ISSN: 0973-7510
E-ISSN: 2581-690X
Two industrial soils around west Godavari districts were analyzed for the concentration of nine heavy metals such as zinc, nickel, copper, manganese, cadmium, chromium, lead, iron and arsenic using atomic absorption spectrophotometer and simultaneously isolated nine morphologically distinct bacterial cultures from the same soil to check for the metal tolerance against copper, zinc and lead. The analyzed data revealed that iron and manganese metals were found to be the most abundant metals in these industrial soils and also noticed that the industrial soil 2 contained high amount of Cu, Zn and Pd. Control sample contained very low concentrations of the above mentioned heavy metals. That is therefore nine bacterial cultures (DPMC1-5 and TSC1-4) were checked for their metal resistance to Cu, Zn and Pb in nutrient broth at various metal concentrations. In in vitro application there was a drastically metal reduction observed in soils inoculated with nine bacterial strains. It can be concluded that this work will be the reference for the bioremediation of heavy metal polluted soils. As the industrialization in Bhimavaram creating the environmental pollution and hence treatment of industrial waste with metal resistant microorganisms called bioremediation is highly essential to clear the metal pollution instead of releasing industrial effluents into nearby water bodies or fields or choosing a costly chemical treatment.
Industrial soils, Heavy metal resistant bacteria, Heavy metals, Bioremediation
West Godavari District has a richly cultivated land among all districts in Andhra Pradesh. West Godavari is popularly known as the Granary of India since about 50% of the state’s rice production comes from the district. Cotton barrage was built on River Godavari at Dhavaleswaram channeling two canals, from which one canal passes through West Godavari, making the soil fertile. In the coastal belt of the district, a large portion of prawns and fish is exported to Japan, and the United States. Bhimavaram is a hub for Prawns export. It is the richest town in the State of Andhra Pradesh. Vendra paper mills in Bhimavaram, and Andhra Sugars, a sugar factory, in Tanuku are some of the more famous industries of the district. Due to the rapid industrialization and urbanization, many industries located in and around the district have released their untreated and semi treated effluents into the environment, more particularly to the nearby agriculture fields and water bodies. Even the pollutants such as heavy metals and other chemicals, which are present in the effluents moved through soil, surface water, sediments of the lake bed and percolated into ground water affecting the soil and groundwater quality. However, given significant industrialization and urbanization with ascendance of random dumping of untreated industrial wastewater and municipal sewage in to the environment, heavy metals have been reported to accumulate to unsafe or lethal concentrations1-4. High concentrations of heavy metals are dangerous to animals, plants and human cause many kinds of diseases. Indeed, even the purported essential components like Zn and Fe, if present in high concentration, are lethal in nature5, 6. All heavy metal are not destructive to people, but rather at high focus levels they are poisonous in nature7-10. Studies have shown that toxic metals in soil influence plant growth11. Many studies show that soluble metal ions in the environment could be captured by microorganisms due to the negatively charged groups attached within their cell wall structure12. Bacteria, algae, and fungi or their separated components have been successfully used as bio-sorbents for heavy metal removal13. Heavy metal resistant bacteria represent a better alternative for heavy metal decontamination and have already been successfully applied for such purposes in the developed world14-16. In the present study, 9 different bacterial species were isolated from two different industrially polluted regions; they are Delta Paper Mill (DPMC1-5 colonies) and Tanuku Sugar Factory (TSC1-4 colonies), Bhimavaram. The metal removing ability of those isolates were investigated by in-vitro applications against copper, lead and zinc heavy metals. So in this investigation, an endeavor is made to set up the pathway of removal of heavy metals present in industrial polluted soils by metal resistant bacteria; thereby establishing the pathway of the bioremediation of metal polluted soils by microorganisms.
