ISSN: 0973-7510
E-ISSN: 2581-690X
The purpose of this study is to explore the soil of the Gujarat, Kachchh region to identify amylase-producing bacteria and characterize them using molecular methods. The unique ecological characteristics of the Kachchh region may facilitate the isolation of these bacteria. Samples were collected from multiple locations within the Kachchh District, including Gandhidham, Rapar, Bhuj, Nakhatrana, Mandvi, and Mundra Talukas. These samples were then screened to isolate amylase-producing bacteria. A total of 27 different types of colonies were identified, out of which 16 exhibited amylase production (M1-M16). Out of 27 colonies identified, 16 showed amylase production. Strains M2, M7, and M13 exhibited high amylase activity, with M2 showing a consistent increase over 72 hours, making it a strong candidate for amylase production. Further identification of M2 stain identified M2 as a Gram-positive, spore-forming, capsulated, and motile bacillus, specifically Bacillus licheniformis. This was confirmed through DNA sequencing and analysis in the NCBI database, which showed a 99.15% similarity with Bacillus licheniformis. The study concludes that Soil in Kachchh is rich with microorganisms that produce amylase, an enzyme with diverse industrial applications. These organisms are valuable for sectors like food, textiles, paper, detergents, pharmaceuticals, and biofuel production.
α-amylase, Bacillus licheniformis, Amylase Production
Alpha-amylase, an enzyme that breaks down starch into simple sugar components, plays a pivotal role in various industries such as starch processing, brewing, sugar manufacturing, textiles, and detergents. In 1894 amylase enzyme produced from a fungal source, was used as a pharmaceutical aid for digestive diseases. Alpha-amylases are among the most critical enzymes, contributing to approximately 30% of the global enzyme production.1
These enzymes are ubiquitous, found in animals, plants, and bacteria. Microorganisms, including bacteria, fungi, and yeast, are known sources of amylase, and their properties have been extensively studied. Among these, Bacillus strains are noteworthy for their ability to produce significant amounts of amylase, accounting for about 60% of commercially available enzymes. Specifically, Bacillus subtilis and Bacillus mesentericus produce the most widely used thermostable amylase in the starch industry.2
Amylase is a key player in the carbon biogeochemical cycle and finds extensive applications in biotechnological food, detergent, and pharmaceutical industries. It can break down glycosidic bonds in polysaccharides. Interestingly, amylase is present in human saliva and initiates the chemical process of digestion in starchy foods like rice and potatoes. This enzymatic conversion of starch into sugar imparts a mildly sweet taste when these foods are consumed. The pancreas also produces alpha-amylase, which hydrolyzes dietary starch into disaccharides and trisaccharides, which are subsequently converted into glucose by other enzymes.3
The use of alpha-amylase extends to various industrial processes, including those in the food, fermentation, and pharmaceutical industries. The selection of the appropriate organism is crucial to ensure a high yield of useful enzymes. The development of new microbial strains for industrial enzyme synthesis using low-cost carbon and nitrogen sources is a constant endeavor. In microorganisms, two primary types of amylases have been identified: alpha-amylase and glucoamylase.4,5
The commercial applications of alpha-amylase have expanded to several domains, including clinical, pharmaceutical, and analytical chemistry, among other extracellular enzymes. Industries such as food, textile, fermentation, and paper heavily rely on starch-degrading bacteria. In the realm of biotechnology, the isolation and manipulation of pure cultures of starch-degrading microorganisms from soil are crucial. The extraction and manipulation of pure culture from various waste materials are vital for several biotechnology sectors.6,7
Significant amylase-producing microbes include Bacillus subtilis, Bacillus licheniformis, Bacillus amyloliquefaciens, Bacillus cereus, and Bacillus megaterium, as well as fungi such as Aspergillus niger, Penicillium, Rhizopus, Cephalosporium, and Neurospora. Microbial alpha-amylases have been demonstrated to be a viable alternative to chemical hydrolysis, although the enzyme’s low yield has always been a challenge in commercial amylase production.8
Amylase-producing bacteria can be found in soil, and exploring various regions can be helpful in finding potent amylase-producing bacteria. The unique nature of the soil may harbor a more diverse range of bacteria. The Kachch soil could be checked for amylase production.
