ISSN: 0973-7510
E-ISSN: 2581-690X
Recent concern for human safety and environmental protection has rekindled interest in natural pigment sources. In comparison to synthetic pigments, microbial pigments show better biodegradability and environmental compatibility and are used in a variety of applications ranging from food to cosmetics. The areas of attention for economical pigment synthesis include the identification of novel microbiological sources and improvement of process parameters. The purpose of this research was to screen and identify microbial isolates capable of generating pigments with antimicrobial activity from a variety of soil samples. A total of six pigment-producing bacterial sps were able to isolate from various soil samples such as bore well digging sites, river shores, river beds, forest areas, dumping yards using the enrichment culture technique. All the isolates were morphologically and biochemically identified as Micrococcus sp producing two-color pigments i.e., yellow and orange, Serratia sp producing red and pink color pigments, Salinococcus sp producing orange color pigment, and Exiguobacterium sp producing yellow color pigment respectively. During optimization studies maximum pigment production was observed at pH 7, agitation at 90 rpm (rotations per minute) and 120 rpm, the temperature of 30°C and 37°C, inoculum size up to 2% with NaCl concentration of 2%, 4%, and 6% respectively. Optimization of nutritional parameters such as carbon source and nitrogen source it was found that glucose (1%) and yeast extract (0.1%) work the best. Extraction of the pigment from the fermented broth was done by solvent-solvent extraction method. UV-Visible spectrophotometry and Silica gel Thin-layer chromatography was used to detect the presence of carotene and prodigiosin in the extracted bacterial pigment. The crude bacterial pigments were tested for antimicrobial activity against clinical pathogens including E. coli, Klebsiella sp, Bacillus sp, Staphylococcus sp, and pseudomonas sp respectively. Among all the isolates, pigments of Micrococcus sp and Salinococcus sp showed comparatively good results. Further purification of the pigment will lead to discovering a promising drug in the pharmaceutical industry.
Microbial pigments, Carotene, optimization, antimicrobial activity
Natural pigments are obtained from plants, insects, ores, and micro-organisms1 Plant and other non-microbial pigments have limitations to use because of their poor water solubility, availability only in some seasons, and instability against light, heat, or acidic pH whereas microbial pigments have more advantages as they are stable, water-soluble and easily produced. Microbiological pigments are of great research importance, which are gaining demand globally and are now one of the rising topics of study. For many years, synthetic coloring compounds have been widely used in a wide range of sectors. However, due to the toxicity of these synthetic colorants on humans and the environment, microbial pigments are gaining importance in the market due to their exclusive properties as antioxidant, antitumor, inhibiting mutagenesis, and acting as a bio-indicator.2 Moreover, microbial pigments are bio-degradable and eco-friendly.3 Various types of microbial pigments were reported until now, including carotenoids, melanin, prodigiosin, violacein, flavins, etc.4,5 Among all the identified microbial pigments carotenoids hold a majority and more complex structure of molecules which shows their availability in various forms of life. To date about 700 types of carotenoid structures were identified that are produced by certain microbes, such as Serratia sp, Bacillus sp, Sarcina sp, Rhodotorula sp, Monascus sp, Streptomyces sp, Penicillium sp, Cryptococcus sp are potential pigment producers. An increase in the use of microbial pigment in dyeing fabrics, cosmetics, and medicinal operations is also observed in recent years. The exploration of diverse microbial pigment sources and their potentials is thus critical.6 Carbon and nitrogen sources with pH and temperature tolerance should be available for an ideal pigment manufacturer.7 The stability against the variations of pH, temperature, and salt concentration is required for the pigment produced in this manner.8 In the different nutritional and cultural circumstances, the capacity of micro-organisms to synthesize pigments may be considerably increased or totally lost. As a result, to maximize growth and pigment production, it is critical to find and optimize the suitable combination of culture conditions.9,10
Considering the wide range of microbial pigments, the current study was conducted with the purpose of isolating and identifying bacterial sp. that produce pigments in the natural environment. In the present work, soil samples from different sources such as borewell digging sites, dried river beds, river shore, etc. were screened for pigment-producing bacteria. Optimization studies were carried out for different physical and nutrient parameters to increase growth and pigment production. The microbial pigments extracted from selected isolates were then evaluated for antimicrobial activity against a few common clinical pathogenic microbes.