Collection of Sample
Soil samples of Delta Paper Mill and Tanuku Sugar Factory from 2 different locations each; superficial and 6 inches depth were collected as polluted soil, Nearby agricultural soil was collected as cross contaminated soil and normal soil was collected as a control soil. Soil samples were collected with a sterile spatula in a polythene bags and were transported to the laboratory.
Preparation of Soil Sample
Under a sterile condition, tenfold serial dilution was carried out in a laminar air flow. The stock suspension was prepared by adding 1 gm of the soil sample in 10 ml of distilled water in a first test tube for each soil sample and is vortexes. 1 ml of this solution is taken and transferred to the second test tube containing 10 ml of distilled water and is vortexes, labeled as 10–2 and from second to third, it was labeled as 10-3 and serial dilution was continued up to 10-8. Hence each soil sample was serially diluted in a laminar airflow.
Isolation of Microorganisms
Nutrient agar medium have chosen to isolate bacteria from the soil samples. Media was prepared as per the composition and was sterilized in an autoclave conditions maintained was 121.50C temperature, 15 lbs pressure for 15minutes. After that media was poured in Petri plates and allowed to solidify and placed in an incubator at 37°C for 24 h in order to check its sterility.
Inoculation of Sample
An amount of 0.1 ml of each soil sample from selected dilutions (10-6, 10-7 and 10-8) was transferred on to previously labeled agar plates by spread plate method, each maintained in triplicate. Plates were incubated at 370C for 18-24hrs to obtain pure colonies.
Sub Culturing of Microorganisms
Isolated bacterial colonies with unique morphological characters were picked and sub cultured on fresh nutrient agar slants using sterile loop using streak plate method in laminar air flow to purify the isolates followed by incubation for 24 h at 37 °C and slants were stored at 40c for further investigation.
Analysis of Heavy metals in soil
Atomic absorption spectrometry was used for metal analysis. Soil samples were analyzed for heavy metal concentration for two times. First time metal analysis was done immediately after the collection of soil samples and second time metal analysis was done after the In vitro application (pot inoculation) to know whether the metals were reduced or not upon treating the metal inoculated soil with the isolated bacterial cultures.
Atomic Absorption spectrum
Model used was OPTIMA 8000, Category ICP-OES
Standard Preparation
Mixed elemental standard was prepared using 100 ppm is as follows:
0.5ppm-0.25ml of 100 ppm mixed standard transferred into a 50 ml volumetric flask and diluted up to mark with milli Q water.
1ppm-05ml of 100 ppm mixed standard transferred into a 50 ml volumetric flask and diluted up to mark with milli Q water.
1.5wg/L -0.75ml of 100 ppm mixed standard transferred into a 50 ml volumetric flask and diluted up to marks with milli Q water.
Sample preparation
5 gm of soil sample was taken and transferred into a beaker to which 5 ml of concentrated HNO3 and 20 ml of water was added. It was digested on a hot plate for about 30 minutes at 800 C; immediately it was cooled and diluted in 100 ml flask with water (Filtered the solution and aspirated it).
Determination of Metal resistance
To test for heavy metal resistance, the metals Cu, Zn and Pb, used in the form of CuCl2, ZnCl2, and PbCl2, were added to sterile nutrient broth medium in varying concentrations viz: 5.0 ppm, 10.0, 20.0, 30.0, 40.0, 50.0, 60.0, 70.0, 80.0, 90.0, and 100.0 ppm/ml. Tubes were inoculated with an equal volume of selected bacterial cultures individually isolated from selected sites (Two from two different industries) and they were incubated at 37°C for 24 to 48 hours17. For comparing the growth response at different concentration, one set of nutrient broth (without the heavy metal) containing test tubes were inoculated with the selected organisms and used as control. Each dilution was maintained in triplicate for three times and Growth rate against metals were determined by taking O.D in turbidometer at 620nm.