Sampling
The research initiative collected soil samples from six distinct Talukas (namely Gandhidham, Rapar, Bhuj, Nakhatrana, Mandvi, and Mundra) in the Kachchh District of Gujarat, India, (GPS coordinates of 23° 44′ 1.4352” N and 69° 51′ 35.0676” E) targeting a diverse range of soil characteristics and microbial ecosystems (Figure 1). The extraction process focused on the subsurface strata at depths of 3 to 4 centimetres, using aseptic techniques to prevent contamination and preserve the indigenous microbial populations. The samples were sealed in sterile plastic bags and transported under strict aseptic conditions to the Mayur Laboratory in Adipur, Kachchh, where they were stored at 4 degrees Celsius to maintain microbial viability. The primary focus was to identify and isolate amylase-producing microorganisms.9
Primary screening
A 10 g soil sample was prepared for amylase isolation, suspended in 90 ml of sterile saline solution containing 0.85% sodium chloride. This isotonic environment maintains the physiological conditions of the microorganisms and prevents osmotic shock. Serial dilutions (10-1 to 10-10) were prepared from the sample and 100 µL suspensions from each dilution were spread on starch agar plates.10,11
The inoculated plates were incubated at a constant 37°C for 72 hours, allowing bacterial colonies to proliferate and express amylase activity. Post incubation, the plates were visually examined for bacterial growth. The presence of amylase activity was confirmed using the Gram’s iodine test. This involved flooding the agar plates with a solution of Gram’s iodine, leading to a blue-black complex formation in the presence of residual starch. This served as an indirect indicator of starch hydrolysis by amylase-producing bacteria.11,12
Secondary screening
After the initial screening identified bacteria capable of producing amylase, the focus shifted to isolating the strain with the highest amylase activity. The transition from primary screening to selecting the most potent strain aims to maximize amylase production efficiency. This involves a thorough evaluation of the amylase’s enzymatic properties and cultivation aspects, paving the way for advancements in industrial and biotechnological applications. The secondary screening selected the isolate with the highest enzyme activity for further applications.13,14
Post-incubation, the crude enzyme was purified by adding 50 ml of a phosphate buffer (50 mM, pH 7.4) to the media. The resulting slurry was strained using a damp cheesecloth and then centrifuged at 11648 RCF for 15 minutes in a cooling centrifuge. This process separated cells, small particles, and spores. As amylase is an exoenzyme, the cell-free supernatant was used as the enzyme. This purification process is crucial for obtaining a clean sample of the enzyme for further analysis.15
Amylase activity was assessed using the 3,5-dinitrosalicylic acid (DNSA) method,16,17 which measures reducing sugars produced during the enzyme-substrate reaction. The amylase assay was conducted with a reaction mixture containing 1% soluble starch in a 50 mM phosphate buffer at pH 7.2. The mixture was incubated for 10 minutes at 37°C, and the reaction was terminated by adding 2 ml of DNSA reagent.
The reaction mixture was then heat-treated at 100°C for 10 minutes and cooled to stop the enzymatic reaction and stabilize the products. The optical density of each sample was measured at 540 nm using a spectrophotometer, allowing for the quantification of the colored reaction product, and providing a direct measurement of amylase activity. Enzyme activity was quantified in units, where 1 unit/ml represents the amount of enzyme that releases 1 µl mole of glucose under the specified assay conditions. This comprehensive methodology reliably assesses the amylase-producing capabilities of bacterial strains.