Screening for pigment-producing bacteria
The soil samples were collected aseptically in sterile plastic bags from borewell drilling sites, dry riverbeds, river shores, and other locations and preserved at 4°C for further use. Serially diluted soil samples were inoculated and incubated for about 2-3 days on a nutrient agar medium. The pigment-producing bacterial isolates were primarily identified by the color of the colony and Gram’s staining technique and later confirmed by biochemical assays. These pure cultures were kept as glycerol stocks in refrigerators.11,12
Optimization studies for enhanced pigment production
The optimization of growth conditions, particularly physical and nutritional parameters are of prime importance in the development of any pigment production process owing to their impact on the economy and practicability of the process. Microbes need an optimal temperature to thrive and produce pigment, hence temperature has a significant impact on pigment synthesis. The pH of the growth medium is crucial in pigment synthesis because it regulates the acidic and basic nature of the medium and provides a comfortable zone for microbial growth. The selected bacterial isolates were inoculated into the nutrient broth and incubated at a different temperatures like 20°C, 25°C, 30°C, 37°C, and 40°C at a pH range of 3, 5, 7, 9, and 11 for about 7 days.13,14 After incubation, the absorbance (OD) of the produced pigments was analyzed using UV-Vis Spectrophotometer against a blank.
Along with temperature and pH of the medium, the other parameters like Concentration of NaCl (0.5%, 1%, 2%, 4%, 6% and 8%), inoculum size (1%, 2%, 3%, 4% and 5%) and agitation (30 rpm, 60 rpm, 90 rpm and 120 rpm) were also studied simultaneously.15
The macro and micronutrients like Carbon, nitrogen, iron, and calcium respectively also play an important role in enhanced growth and pigment production.16 Different carbon sources such as fructose, glucose, galactose, lactose, sucrose, and maltose at the concentration of 1% along with different nitrogen sources including KNO3, beef extract, peptone, and yeast extract were taken at the concentration of 0.1% of broth. To this Carbohydrate, the medium inoculum was added and incubated.17 After incubation pigment production was recorded colorimetrically.
Extraction of pigment from the culture broth
After incubation, the culture broth was centrifuged at 7000 rpm at 0°C for 20 minutes. To the obtained cell pellets add an equal volume of solvents such as ethanol, methanol, petroleum ether, and chloroform. The cell suspension was vortexed for a few minutes and heated in a water bath at 60°C until cell pellets loses its color. The colored supernatant was filtered through a Millipore syringe and subjected to spectrophotometer analysis and Thin layer chromatography for separation and identification of pigment.18
UV- Visible spectrophotometric analysis
The maximum absorption spectrum of the extracted pigment was observed by means of UV- A visible spectrophotometer within the range of 300-800 nm using methanol as blank.19,20
Thin-layer chromatography (TLC)
Purification of the extracted pigment was performed by TLC using silica gel (Merk, Mumbai, India) G-60 F25~. The solvent system used was methanol, ethyl acetate, and chloroform in the ratio of 6:3:1 (v/v). The pigment was loaded onto the gel sheet using a sterile capillary tube and dipped in the solvent system.21 When the solvent system reached the 2/3rd of the TLC sheet the plate was removed and air-dried and Retention factor was calculated as follows,
The relative Rf values were calculated as:
Rf = (sample) / d(solvent)
Where d(sample) is the distance traveled by the sample and d(solvent) is the distance traveled by the solvent system.
