R; 5-GGT TAC CTT GTT ACG ACT T-3). The PCR mixture contained 1 ul template, 2.5 ul of 10 X Taq DNA polymerase buffer, 5 mM MgCl2, 1 ul of dNTP at 2.5 mM, 0.5 ul of 2.5 unit Taq DNA polymerase, 3.75pmol of each primers, and 0.5 ul of 2.5 unit Taq polymerase. The cycling conditions were, initial denaturation at 94 0C for 5 min, followed by 30 cycles of 94 0C for 30 sec, 54 0C for 30 sec, and 72 0C for 1.5 min, followed by a final extension performed at 72 0C for 5 min. The PCR products were purified and sequenced by the SciGenome lab. The sequences were compared with known sequences in the Gene Bank nucleotide database and identified as the nearest phylogenetic neighbor with the highest similarity. Based on maximum identity score first ten sequences were aligned using multiple alignment software program Clustal W. The phylogenetic tree was constructed using MEGA7 (Tamura et al. 2007). The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei 1987). The evolutionary distances were computed using the Kimura 2-parameter method (Kimura 1980) and are in the units of the number of base substitutions per site. The rate variation among sites was modeled with a gamma distribution (shape parameter = 1).
Determination of minimum inhibitory concentration of heavy metals
Minimum inhibitory concentration or MIC is known as the lowest concentration of metal salts at which no CFU is observed (Mergeay et al. 1985). Appropriate dilutions were plated in LB agar (Hi Media, India) supplemented with increasing concentrations of heavy metals (copper as CuSO4, 5H2O (0-25mM); cadmium as CdCl2,5H2O (0-5 mmol); zinc as ZnSO4, 6H2O (0 -25mM); cobalt as Co(NO3)2,6H2O (2-15mM); lead as Pb(NO3)2 (0-15mM), NaAsO2 (5-30mM), chromium as K2Cr2O7 (2-15mM). The plates were incubated at 30ºC for 24-48 hours to observe bacterial growth. MIC values were measured individually in test tubes and LA plates. Tubes/plates were incubated at 300C and observations for growth/colonies were monitored for 4 days. The optical density of the cultures, as a measure of microbial growth, was detected at a wavelength of 600 nm by an UV-visible spectrophotometer (Systronics). A blank with the sole medium culture, without bacteria, was kept as control. Experiments were carried out in triplicates. To determine the MTCs (maximal tolerated concentration) for the above mentioned heavy metals, bacteria were grown on 10 ml of LB broth in the presence of different concentrations of different metals, ZnCl2, CuSO4, Pb(NO3)2, CdSO4, Co(No3)2, NaAsO2 and K2Cr2O7 at 300C under shaking. The MTCs corresponded to the highest concentration of each metal at which growth was still observed (Page‘s et al. 2007). Experiments were performed in triplicate for each condition.
Antibiotics resistance assay
The antibiotics susceptibility testing of the strain was done in Mueller Hinton agar by agar disc diffusion technique (Bauer et al. 1966). Hexa G antibiotics discs (HIMEDIA), vancomycin and rifampicin discs were used. Table 2 shows the composition and concentration of individual antibiotics in the disks. MH agar plates were poured with the fresh 24 hours culture (2ml culture in 100 ml of sterile MH agar) and discs were placed with the help of the sterile forcep on top of the agar after solidification. After 24 hours, zone of inhibition was measured for each antibiotics disc. The isolate was classified as sensitive or resistant according to the diameter of inhibition zone given in standard antibiotic disc chart published by CLSI (formerly NCCLS).