Identification of amylase producing bacterial strains
The identification of the most potent amylase-producing bacteria involved key steps: Colony Morphology characterization, Microscopic characterization, Biochemical characterization and Molecular characterization.18
Colony morphological characterization
Colony Morphology Analysis provided a comprehensive characterization of bacterial isolates, examining color, shape, size, colony nature, and pigmentation. These parameters offered insights into the bacteria’s nature, growth patterns, structural characteristics, physiological attributes, and environmental adaptations.19
Microscopic characterization
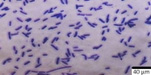
Microscopic Observation revealed specific structural features of the bacterial cells. Techniques such as Gram staining, capsule staining, endospore staining, and the motility test provided a detailed view of the structural and functional attributes of the bacterial isolates. These methods collectively contributed to a nuanced understanding of the isolates, laying the groundwork for further investigations into their physiological and ecological characteristics. This systematic approach was crucial in identifying the most potent amylase-producing bacteria.20
Biochemical characterization
Biochemical characterization of bacterial isolates was performed both manually and using the Vitek system. The Vitek system, with its comprehensive library of biochemical profiles, facilitated a thorough analysis of the isolates’ unique biochemical traits.
The process involved preparing bacterial isolates for testing, setting up the Vitek system, and conducting various biochemical tests. The Vitek system automated the analysis of the biochemical reactions, comparing the observed reactions with the patterns stored in its database. This resulted in outputs based on the unique biochemical characteristics of each isolate, aiding in the classification, and understanding of the microbial community. The extensive biochemical library ensured a high level of accuracy and precision in the identification process. The Vitek system covered a broad spectrum of biochemical reactions, providing a comprehensive analysis of the isolates’ metabolic and enzymatic activities. This contributed to a more detailed understanding of the bacterial community.21
Molecular characterization
Molecular identification by DNA sequencing is the most precise tool for identifying microbial strains. The DNA sequencing of the isolated strain was carried out in several steps20,22
DNA Purification: A ready-to-use kit was used to purify DNA from bacterial cells. The purification process involved a spin column-based nucleic acid purification method, utilizing solid-phase extraction to rapidly purify nucleic acids. The method consisted of four main stages: lysing, binding, washing, and eluting.
DNA amplification
PCR primers were used for the amplification of DNA. The primers used were 16S rRNA FP: 5’-AGAGTTTGATCCTGGCTCAG-3’ and 16S rRNA RP: 5’-ACGGCTACCTTGTTACGACTT-3’.
Quality test of PCR product
After the completion of the PCR reaction, the resulting products were visualized through 2.5% agarose gel electrophoresis under ultraviolet (UV) light.
DNA sequencing
The procedure for DNA sequencing was done by Sanger sequencing. The sequencing was performed using a Thermo Fisher DNA sequencer (Model No: 3730xl DNA Analyzer).
DNA sequencing data analysis
Data obtained from DNA sequencing were analysed in the NCBI database. The BLAST Web Interface was accessed as per the procedure. This comprehensive approach allowed for the identification of the most potent amylase-producing bacteria.
Isolation of amylase producing bacteria
Primary screening
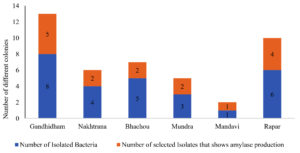
Post-incubation, different colonies were counted from six sampling sites: Gandhidham, Nakhtrana, Bhachou, Mundra, Mandavi, and Rapar. A total of 27 different types of colonies were identified. For instance, Gandhidham yielded 8 bacterial isolates, Nakhtrana had 4, Bhachou had 5, Mundra had 3, Mandavi had 1, and Rapar had 6 (Figure 2).
The Gram’s iodine test was applied to each plate for primary screening of amylase-producing bacteria. Out of the 27 colonies, 16 showed a clear zone around them, indicating amylase production. For example, out of the 8 bacteria isolated in Gandhidham, 5 exhibited amylase production. Similarly, Nakhtrana yielded 2 amylase-producing isolates out of 4. A similar pattern was observed for Bhachou, Mundra, Mandavi, and Rapar. This systematic approach was crucial in identifying the most potent amylase-producing bacteria.
The colony characteristics of the 16 amylase-producing strains were analysed across multiple parameters, including size, shape, colour, elevation, texture, margin, and optical properties. Each strain exhibited unique features, contributing to a nuanced understanding of their visual attributes.