Antimicrobial activity
The antimicrobial activity of the extracted pigments was tested with lab isolates like gram-positive (Staphylococcus sp and Bacillus sp) and gram-negative bacteria (Escherichia coli, Pseudomonas sp and Klebsiella sp) using agar well diffusion assay.22 All the bacterial isolates were grown on Muller Hinton agar media and incubated at 37°C for 24 hrs.23 Later, 25 µl of the pigment sample were loaded in the wells and incubated for 24 hrs. After incubation, observation was made by measuring the zone of inhibition around the wells.24 The above process was carried out in triplicates.
Isolation and identification of pigment-producing bacteria
In our study, six pigment-producing bacteria were isolated from soil samples which showed luxuriant growth and high color intensity on nutrient agar media. The isolated bacteria were identified and characterized with the help of morphological characteristics and biochemical reactions (Tables 1 and 2) and their genus-level identification was done using Bergey’s Manual of Determinative Bacteriology.
Table (1):
Morphological and physiological characteristics of pigment-producing bacterial isolates.
No. |
Bacterial isolates |
Pigment Color |
Grams reaction |
Cell shape |
Texture |
Margin |
Colony |
Motility |
|---|---|---|---|---|---|---|---|---|
1. |
Exiguobacterium sp |
Yellow |
+ve |
Bacilli |
Convex |
Irregular |
Circular |
+ve |
2. |
Micrococcus sp 1 |
Yellow |
+ve |
Cocci |
Convex |
Irregular |
Circular |
-ve |
3. |
Micrococcus sp 2 |
Orange |
+ve |
Cocci |
Convex |
Regular |
Circular |
-ve |
4. |
Serratia sp 1 |
Red |
-ve |
Bacilli |
Non-sticky |
Entire |
Circular |
+ve |
5. |
Serratia sp 2 |
Pink |
-ve |
Bacilli |
Non-sticky |
Entire |
Circular |
+ve |
6. |
Salinococcus sp |
Orange |
+ve |
Cocci |
Flat |
Regular |
Pin-pointed |
-ve |
Table (2):
Biochemical reactions of pigment-producing isolates.
No. |
Bacterial isolates |
Indole |
MR |
VP |
Citrate |
Oxidase |
Catalase |
Urease |
Nitrate reduction |
|---|---|---|---|---|---|---|---|---|---|
1. |
Exiguobacterium sp |
– |
+ |
+ |
– |
+ |
+ |
– |
+ |
2. |
Micrococcus sp 1 |
– |
+ |
– |
+ |
+ |
+ |
– |
– |
3. |
Micrococcus sp 2 |
– |
+ |
– |
+ |
+ |
+ |
– |
– |
4. |
Serratia sp 1 |
– |
+ |
+ |
+ |
+ |
+ |
– |
– |
5. |
Serratia sp 2 |
– |
+ |
+ |
+ |
+ |
+ |
– |
+ |
6. |
Salinococcus sp |
– |
+ |
– |
+ |
+ |
+ |
– |
– |
(+) positive and (-) negative.
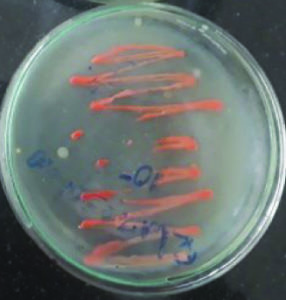
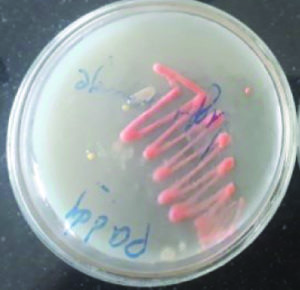
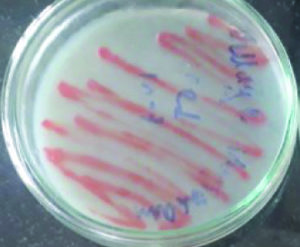
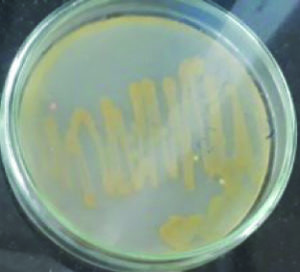
These isolates were identified as two Micrococcus sp producing color pigments i.e., yellow and orange (Fig. 1 and 2), Serratia sp producing another two color pigments red and pink (Fig. 3 and 4), Salinococcus sp producing orange color pigment (Fig. 5), and Exiguobacterium sp producing yellow pigment, respectively (Fig. 6).