In-vitro application (Pot inoculation)
For evaluation of bioremediation efficiency among selected bacterial cultures against copper, zinc and lead an attempt was made in the laboratory. Bioremediation of metal polluted soils was carried out in sterile clay pots. After exposure to bacterial cultures metal reduction were determined by atomic absorption spectrophotometer as described previously18. Briefly, 10 ml bacterial suspension which previously grown in 200 mL of sterile nutrient broth overnight were inoculated in to sterile soil contaminated with 60ppm, 68ppm and 70ppm, 0f Cu, Zn and Pb respectively in sterile clay pots for 4 days. After incubation soil sample was dried in oven at 60°C for 10 hours. 10 mg dried soils were dissolved in 1 ml of concentrated nitric acid and diluted to 10 ml with DD/W. Blanks were treated in the same way and analyzed by atomic absorption spectrophotometer. Calibration curve of each heavy metal was drawn with working standard solution before testing. All the statistical analyses were carried out using Model no: Optima 8000 Spectrometer (Atomic): AES: ICP-OES.
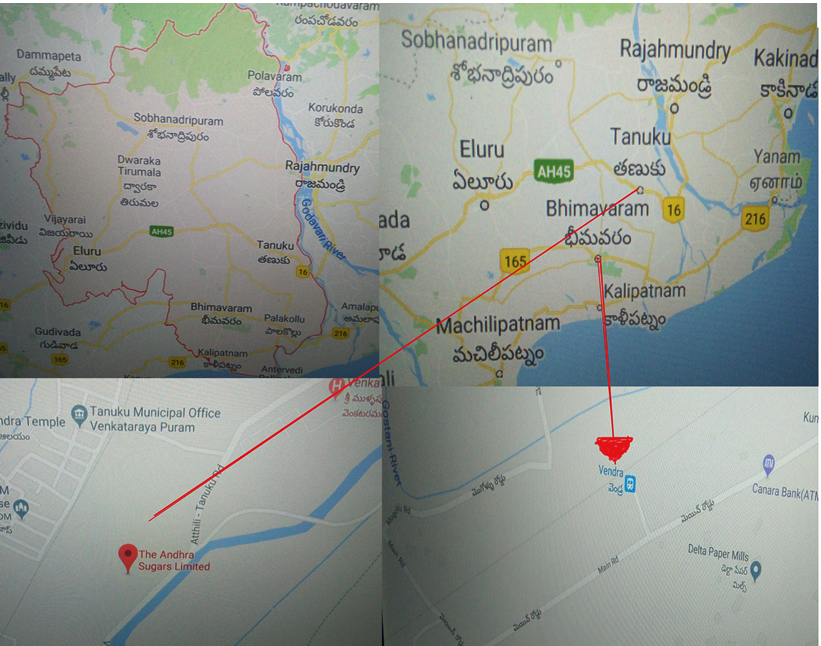
ASS model No: ST-EQ-084 was used for determination of the heavy metal contents of the contaminated soils. Soil samples were taken from 4 different locations; in the vicinity of a heavy metal industrial area in Vendra, Tanuku, Agricultural field nearby industrial effluent out let and soil away from pollutions to take as control; Bhimavaram. (Figure 1).