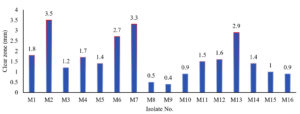
The primary screening for amylase production involved measuring the clear zones formed after the application of iodine on starch agar plates. These clear zones indicate the extent to which the 16 isolated strains, labelled as M1 to M16, are capable of hydrolysing starch. A larger clear zone generally suggests higher amylase activity and more potent amylase production.
The clear zone measurements exhibited notable variability among the strains, ranging from 0.4 mm (M9) to 3.5 mm (M2). This diversity suggests differences in the amylase-producing capabilities of the isolates. Strains M2, M6, M7, and M13 stood out as potentially potent amylase producers, showcasing clear zones of 3.5 mm, 2.7 mm, 3.3 mm, and 2.9 mm, respectively (Figure 3).
The distinct clear zone sizes for each strain indicate that amylase production is not uniform across the microbial isolates. This information is crucial for selecting specific strains with desirable amylase-producing capabilities.
Secondary screening
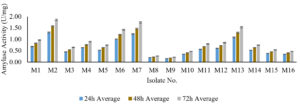
Following the initial screening, 16 amylase-producing bacterial strains underwent a secondary screening to evaluate their specific amylase activity across different time intervals (24 hours, 48 hours, and 72 hours). The results provided comprehensive insights into the temporal dynamics of amylase production.
Notably, strains M2, M7, and M13 consistently demonstrated elevated amylase activity, suggesting their potential as robust amylase producers. However, M2 at 48 hours and M7 at 72 hours recorded unusually low amylase activity values, indicating biological variability within these strains.
An ascending trend in amylase activity was observed from 24 hours to 72 hours across many strains, with particularly pronounced time-dependent increases in M2, M6, M7, M11, M13, and M14. Conversely, strains M8 and M9 consistently exhibited relatively low amylase activity across all time points (Figure 4).
Figure 4. Amylase activity of produced amylase from amylase producing bacteria at different time interval
Upon closer examination, certain strains, notably M2, showed an incremental trend in amylase activity over the 72-hour period, positioning M2 as a robust and persistent amylase producer. These findings aid in identifying promising bacterial strains for further exploration or potential industrial applications. Strains demonstrating both elevated overall amylase activity and temporal stability, such as M2, merit careful consideration for their suitability in practical applications. Following the secondary screening process, the M2 strain was specifically chosen for subsequent phases of identification.
Identification of amylase producing bacterial strains
The M2 strain, identified as the most potent amylase-producing bacteria, was characterized by several distinctive features.
Colony morphology
The colony of the M2 strain is medium-sized, irregular, and powdery in nature. It displays a spectrum of colours, ranging from white to beige and cream. The colony is flat or slightly convex in terms of elevation, and its texture is described as powdery. The margin of the colony is irregular and undulate, exhibiting a wavy pattern. Notably, no specific optical properties were identified for the M2 colony.
Microscopic observation
The M2 strain, based on microscopic examinations, is identified as a Gram-positive bacillus capable of forming endospores and capsules. Furthermore, the rod-shaped bacteria occur in chain formations, indicating a particular structural arrangement. The motility test confirmed the ability of the M2 strain to exhibit movement. These detailed observations provide valuable insights into the morphological diversity of the isolates, aiding in the identification of the most potent amylase-producing bacteria.
The M2 strain underwent a comprehensive biochemical characterization, performed both manually and using the Vitek system (Table).
Table:
Microscopic observation of amylase producing bacteria
Gram staining |
Gram-positive bacilli |
|
Endospore staining |
Spore forming bacilli |
|
Capsule staining |
Capsulated bacilli |
|
Motility |
Motile |
Manual biochemical tests
The results provided valuable information about the biochemical profile of the organism. The organism exhibited a negative response for the Indole test, indicating the absence of indole production. Conversely, it demonstrated positive reactions for various tests, including Methyl Red, Citrate Utilization, Sugar Fermentation (both glucose and sucrose), Catalase, Oxidase, Starch Hydrolysis, Nitrate Reduction, and Glucose Fermentation. These positive reactions suggest the organism’s ability to metabolize certain substrates or produce specific enzymes.