Optimization studies to enhance pigment production
The influence of various factors in enhancing production of pigments by selected isolates was studied taking into consideration of the temperature, pH, salt concentration, inoculum size, agitation, and nutrient sources (carbon and nitrogen) over a range. A gradual increase in the production of pigment was observed with an increase in the factor under study and decreased when the factor was increased beyond the optimum level.
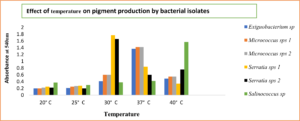
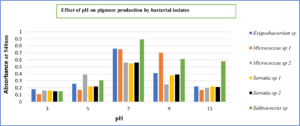
It was observed in our studies that when pigment producing bacteria were inoculated in nutrient broth and incubated at different temperatures, maximum pigment production was seen at 37°C for Exiguobacterium sp (yellow pigment) and Micrococcus sp (yellow and orange pigment)., maximum pigment production was observed by Exiguobacterium sp (yellow) and Micrococcus sp (yellow and orange) at an incubation temperature of about 37°C. Mohana et al25 reported that optimum growth for Micrococcus roseus was obtained at 37°C at pH 8. Also, the optimal temperature was in accordance with the data of Exiguobacterium sp PMA by P.K. Arora et al.26 It was also observed that Serratia sp produced (red and pink) pigment at 30°C in accordance with Williams et al., reported27 that maximum pigment production by Serratia sp was seen at 27°C and no pigmentation was observed when the cultures were incubated at 38°C. The other bacterial isolate Salinococcus sp produced (orange) pigment at 40°C. The same results were obtained by Hizbullahi et al.12 that Salinococcus roseus produced maximum pigment at 40°C. The maximum color intensity of the pigment was observed at pH 7 (Fig. 7 and 8). Bhatt and Marar28 reported the pigment production of Salinococcus sp at pH 7 at 30°C.
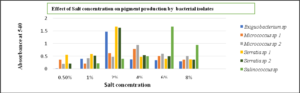
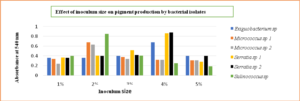
The effect of NaCl concentration varies with different species of bacterial isolates. Exiguobacterium sp and Serratia sp required 2% of salt concentration whereas Micrococcus sp utilized 4% respectively. And the Salinococcus sp was observed to be halophilic as it produced maximum pigment at 6% NaCl concentration (Fig. 9)
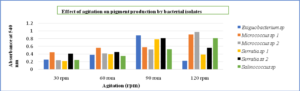
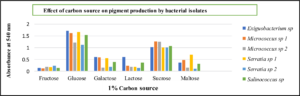
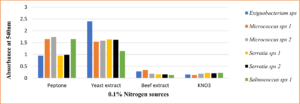
Maximizing growth and pigment production while minimizing inoculum size had the opposite effect. Micrococcus sp and Salinococcus sp showed high pigmentation at 2% inoculum. Whereas Serratia sp and Exiguobacterium sp produced maximum pigment at 4% inoculum (Fig. 10) respectively. The decrease in the growth was due to the accumulations of the by-products and extortion of nutrients. It was also observed that optimum agitation was 120 rpm for Micrococcus sp and Salinococcus sp. For Serratia sp and Exiguobacterium sp 90 rpm was proved to be effective in maximum pigment production (Fig. 11). All the bacterial isolates showed maximum pigment production after 48 hours of incubation when the growth medium was supplemented with 1% glucose and sucrose (Fig. 12). Yeast extract and peptone proved to be good nitrogen sources when supplemented with 0.1 % in growth medium (Fig. 13).