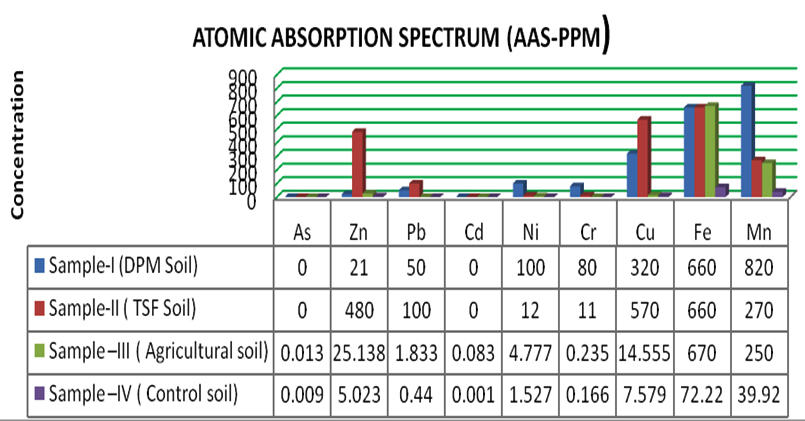
The heavy metal concentrations of these soils were included in the Table 1. The concentrations of Zn, Pb, Cd, Ni, Cr, Cu, Fe and Mn in the industrial soils and agricultural soils were high as compared with the values of the control soil. However, the concentration of As and Cd in the soils was found to be BDL when compared with the Agricutrl soil and control soil. Fe was found to be more in agricultural soil, and it was also be obseved that there was a major difference in the concentration of Zn, Pb Cu and Mn between two industrial samples i.e 21ppm in DPM soil and 480 ppm in TSC soil, 50ppm in DPM soil and 100ppm in TSC soil, 320ppm in DPM soil and 570ppm in TSC soil and 820ppm in DPM soil and 270 in TSC soil respectively. The concentration of Zn, Cu and Pb was high in TSC soils than in DPM soils hence an attempt was made for bioremediation of these three metals in metal-polluted TSC soils by the isolation of bacterrial cultures against Cu, Zn and Pb specifically taking them in its chloride forms as CuCl2, ZnCl2 and PbCl2.
Table (1):
Heavy metal analysis data (Atomic Absorption spectrum).
Soil sample type |
As |
Zn |
Pb |
Cd |
Ni |
Cr |
Cu |
Fe |
Mn |
|---|---|---|---|---|---|---|---|---|---|
Sample-I (DPM Soil) |
BDL |
21 |
50 |
BDL |
10 |
80 |
320 |
660 |
820 |
Sample-II ( TSC Soil) |
BDL |
480 |
100 |
BDL |
12 |
11 |
570 |
660 |
270 |
Sample –III ( Agricultural soil) |
0.013 |
25.138 |
1.833 |
0.083 |
4.777 |
0.2350 |
14.555 |
670 |
250 |
Sample –IV ( Control soil) |
0.009 |
5.023 |
0.44 |
0.001 |
1.527 |
0.166 |
7.579 |
72.22 |
39.92 |
Two different soil samples were innoculated on general purpose medium that is nutrient agar the temperature and pH of the collected samples were maintained at constant conditions that is 37°C and 7.2 respectively. The total bacterial count of each sample are given in Table 2. The total bacterial count ranged from 06 x 107 to 256 x 105(cfu/10 ml of sample).
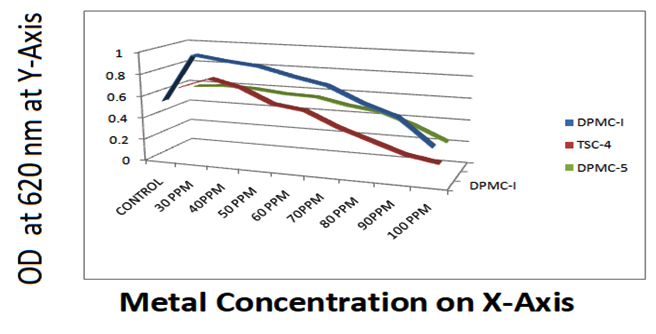
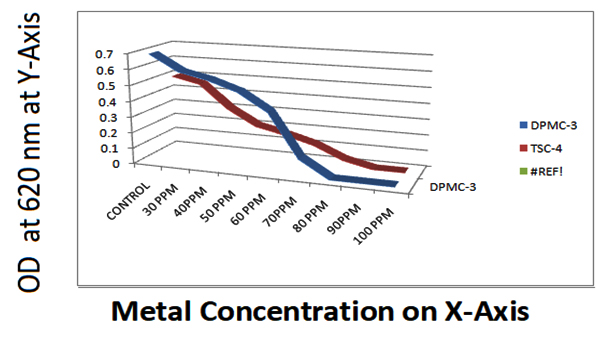
During the period of study, a total number of 256 bacterial colonies were isolated from two industrial samples of two different locations each. Out of the 256 isolates, only 18 isolates were selected for further study on the basis of their morphological diversities. Among 18, 09 isolates, showed different ranges of metal resistant against three different heavy metals namely CuCl2, ZnCl2 and PbCl2 from a minium concentration of 10ppm to 100ppm. At the point when common habitats are discharged