Biochemical test with VITEK analysis
The VITEK analysis of the M2 strain yielded a wealth of biochemical results, providing a detailed overview of its metabolic capabilities. Key observations from the VITEK analysis include positive results for tests such as Beta-Xylosidase, Phenylalanine Arylamidase, Beta-Galactosidase, L-Pyrrolydonyl-Arylamidase, Alpha-Galactosidase, Tyrosine Arylamidase, Cycloheximide utilization, Glycogen, Myo-Inositol, Methyl-α-D-glucopyranoside, Ellman reduction, Maltotriose, D-Mannitol, D-Mannose, N-Acetyl-D-Glucosamine, Palatinose, Beta-Glucosidase, Alpha-Glucosidase, D-Tagatose, D-Trehalose, Inulin, D-Glucose, D-Ribose, Growth in 6.5% NaCl, Kanamycin resistance, Oleandomycin resistance, Esculin hydrolysis, Tetrazolium red, and Polymyxin B resistance. The results also indicate negative outcomes for tests such as L-Lysine-Arylamidase, L-Aspartate Arylamidase, Leucine Arylamidase, L-Proline Arylamidase, Alanine Arylamidase, Beta-N-Acetyl-Glucosaminidase, Ala-Phe-Pro Arylamidase, Methyl-D-Xyloside, Alpha-Mannosidase, Glycine Arylamidase, D-Melezitose, L-rhamnose, Beta-Mannosidase, Phosphoryl Choline, Pyruvate, and Putrescine assimilation.
The culmination of these comprehensive biochemical tests and the VITEK 2 data analysis confidently identified the M2 strain as Bacillus licheniformis. This taxonomic classification provides essential information for understanding the strain’s physiological and biochemical characteristics. The positive outcomes in various enzymatic assays suggest the strain’s potential utility in industrial applications, particularly in enzyme production. These findings contribute not only to the taxonomic knowledge of the M2 strain but also have implications for potential biotechnological and industrial applications in areas such as enzyme production and microbial processes.
Molecular characterization
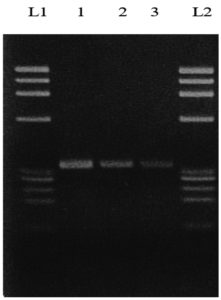
The M2 strain was further confirmed by a molecular method, specifically DNA sequencing. After the purification of DNA from the isolate, it was multiplied using the PCR method to increase the quantity. The purification and amplification of DNA were confirmed by electrophoresis (Figure 5).
Figure 5. Electrophoresis of DNA (4th sample)
L1: Leader 1, 1: Replicate 1, 2: Replicate 2, 3: Replicate 3, L2: Leader 2
The DNA sequencing results were analysed in the NCBI database to identify the most suitable microorganisms through BLAST. The results showed that Bacillus licheniformis had a 99.15% similarity with the test microorganisms. Specifically, the Bacillus licheniformis strain DLSB-13 16S ribosomal RNA gene partial sequence (Accession: MK795391.1) scored 2124, with a 100% query cover and an E-value of 0.0. This confirms the identification of the M2 strain as Bacillus licheniformis, providing essential information for understanding the strain’s physiological and biochemical characteristics. These findings have implications for potential biotechnological and industrial applications in areas such as enzyme production and microbial processes.
Study’s revelation of the rich microbial diversity within the soil samples from the Kachchh region is a significant contribution to our understanding of the area’s ecological dynamics. The identification of amylase-producing microorganisms as a prominent group within this diversity underscores their potential role in the region’s biogeochemical cycles and industrial applications.
Our findings are in harmony with the research conducted by Joshi and Trivedi, which also highlighted the microbial richness of Kachchh soil. Their work, which isolated 48 bacterial types with 36 identified as amylase producers, provides a broader context for our study. It suggests that the Kachchh region may be a hotspot for microbial diversity, particularly for strains capable of producing industrially relevant enzymes like α-amylase.23 It also emphasizes the need for further research into the specific conditions that foster such high levels of microbial diversity and enzyme production. Understanding these factors could lead to more efficient exploitation of these resources for industrial purposes, such as in the production of biofuels, bioplastics, and other value-added products.