Table 2. Antibiotic resistance profile of S.maltophila strain BN1
| Hexa G-plus 6 HX022 | |||
| Disk potency (µg) | Zone of inhibition
(mm) |
Susceptible (S)/
Intermediate (I)/ Resistant (R) |
|
| Ampicillin (AMP) | 10 | 7 | R |
| Chloramphenicol (C) | 25 | 14 | I |
| Penicillin G (P) | 1 unit | 7 | R |
| Streptomycin (S) | 10 | 6 | R |
| Sulphatriad (S3) | 300 | 20 | S |
| Tetracycline (TE) | 25 | 18 | S |
| Hexa G-minus 2 HX007 | |||
| Ceftazidime (CAZ) | 30 | 20 | S |
| Ciprofloxacin (CIP) | 5 | 25 | S |
| Amikacin (AK) | 30 | 7 | R |
| Nitrofurantoin (NIT) | 300 | 7 | R |
| Netillin (NET) | 30 | 7 | R |
| Nalidixic acid (NA) | 30 | 14 | I |
| Hexa G- minus 3 HX008 | |||
| Co-Trimoxazole (COT) | 25 | 13 | I |
| Amoxyclav (AMC) | 30 | 8 | R |
| Gentamicin (GEN) | 10 | 8 | R |
| Tetracycline (TE) | 30 | 20 | S |
| Ofloxacin (OF) | 5 | 22 | S |
| Cefuroxime (CXM) | 30 | 20 | S |
| Hexa G- minus 5 HX010 | |||
| Cefotaxime (CTX) | 30 | 14 | I |
| Levofloxacin (LE) | 5 | 22 | S |
| Aztreonam (AT) | 30 | 13 | I |
| Imipenem (IPM) | 10 | 7 | R |
| Amikacin (AK) | 30 | 8 | R |
| Ceftazidime (CAZ) | 30 | 7 | R |
Resistant (R), Susceptible (S) or Intermediate (I) has been declared based on the zone of inhibition proposed by CLSI.
Growth optimization and growth curve determination
To see the effect of NaCl on growth of S.maltophila, isolated culture of the strain was inoculated into sterilized MGM medium with different NaCl concentration (12-25% MGM) (Dyall-Smith 2008) the inoculated samples were placed on incubated shaker at 300C. Samples were collected every 12 h up to 5 days. Collected samples were examined for absorbance values at 660 nm using spectrophotometer. To see the effect of pH cultures were set up in different pH from 5-10 range. Effect of temperature was studied by incubating the strain at different temperatures from 200c-500C.
To see the effect of heavy metals on growth profile of the strain growth curve was prepared in the absence (control) and presence of different heavy metals such as lead nitrate, zinc sulfate and copper sulfate with 50 µg/ml concentration. The liquid medium (LB broth), non-amended (controls) or amended by adding the heavy metals from stock solutions, was inoculated with equal volume of overnight grown the strain Stenotrophomonas maltophilia strain BN1. All four media were kept on a shaker at room temperature and the OD was taken in different time intervals to assess the growth of the culture. The OD readings of all four media were taken at 660 nm in a double beam UV/VIS spectrophotometer.
Plant growth promoting traits of the isolated strain
The plant growth promoting activities of the MDR strain was tested for Indole Acetic acid (IAA) production, ammonia production, siderophore production and phosphate solubilizing activity.
Production of IAA by the bacterium was determined both qualitatively and quantitatively using the protocol of Gordon and Weber (1951). The bacterial culture with tryptophan (200 µg/ml) was grown for 24-48 hours at 30ºC in LB broth and centrifuged to get the supernatant. 2 ml of the supernatant was mixed with two drops of orthophosphoric acid and 4 ml of Salkowski reagent (50 ml, 35% of perchloric acid, 1ml 0.5M FeCl3 solution) and kept in the dark for ~25 minutes. A pink color developed that indicated production of IAA. The optical density (OD) was taken at 530 nm in a spectrophotometer and concentration of IAA produced by the bacterial isolates was compared to the standard curve of IAA.
The assay of ammonia production was done as described by Cappuccino and Sherman (1992). For the assay of ammonia production, bacterial culture was grown on peptone water for 48-72 hours at 30ºC. After that, Nessler’s reagent was added (500 µl) and observed for a color change of brown to yellow.
Siderophore production test was carried out by the method described by Farah Ahmad et al. (2008).
For phosphate solubilization test, Pikovskaya’s (PKV) agar medium (glucose 10 g/l; tricalcium phosphate (TCP) 5 g/l; ammonium sulphate 0.5 g/l; sodium chloride 0.2 g/l; potassium chloride 0.2 g/l; magnesium sulphate 0.1 g/l; yeast extract 0.5 g/l; manganese sulphate trace; ferrous sulphate trace; agar 2%) was prepared and pH was adjusted to 7.0 before sterilization (Picovskaya 1948). The strain was spot inoculated on the PKV agar plate aseptically and incubated at 30°C for 7 days. A clear zone surrounding the colony indicated the phosphate solubilization and was measured as phosphate solubilization index (PSI). The pH of the media was checked at regular intervals to verify production of organic acids. Proteolytic activity of S. maltophilia was tested on skimmed milk medium. A zone of halo surrounding the colony was indicator of extracellular protease.