with metal pollutants, the indigenous microbial networks are probably going to contain microbial population of various taxonomic qualities, which are equipped for reducing the discharged chemicals 19. Since industrial effluents contain extensive measure of substantial metals, the impact of Cu, Zn and Pb on bacterial development and the capacity to detoxify the toxins in vitro was the critical piece of the present examination. Metal resistance among microorganisms against Cu, Zn and Pb was considered evidently. To study metal resistance among selected colonies, initially bacterial cultures were allowed to grown on Nutrient medium contain Cu and Pb but the results got on the medium containing Cu was unsatisfactory since Cu can bound the agar components 20,21. That is therefore in our investigation, the test was done in nutrient broth supplemented with Cu, Zn and Pb of various dilutions ranges from 10 ppm to 100 ppm. It was discovered that, the development of all the bacterial growth diminished with increasing metal concentration and the growth of each dilution were maintained in three replicates for three times and O.D was measured in turbidimetry at 620 nm and results were tabulated (Table 3, 4 and 5)
Table (2):
Microbial count of the industrial soil samples.
Sample types |
Location |
p H |
Temperature |
Colony forming units |
|---|---|---|---|---|
DPM |
Site -I |
7.0 |
370C |
256×105 |
Site-II |
7.0 |
370C |
83×107 |
|
TSC |
Site-I |
7.0 |
370c |
10×106 |
Site-II |
7.0 |
370c |
6×107 |
Table (3):
Effect of Copper on bacterial growth.
S.No |
Culture type |
Control |
CuCl2 (30ppm/ml) |
CuCl2 (40ppm/ml) |
CuCl2 (50ppm/ml) |
CuCl2 (60ppm/ml) |
CuCl2 (70ppm/ml) |
CuCl2 (80ppm/ml) |
CuCl2 (90ppm/ml) |
CuCl2 (100ppm/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
01 |
DPMC-I |
0.55 |
0.98 |
0.94 |
0.91 |
0.84 |
0.78 |
0.65 |
0.55 |
0.32 |
02 |
DPMC-2 |
0.49 |
0.57 |
0.58 |
0.48 |
0.38 |
0.38 |
0.22 |
0.10 |
0.06 |
03 |
DPMC-3 |
0.54 |
0.58 |
0.52 |
0.48 |
0.46 |
0.38 |
0.26 |
0.10 |
0.09 |
04 |
DPMC-4 |
0.56 |
0.60 |
0.55 |
0.55 |
0.44 |
0.32 |
0.22 |
0.10 |
0.07 |
05 |
DPMC-5 |
0.58 |
0.60 |
0.59 |
0.56 |
0.55 |
0.49 |
0.45 |
0.35 |
0.22 |
06 |
TSC-1 |
0.49 |
0.48 |
0.37 |
0.28 |
0.27 |
0.23 |
0.14 |
0.04 |
0.00 |
07 |
TSC-2 |
0.40 |
0.47 |
0.28 |
0.23 |
0.18 |
0.13 |
0.09 |
0.00 |
0.00 |
08 |
TSC-3 |
0.45 |
0.50 |
0.45 |
0.35 |
0.30 |
0.18 |
0.14 |
0.06 |
0.00 |
09 |
TSC-4 |
0.62 |
0.72 |
0.65 |
0.52 |
0.48 |
0.35 |
0.25 |
0.15 |
0.10 |
Table (4):
Effect of Zinc on bacterial growth (OD at 620nm).