Another study conducted in the unexplored Nasinuan Forest of Thailand has led to the discovery of 13 bacterial isolates capable of producing the amylase enzyme. These isolates were identified using 16S rRNA sequencing on 1% starch agar, revealing a diverse group of bacteria with amylase-producing potential.24
The study’s findings are noteworthy as they mirror our research where the M2 strain was identified as Bacillus licheniformis through DNA sequencing and 16S rRNA gene analysis. This consistency in results underscores the reliability of these molecular techniques in identifying bacterial strains with specific enzymatic activities.
The implications of such discoveries are significant, particularly for industries that rely on amylase enzymes, such as food and agriculture.1 The ability to harness these enzymes from natural sources can lead to more sustainable and cost-effective production methods.6
The study conducted in Sudan, which focused on the isolation and characterization of α-amylase producing microorganisms, has yielded results that are noteworthy for their implications in both scientific research and industrial applications. The identification of Bacillus cereus and Bacillus licheniformis as starch hydrolyzing bacteria with amylase activity is particularly significant. This finding is consistent with our current study, where B. licheniformis was observed to produce a clear zone indicative of amylase activity.20
The presence of these bacteria in Sudanese soil samples suggests a potential for harnessing their enzymatic capabilities for various biotechnological processes. The ability of B. licheniformis to produce amylase is not only crucial for the natural degradation of starch but also holds promise for industrial applications such as the production of bioethanol, where amylase plays a key role in breaking down starch into fermentable sugars.14
Moreover, the similarity between the findings of the Sudanese study and our own underscores the global nature of microbial diversity and its potential for enzyme production. It highlights the importance of cross-regional studies in identifying and utilizing microbial resources for sustainable development.17
In conclusion, our study has isolated and identified Bacillus licheniformis from Kachch soil as a potent amylase producer. The comprehensive morphological, microscopic, and biochemical characterization of the strain, coupled with DNA sequencing confirmation, has provided a robust identification of this microorganism.
The amylase activity of Bacillus licheniformis holds significant promise for industrial applications, particularly in the field of enzyme production. This discovery not only enriches the existing knowledge base but also opens new avenues for research into the sustainable exploitation of microbial resources in the Kachchh region.
Future research should focus on exploring the genetic and environmental factors that contribute to the prevalence of amylase-producing bacteria in this ecosystem. Such studies could lead to optimized conditions for enzyme production and contribute to the development of sustainable biotechnological processes. The potential of Kachchh soil as a reservoir for industrially relevant microorganisms is immense, and continued investigation will undoubtedly yield further valuable insights.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- de Souza PM, de Oliveira Magalhaes P. Application of microbial α-amylase in industry – A review. Braz J Microbiol. 2010;41(4):850-861.
Crossref - Solanki P, Putatunda C, Kumar A, Bhatia R, Walia A. Microbial proteases: ubiquitous enzymes with innumerable uses. 3 Biotech. 2021;11(10):428.
Crossref - Vaikundamoorthy R, Rajendran R, Selvaraju A, Moorthy K, Perumal S. Development of thermostable amylase enzyme from Bacillus cereus for potential antibiofilm activity. Bioorg Chem. 2018;77:494-506.
Crossref - Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B. Microbial α-amylases: a biotechnological perspective. Process Biochem. 2003;38(11):1599-1616.
Crossref - El-Fallal A, Abou M, El-Sayed A, Omar N. Starch and Microbial α-Amylases: Concepts to Biotechnological Applications. Carbohydrates – Comprehensive Studies on Glycobiology and Glycotechnology. InTech. 2012.
Crossref - Mesbah NM. Industrial Biotechnology Based on Enzymes From Extreme Environments. Front Bioeng Biotechnol. 2022;10:870083.
Crossref - Maghraby YR, El-Shabasy RM, Ibrahim AH, Azzazy HMES. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega. 2023;8(6):5184-5196.