Fig. 14. (a) The extracted yellow pigment of Exiguobacterium sp, (b) Extracted yellow and orange pigment of Micrococcus sp, (c) and (d) extracted pink and red pigment of Serratia sp and (e) extracted yellow pigment of Salinococcus sp.
Extraction of pigment from the culture medium
Various methods had been used for the extraction of pigment from the selected isolates like filtration and centrifugation with ethanol addition so that cell gets lysed and intracellular pigment can be easily extracted. The extracted pigments were observed as yellow and orange in the color of Micrococcus sp., pink and red in color of Serratia sp., orange color from Salinococcus sp, and yellow color from Exiguobacterium sp. respectively (Fig. 14). The intracellular pigments were completely dissolved in ethanol. The optical density (OD) of the pigments had been measured and the colored solutions were further considered for TLC analysis.
UV-Visible spectrophotometer analysis
Maximum absorbance in the region of 300 to 800 nm was measured in the solvent extracted pigments. Exiguobacterium sp pigment has a maximum at 450 nm, which was consistent with the finding of Arunkumar et al.29 The maximum OD value of Micrococcus sp pigments is 450 nm. The highest OD of Serratia sp pigment is 450 nm. RR Rakh et al30 found that the prodigiosin pigment generated Serratia rubidea JCM 1204T has a maximum absorption at 470 nm. At 450 nm, the pigment of Salinococcus sp displayed maximum absorption. The findings were comparable to those of Bhat and Marar28, that pigment generated by Salinococcus roseus had the largest peak at 450nm, which is typical of carotenoid pigment.
Thin-layer chromatography
The pigment contained in the solvent system was subjected to thin-layer chromatography (Fig. 15). It was discovered that the pigment produced by Exiguobacterium sp was carotenoid (yellow pigment) as it showed Rf= 0.83. Micrococcus sp extracted pigments were also carotenoid as (yellow pigment) it has Rf= 0.75 and (orange pigment) Rf=0.7. A group led by Godinho et al30 found that Microbacterium arborescens-AGSB contained carotenoids. The pigment produced by Serratia sp was identified as prodigiosin Rf= 0.71 (red pigment) and Rf= 0.73 (pink pigment). Salinococcus sp (orange pigment) Rf= 0.65 indicates the presence of xanthophyll which coincides with the results obtained by Marar and Bhatt.28
Antimicrobial activity of the extracted pigments
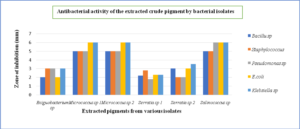
In our investigation, the ethanol-extracted pigment of Micrococcus sp 1 and 2 were effective against Bacillus sp (5mm), Staphylococcus aureus (5mm), Escherichia coli (6mm), Pseudomonas aeruginosa (5mm) and Klebsiella pneumonia (6mm) followed by Salinococcus sp, Serratia sp, and Exiguobacterium sp (Fig. 16). The current findings are in line with a recent study that found that pigment generated by Micrococcus sp has inhibitory action against gram-positive bacteria.22 Furthermore, the Rhodotorula glutinis Yolmeh Mahmoud et al32 pigment showed substantial antibacterial activity against both gram-positive and gram-negative bacteria, with Bacillus sp and Salmonella enteritidis having the lowest and highest susceptibility to this pigment, respectively. The present study also indicates that the antibacterial metabolites were generated by selected isolates were extracellular and diffusible in growth media.
As bacterial pigment synthesis is a hot topic in recent years, the development of its discovery and production are both in high demand, particularly the studies in producing a low-cost and appropriate growing medium to reduce the cost in production processes as well as improving compatibility for industrial manufacturing. While microbial pigments are commonly utilized in food coloring, flavoring agents, and colors, they are also used in clinical therapeutic against infection diseases. The emergence of antimicrobial resistance has become a major issue in clinical practice. Thus, screening microbial isolates for improved pigment synthesis is a pressing requirement. Microbial pigments are the least studied category of secondary metabolites having antibiotic activity in this regard. Several growth variables, including temperature, pH, NaCl concentration, inoculum size, agitation, and nutrients including carbon and nitrogen sources, have a vital influence, according to the findings of this study. The screened isolates produced carotene and prodigiosin pigments within 48 hours and were easily extracted. All the extracted pigments from our study showed good antimicrobial activity against bacterial pathogens. These pigments might also have potential activities of anti-biofilm and anti-tumor thus will be further studied.