Scanning electron microscopy
The specimens were mounted on aluminum stubs with conductive carbon cement, allowed to dry for 3 h, and then coated with 15-nm platinum film with an agar automatic sputter coater. After processing, samples were observed with JEOL JSM-6700F field emission scanning electron microscope in the high-vacuum mode at 15 kV.SEM images were processed for display using Photoshop software (Adobe Systems Inc., Mountain View, Calif.).
RESULTS
Isolation and identification
During the screening of heavy metal resistant rhizobacterial isolates from the rhizosphere of I. pes-caprae the multiple heavy metal resistant isolate was isolated. The strain was an aerobic, gram-negative rod, motile and non-spore-forming. Colonies grew in LB agar and were cream, low convex, smooth and circular. Fig. 1 shows the plant I.pes-capreae collected from Bonnie Camp of Indian Sundarbans (1A) and the rhizobacterial strain (1B) isolated from its rhizosphere for further study. Table 1 lists the biochemical features noted. Phylogenetic analysis identified the strain as Stenotrophomonas maltophilia strain BN1. Accession number obtained from GenBank is KT238973.1.A phylogenetic tree was constructed by multiple alignment and neighbor joining method using MEGA7 (Fig. 2).
Heavy metal MIC of the strain
The isolate was resistant to all the heavy metals tested (Cr, Cu, Cd, Co, Zn, Pb and As). Fig. 3 shows the MIC values of the different heavy metals.
Antibiotic susceptibility testing
The isolate showed resistance to multiple antibiotics supplied with the hexa G antibiotic discs (HiMedia laboratories, India) viz .ampicillin, penicillin, streptomycin, amikacin, nitrofurantoin, netillin, amoxyclav, gentamycin, cefuroxime etc. Each disc contains 6 antibiotics. The results of antibiotic susceptibility testing of the isolate are given in the Table 2 and Figure 4.
Growth optimization and effect of heavy metals on growth curve
Growth occurred in a wide range of pH, temperature and salt stress. It tolerated temperature from 150C to 450C. However, optimum growth was noticed at 30ºC. Although it showed growth at various pH from 7 to 11 but optimum pH noticed for growth was 6-8. The optimum NaCl concentration for growth was 1-5 % (w/v). However it could tolerate NaCl concentration of up to 10%. Figure 5 shows the effect of heavy metals on the growth of the strain. In the presence of zinc or chromium or arsenic the strain obtained a maximum optical density of 2.24 whereas in the absence of the heavy metals the strain attained a slightly higher OD (2.7).
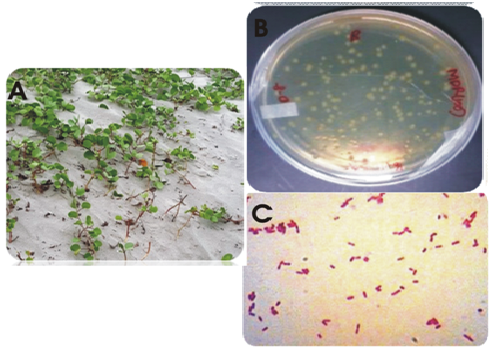
Fig.1. (A) Ipomoea pes-capreae (B) Isolated strain S.maltophila strain BN1 in LB agar plate (C) Gram stain image of the strain
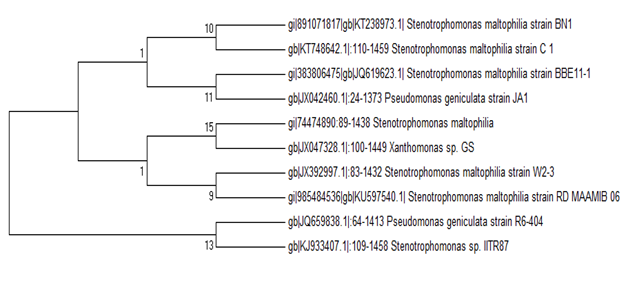
Fig.2. Evolutionary relationships of S.maltophila strain strain BN1
Plant growth promoting traits of the isolate
The isolate produced IAA and other plant growth promoting traits as observed from the tests performed.Concentration of IAA produced by the bacterial isolate was compared to the standard curve of commercial IAA. The isolate produced IAA in the range of 3-5.7 µg/ml. In addition, the isolate produced ammonia as indicated from the color change to yellow of the culture after adding Nessler’s reagent. The isolate also solubilized phosphate as indicated from the zone formed on PKV medium and the pH change of the culture in Pikovskaya’s broth. The initial pH of the medium was 7.0; pH after 10 days was 6.88 and pH after 13 days- was 5.68. The lowering of pH indicates production of organic acid in the media. The strain showed a large halo in casein agar medium indicating it may act antagonistically against phytopathogens. Figure 6 shows images of siderophore formation and production of extracellular protease.