Crossref - Nimisha P, Moksha S, Gangawane AK. Amylase Activity of Starch Degrading Bacteria Isolated from Soil. Int J Curr Microbiol Appl Sci. 2019;8(04):659-671.
Crossref - Srivathsan V, Bhandari M, Swaminathan P. Isolation and characterization of starch degrading bacteria from disparate soil samples. J Appl Biol Biotechnol. 2022;10(5):193-197.
Crossref - Yassin SN, Jiru TM, Indracanti M. Screening and Characterization of Thermostable Amylase-Producing Bacteria Isolated from Soil Samples of Afdera, Afar Region, and Molecular Detection of Amylase-Coding Gene. Int J Microbiol. 2021;2021:5592885.
Crossref - Liu JH, Guo JN, Lu H, Lin J. Activity-Based Screening of Soil Samples from Nyingchi, Tibet, for Amylase-Producing Bacteria and Other Multifunctional Enzyme Capacities. Int J Microbiol. 2022;2022:2401766.
Crossref - Davis KER, Joseph SJ, Janssen PH. Effects of Growth Medium, Inoculum Size, and Incubation Time on Culturability and Isolation of Soil Bacteria. Appl Environ Microbiol. 2005;71(2):826-834.
Crossref - Simandjuntak S, Samuel M. Isolation and Identification of Thermophilic Bacteria, Producer of Amylase Enzyme, from Lake Linow, North Sulawesi. J Pure Appl Microbiol. 2018;12(2):543-554.
Crossref - Kumar P, Padmadeo SR, Jha V, Prasad B. Screening and identification of amylase producing strains of Bacillus. J Appl Biol Biotechnol. 2019;7(4):57-62.
Crossref - Annamalai N, Thavasi R, Vijayalakshmi S, Balasubramanian T. Extraction, Purification and Characterization of Thermostable, Alkaline Tolerant α-Amylase from Bacillus cereus. Indian J Microbiol. 2011;51(4):424-429.
Crossref - Wickramaratne MN, Punchihewa JC, Wickramaratne DBM. In vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement Altern Med. 2016;16(1):466.
Crossref - Elyasi Far B, Ahmadi Y, Yari Khosroshahi A, Dilmaghani A. Microbial Alpha-Amylase Production: Progress, Challenges and Perspectives. Adv Pharm Bull. 2020;10(3):350-358.
Crossref - Ullah I, Khan MS, Khan SS, et al. Identification and characterization of thermophilic amylase producing bacterial isolates from the brick kiln soil. Saudi J Biol Sci. 2021;28(1):970-979.
Crossref - Sousa AM, Machado I, Nicolau A, Pereira MO. Improvements on colony morphology identification towards bacterial profiling. J Microbiol Methods. 2013;95(3):327-335.
Crossref - Rakaz MA, Hussien MO, Ibrahim HM. Isolation, Extraction, Purification, and Molecular Characterization for Thermostable α-Amylase from Locally Isolated Bacillus Species in Sudan. Biochem Res Int. 2021;2021:6670380.
Crossref - Altheide ST. Biochemical and Culture-based Approaches to Identification in the Diagnostic Microbiology Laboratory. American Society for Clinical Laboratory Science. 2019;32(4):166-175.
Crossref - Galkiewicz JP, Kellogg CA. Cross-Kingdom Amplification Using Bacteria -Specific Primers: Complications for Studies of Coral Microbial Ecology. Appl Environ Microbiol. 2008;74(24):7828-7831.
Crossref - Joshi CM, Trivedi NS. Isolation, Identification and Characterization of Microorganisms Isolated from Unexplored Saline Regions of Kutch, Gujarat, India. ACTA Scientific Microbiology. 2020;3(7):03-09.
Crossref - Luang-In V, Yotchaisarn M, Saengha W, Udomwong P, Deeseenthum S, Maneewan K. Isolation and Identification of Amylase-producing Bacteria from Soil in Nasinuan Community Forest, Maha Sarakham, Thailand. Biomedical & Pharmacology Journal. 2019;12(3):1061-1068.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.