ACKNOWLEDGMENTS
The authors would like to thank Bhavans Vivekananda College of Science, Humanities and Commerce, Sainikpuri, Secundrabad and Department of Microbiology, Osmania University, Hyderabad, India and College of Technology, Osmania University for providing assistance on analysis.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MF and KA designed the experiments. MF compiled information from the literature and designed the Figures and Tables and drafted the manuscript. KA supervised and reviewed the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Prasad MP, Swaroop JK, Maitraya B, Nadir S, Kumar AP. Optimization of physico-chemical parameters for pigment production in bacteria isolated from different effluent water samples. Int Res J Pharm Appl Sci. 2013;3(6):27-30. https://www.scienztech.org/index.php/irjpas/article/view/584 in reference no 1
- Venil CK, Dufosse L, Renuka Devi P. Bacterial pigments: sustainable compounds with market potential for pharma and food industry. Frontiers in Sustainable Food Systems. 2020;4:100.
Crossref - Bennett JW, Bentley R. Seeing red: the story of prodigiosin. Adv Appl Microbiol. 2000;47:1-32.
Crossref - Celedon RS, Diaz LB. Natural Pigments of Bacterial Origin and Their Possible Biomedical Applications. Microorganisms. 2021;9(4):739.
Crossref - Liu S-R, Zhang W-R. Optimization of submerged culture conditions involving a developed fine powder solid seed for exopolysaccharide production by the medicinal mushroom Ganoderma lucidum. Food Sci Biotechnol. 2019;28:1135-1145.
Crossref - Sen T, Barrow CJ, Deshmukh SK. Microbial pigments in the food industry-challenges and the way forward. Front Nutr. 2019;6:7.
Crossref - Usman HM, Abdulkadir N, Gani M, Maiturare HM. Bacterial pigments and its significance.
J Bioequivalence Bioavailab. 2017;4(3):285-288.
Crossref - Cho YJ, Park JP, Hwang HJ, Kim SW, Choi JW, Yun JW. Production of red pigment by submerged culture of Paecilomyces sinclairii. Lett Appl Microbiol. 2002;35(3):195-202.
Crossref - Majumdar S, Priyadarshinee R, Kumar A, Mandal T, Mandal DD. Exploring Planococcus sp. TRC1, a bacterial isolate, for carotenoid pigment production and detoxification of paper mill effluent in immobilized fluidized bed reactor. J. Clean. Prod. 2019; 211:1389–1402.
Crossref - Arulselvi PI, Umamaheswari S, Ranandkumar SG, Karthik C, Jayakrishna C. Screening of yellow pigment producing bacterial isolates from various eco-climatic areas and analysis of the carotenoid produced by the isolate. J Food Process Technol. 2014;5:1.
Crossref - Chakraborty I, Redkar P, Munjal M, Kumar SS, Rao KB. Isolation and characterization of pigment producing marine actinobacteria from mangrove soil and applications of bio-pigments. Der Pharm Lett. 2015;7(4):93-100.
- Usman HM, Farouq AA, Baki AS, Abdulkadir N, Mustapha G. Production and characterization of orange pigment produced by Halophilic bacterium Salinococcus roseus isolated from abattoir soil. J Microbiol Exp. 2018;6(6):238-43.
Crossref - Choubey S, Chapade SV, Garud SA. Optimization of Pigment Production by Micrococcus and Arthrobacter species Isolated from Soil and Water. Int J Res Pharm Sci. 2021;12(3):1902-1907.
Crossref - Prasad MP. Optimization of media parameters for pigment production in bacteria from effluent water samples. Bio life. 2015;3(2):428-33.