FE SEM analysis
The FE SEM analysis of the strain was done after growing the isolate on LB broth amended with antibiotics. The result is depicted in the Fig. 7. Many irregular spheres like structures are visible in the cell surface.
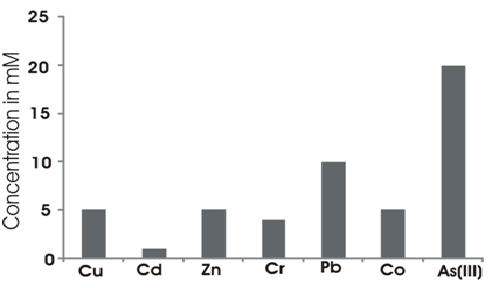
Fig.3. MIC of the different heavy metals by S.maltophila strain strain BN1
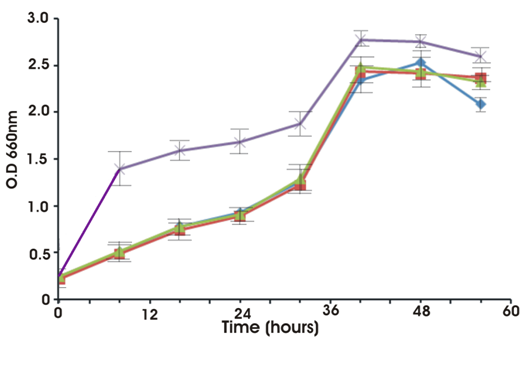
Fig. 4. Growth of isolate S.maltiphila strain BN1 with and without heavy metals.
Violet line corresponds to control (without metal); blue, green and red corresponds to zinc, chromium and arsenic.
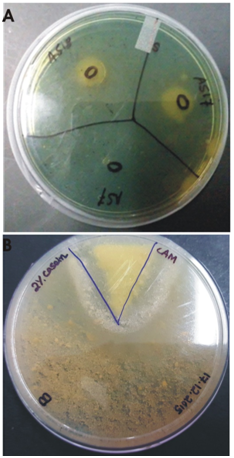
Fig. 5. (A) Siderophore formation (B) Protease production by the strain S.maltophila BN1
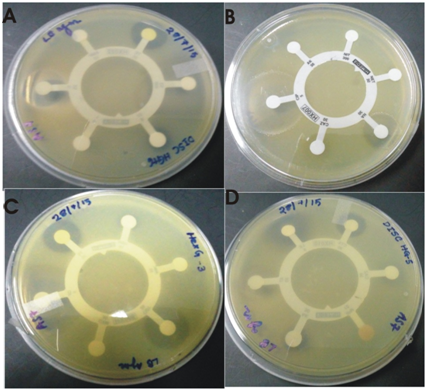
Fig.6. Zone of inhibition against tested antibiotics by S.maltophila strain BN1.
(A) Hexa G plus 6 (Chloramphenicol, Ampicillin, Penicillin G, Streptomycin, Sulphatriad, Tetracycline) (B) Hexa G minus 2 (Ceftazidime, Ciprofloxacin, Amikacin, Nitrofurantoin, Netillin, Nalidixic acid)(C) Hexa G Minus 3 (Co-Trimoxazole, Amoxyclav, Gentamicin, Tetracycline, Ofloxacin, Cefuroxime) (D) Hexa G Minus 5 (Cefotaxime, Levofloxacin, Aztreonam, Imipenem, Amikacin, Ceftazidime).