S.No |
Culture type |
ZnCl2 (20ppm/ml) |
ZnCl2 (30ppm/ml) |
ZnCl2 (40ppm/ml) |
ZnCl2 (50ppm/ml) |
ZnCl2 (60ppm/ml) |
ZnCl2 (70ppm/ml) |
ZnCl2 (80ppm/ml) |
ZnCl2 (90ppm/ml) |
ZnCl2 (100ppm/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
01 |
DPMC-I |
0.45 |
0.38 |
0.21 |
0.06 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
02 |
DPMC-2 |
0.43 |
0.34 |
0.20 |
0.06 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
03 |
DPMC-3 |
0.69 |
0.59 |
0.55 |
0.49 |
0.38 |
0.11 |
0.00 |
0.00 |
0.00 |
04 |
DPMC-4 |
0.48 |
0.43 |
0.27 |
0.09 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
05 |
DPMC-5 |
0.40 |
0.37 |
0.25 |
0.07 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
06 |
TSC-1 |
0.61 |
0.50 |
0.31 |
0.14 |
0.09 |
0.05 |
0.00 |
0.00 |
0.00 |
07 |
TSC-2 |
0.43 |
0.36 |
0.23 |
0.16 |
0.10 |
0.01 |
0.00 |
0.00 |
0.00 |
08 |
TSC-3 |
0.39 |
0.26 |
0.20 |
0.15 |
0.07 |
0.05 |
0.00 |
0.00 |
0.00 |
09 |
TSC-4 |
0.50 |
0.46 |
0.31 |
0.21 |
0.17 |
0.12 |
0.04 |
0.00 |
0.00 |
Table (5):
Effect of Lead on bacterial growth (OD at 620nm).
S.No |
Culture type |
PbCl2 (20ppm /ml) |
PbCl2 (30ppm /ml) |
PbCl2 (40ppm /ml) |
PbCl2 (50ppm/ml) |
PbCl2 (60ppm /ml) |
PbCl2 (70ppm/ml) |
PbCl2 (80ppm/ml) |
PbCl2 (90ppm/ml) |
ZnCl2 (100ppm/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
01 |
DPMC-I |
0.33 |
0.22 |
0.20 |
0.15 |
0.14 |
0.00 |
0.00 |
0.00 |
0.00 |
02 |
DPMC-2 |
0.25 |
0.19 |
0.20 |
0.15 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
03 |
DPMC-3 |
0.31 |
0.31 |
0.18 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
04 |
DPMC-4 |
0.49 |
0.34 |
0.23 |
0.19 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
05 |
DPMC-5 |
0.50 |
0.28 |
0.15 |
0.10 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
06 |
TSC-1 |
0.42 |
0.33 |
0.25 |
0.10 |
0.06 |
0.00 |
0.00 |
0.00 |
0.00 |
07 |
TSC-2 |
0.48 |
0.30 |
0.20 |
0.10 |
0.02 |
0.00 |
0.00 |
0.00 |
0.00 |
08 |
TSC-3 |
0.55 |
0.47 |
0.28 |
0.17 |
0.01 |
0.00 |
0.00 |
0.00 |
0.00 |
09 |
TSC-4 |
0.64 |
0.55 |
0.46 |
0.39 |
0.25 |
0.16 |
0.09 |
0.00 |
0.00 |
Isolate DPMC1 showed highest resistant against CuCl2 than ZnCl2 and PbCl2, where as TSC4 showed metal resistant against CuCl2, ZnCl2 and PbCl2. DPMC2 was showed metal resistant agisnt CuCl2 and ZnCl2 and least resistant among all against PbCl2.