Crossref - Ram MS, Kumar NP, Bhaskar CV, Audipudi AV. Optimization and characterization of intracellular orange fluorescent pigment from Bacillus endophyticus (AVP-9 (Kf527823)). Int J Curr Pharm Res. 2017;9(5):67-74.
Crossref - Gunasekaran S, Poorniammal R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr J Biotechnol. 2008;7(12):1894-1898.
Crossref - Poddar K, Padhan B, Sarkar D, Sarkar A. Purification and optimization of pink pigment produced by newly isolated bacterial strain Enterobacter sp. PWN1. SN Appl Sci. 2021;3(1):1.
Crossref - Elkenawy NM, Yassin AS, Elhifnawy HN, Amin MA. Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol Rep. 2017;14:47-53.
Crossref - Suryawanshi RK, Patil CD, Borase HP, Salunke BK, Patil SV. Studies on production and biological potential of prodigiosin by Serratia marcescens. Appl Biochem Biotechnol. 2014;173(5):1209-1221.
Crossref - Biswa P, Doble M. Production of acylated homoserine lactone by Gram-positive bacteria isolated from marine water. FEMS Microbiol Lett. 2013;343(1):34-41.
Crossref - Renukadevi KP, Vineeth M. Extraction, characterization and optimization of pigment produced from serratia marcescens, 2015. https://www.researchgate.net/publication/327513206_extraction_
characterization_and_optimization_of_pigment_produced_from_
serratia_marcescens - Sinha S, Choubey S, Ajay Kumar A, Bhosale P. Identification, characterization of pigment producing bacteria from soil and water and testing of antimicrobial activity of bacterial pigments. Int J Pharm Sci Rev Res. 2017;42(2):119-24.
- Shatila F, Yusef H, Holail H. Pigment production by Exiguobacterium aurantiacum FH, a novel Lebanese strain. Int J Curr Microbiol App Sci. 2013;2(12):176-91. http://www.ijcmas.com/
- Siddharthan N, Sandhiya R, Hemalatha N. Extraction and Characterization of Antibacterial Pigment from Roseomonas Gilardii YP1 Strain in Yercaud Soil. Asian J Pharm Clin Res. 2020;13(3):116-120.
Crossref - Mohana DC, Thippeswamy S, Abhishek RU. Antioxidant, antibacterial, and ultraviolet-protective properties of carotenoids isolated from Micrococcus spp. Radiation Protection and Environment. 2013;36(4):168.
Crossref - AP Kumar. Bacilli-mediated degradation of xenobiotic compounds and heavy metals. Frontiers in Bioengineering and Biotechnology. 2020; 1100.
Crossref - Williams RP, Quadri SM. The pigments of Serratia. In The Genus Serratia, pp. 31–75. Edited by A. Von Graevenitz & S. J.Rubin. Boca Raton, FL: CRC Press Inc, 1980. https://ci.nii.ac.jp/naid/10013583109/
- Bhat MR, Marar T. Media optimization, extraction and partial characterization of an orange pigment from Salinicoccus sp. MKJ 997975. International journal of life sciences biotechnology and pharma research, 4(2): p.85. http://www.ijlbpr.com/papers/12-IJLBPR-A0500.pdf
- Ramasamy AK, Udayasuriyan V. Isolation and Characterization of a Yellow Pigmented Colony Forming Bacterium for Carotenogenesis. Biotechnology, 2006 ;5(1), pp.79-82.
Crossref - Rakh RR, Dalvi SM, Musle BB, Raut LS. Production, extraction and characterization of red pigment produced by Serratia rubidaea JCM 1240T isolated from soil. Int J Curr Microbiol App Sci, 2017;6(1), pp.143-154.
Crossref - Godinho A, Bhosle S. Carotenes produced by alkaliphilic orange-pigmented strain of Microbacterium arborescens-AGSB isolated from coastal sand dunes. 2008 http://hdl.handle.net/123456789/2053
- Yolmeh M. Antimicrobial activity of pigments extracted from Rhodotorula glutinis against some bacteria and fungi. 2016.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.