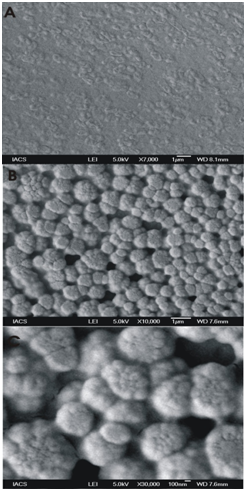
Fig.7. FeSEM analysis of S. maltophila BN1
The magnifications are (A) 7000 (B) 10,000 and (C) 30,000. (A) shows small rod shaped cells embedded in a matrix (B) shows the cell surface and (C) is the enlarged view of (B).
DISCUSSION
Among the eight species of Stenotrophomonas, S. maltophila is the most famous for its plant growth promoting and xenobiotic compound degrading abilities (Pages et al. 2008). In previous studies S.maltophila has been isolated from the rhizosphere of wheat, oat, cucumber, maize, oilseed rape, and potato (Berg et al. 1994; Debette and Blondeau 1980; Heuer and Smalla1999; Lambert and Joos 1989). However, it has bad reputation for serving as multidrug resistant bacteria in hospital patients and causing thousands of death every year (Brooke 2014). They are naturally resistant to many broad spectrum antibiotics such as cephalosporins, carbapenems, and aminoglycosides which causes treatments against the infection very difficult. Along with the heavy metal resistance property (Pages et al. 2008), S. maltophilia is also able to degrade other xenobiotic compounds (Binks et al. 1995; Lee et al. 2002) and detoxify high molecular weight polycyclic aromatic hydrocarbons (Juhasz et al. 2000). S. maltophila has been isolated from different pristine environments like Antarctica to (Kuddus et al. 2009) and isolation of the bacterium from mangrove habitat again proves the highly adaptive nature of the bacterium.
Our present study has demonstrated the presence of protease production ability of the strain. Many clinical isolates of S.maltophila has shown proteolysis as the mechanism of pathogenesis (Pages et al. 2008; Berg et al.1994; Debette et al. 1980; Heuer et al.1999). In environmental isolates the protease act antagonistically against phytopathogens. It may be predicted that same proteolysis mechanisms have given the strain a weapon against plant pathogens and establish itself in the highly competitive environment of the rhizobiome. Most rhizobacterial S.maltophila strains that act as biocontrol agent is protease positive. This genus is ideal for preparation of biopesticides. Although in this study we have not analyzed biocontrol activities of the strain but in future we would carry out in vivo and in vitro biocontrol activities. Present study has implication in the understanding of the adaptive mechanism of strain BN1 in the highly saline environment of Sundarbans. Ability of the strain to show good growth in a wide range of salt concentration explains the adaptability and osmotolerant nature of the strain in this unique environment. The heavy metal resistance and plant growth promoting properties of the strain are in line with previous findings by other authors (Pages et al. 2008; Wierzba 2015; Berg et al.1994; Debette et al. 1980; Heuer et al.1999). But still more research should be carried out with the strain thinking the novel bioactive compounds produced by the strains found by other researchers (Mukherjee and Roy 2016).
REFERENCES
- Ahmad, F., Ahmad, I., Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res, 2008; 163(2): 173–181.
- Bauer, A. W., Kirby, W. M. M., Sherris, J. C. Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol, 1966; 45(4): 493.
- Berg, G., Knaape, C., Ballin, G., Seidel, D. Biological control of Verticillium dahliae KLEB by naturally occurring rhizosphere bacteria. Arch Phytopathol Dis Prot, 1994; 29: 249–262.
- Binks, P.R., Nicklin, S., Bruce. N.C. Degradation of RDX by Stenotrophomonas maltophilia PB1. Appl Environ Microbiol 1995; 61: 1813–1822.
- Bragadeeswaran, S., Prabhu, K., Rani, S. S., Priyadharsini, S. Vembu, N. Biomedical application of beach morning glory Ipomoea pes-caprae. Int J Tropical Medicine. 2010; 5(4): 81-85.
- Brooke, J.S.New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert. Rev.Anti. Infect. Ther. 2014; 12: 1–4.
- Cappuccino, J. C., Sherman, N. In: Microbiology: A Laboratory Manual, New York, 1992: 125–179.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, Twenty Second Informational Supplement M100–S22. Wayne, Pennsylvania, Clinical and Laboratory Standards Institute, 2012, 31(1).
- Debette, J., Blondeau, R. Presence of Pseudomonas maltophilia in the rhizosphere of several cultivated plants. Can J Microbiol 1980; 26: 460–463.
- Devall, M. S. The biological flora of coastal dunes and wetlands. 2. Ipomoea pes-caprae (L.) Roth. J. Coast. Res. 1992; 442-456.
- Dyall-Smith, M.L. The Halohandbook: Protocols for halobacterial genetics (online book) 2008.
- Gordon, S. A. Weber, R. P. Colorimetric estimation of indoleacetic acid. Plant physiol. 1951; 26 (1): 192.
- Gopal, B., Chauhan, M. Biodiversity and its conservation in the Sundarban Mangrove Ecosystem. Aquatic Sciences, 2006; 68(3): 338–354.
- Heuer, H., Smalla, K. Bacterial phyllosphere communities of Solanum tuberosum L and T4-lysozyme producing genetic variants. FEMS Microbiol Ecol 1999; 28: 357–371.
- Juhasz, A.L., Stanley, G.A., Britz, M.L. Microbial degradation and detoxification of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltophilia strain VUN 10,003. Lett Appl Microbiol 2000; 30: 396–401.
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol, 1980; 16:111-120.
- Krieg, N.R., Holt, J.G. Bergy’s manual of determinative bacteriology, volume 1, Baltimore, The Williams and Wilkins Co.
- Kuddus M, Ramteke PW. Cold-active extracellular alkaline protease from an alkaliphilic Stenotrophomonas maltophilia: production of enzyme and its industrial applications. Can J Microbiol, 2009; 55(11):1294-301.
- Lambert, B., Joos, H. Fundamental aspects of rhizobacterial plant growth promotion research. Trends Biotechnol., 1989; 7: 215–219.
- Lee, E.Y., Jun, Y.S., Cho, K.S., Ryu, H.W. Degradation characteristics of toluene, benzene, ethylbenzene, and xylene by Stenotrophomonas maltophilia T3-c. J. Air. Waste. Manag. Assoc., 2002; 52: 400–406.
- Mergeay, M., Nies, D., Schlegel, H. G., Gerits, J., Charles, P. Van Gijsegem, F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bac. 1985; 162(1): 328-334.
- Mukherjee, P., Roy, P. Genomic Potential of Stenotrophomonas maltophilia in Bioremediation with an Assessment of Its Multifaceted Role in Our Environment. Environment. Front. Microbiol, 2016; 7:967.
- Pages, D., Rose, J., Conrod, S., Cuine, S., Carrier, P., et al Heavy Metal Tolerance in Stenotrophomonas maltophilia. PLoS ONE 2008; 3(2): e1539.
- Pikovskaya, R.I. Mobilization of phosphorous in soil in the connection with vital activity of some microbial species. Mikorobiologiya 1948; 17: 362-370.
- Robert P. Ryan, Sebastien Monchy, Massimiliano Cardinale, Safiyh Taghavi, Lisa Crossman, Matthew B. Avison, et al. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nature Rev. Microbiol. 2009; 7, 514-525.
- Saitou, N., Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol, 1987; 4:406-425.
- Souzaa, M.M.D., Madeiraa, A., Bertia, C., Kroghb, R., Yunesb, R.A., Cechinel-Filho, V. Antinociceptive properties of the methanolic extract obtained from Ipomoea pes-caprae (L.) R. Br. J Ethnopharmacol. 2000; 69: 1 85–90
- Tamura, K., Dudley, J., Nei, M. and Kumar, S.MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007; 24(8): 1596-1599.
- Wierzba,S. Biosorption of lead(II), zinc(II) and nickel(II) from industrial waste water by Stenotrophomonas maltophilia and Bacillus subtilis. Pol. J.Chem.Technol. 2015; 17: 79–87