DPMC3 and TSC4 have showed metal resistant more against ZnCl2 than other cultures. DPMC4 and DPMC5 have showed metal resistant more agiainst CuCl2 and PbCl2 and least resistant against ZnCl2 among all. TSC2 have showed least resistant among all for 3 heavy metals. TSC1 showed metal resistant aginst Zncl2 and Pbcl2 and least against CuCl2 like that of TSC2.
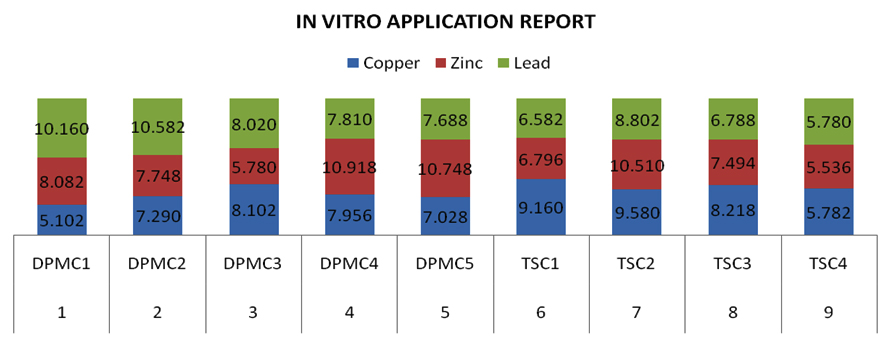
The study was extended next to in vitro application for proving the cultures can be adopt for bioremediation of metal-polluted soil. The data regarding in vitro application was tabulated in Table 6. There was a drastical metal reduction was observed in the atomic absorption report. And it was obsreved and proved that the cultures which showed metal resistant in tube dilutions more, have also be reduced metal concentration in in vitro application (Fig. 6). Additionally investigation of the impacts of various supplements and conditions in their development is expected to distinguish their effectiveness as bioremediation specialists, where optimization of pH, temperature, and incubation time can impact metal obstruction limit22.
Table (6):
In vitro Application Report.
S. No |
Type of Bacterial culture |
Copper |
Zinc |
Lead |
|---|---|---|---|---|
1 |
DPMC1 |
5.102 |
8.082 |
10.160 |
2 |
DPMC2 |
7.290 |
7.748 |
10.582 |
3 |
DPMC3 |
8.102 |
5.780 |
8.020 |
4 |
DPMC4 |
7.956 |
10.918 |
7.810 |
5 |
DPMC5 |
7.028 |
10.748 |
7.688 |
6 |
TSC1 |
9.160 |
6.796 |
6.582 |
7 |
TSC2 |
9.580 |
10.510 |
8.802 |
8 |
TSC3 |
8.218 |
7.494 |
6.788 |
9 |
TSC4 |
5.782 |
5.536 |
5.780 |
The polluted soils are high medium to develop and spread of resistant microbial populations, which are resistant to various metals. The recognizable proof of opposition against various metals may give a valuable device to the synchronous observing of a few harmful contamination in the earth. It was demonstrated that industrial waste are the source of highly resistant forms. All the bacterial strains isolated in this investigation demonstrated against copper, zinc and lead have showed high levels of metal resistance. The future prospect lies in the use of these microorganisms for purposes like overwhelming metal remediation of metal-polluted soils. All the results formulated in this study proved that DPMC1, DPMC5 and TSC4 cultures showed varing degree of metal resistant against Cu, Pb and Zn and that is therefore these cultures might be helpful for the detoxification of heavy metal pollution in the industrial effluents at industrial surroundings.
ACKNOWLEDGMENTS
The authors are highly appreciative to the Department of Microbiology Acharya Nagarjuna University Nagarjuna Nagar, ASN aqua labs Bhimavaram and B.V.Raju College Vishnupur Bhimavaram for their help and continues encouragement in the fruit full completion of this project work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Iwegbue, C. M. A., Nwajei, G. E., Ogala, J. E. and Overah, C. L. Determination of trace metal concentrations in soil profiles of municipal waste dumps in Nigeria. Environmental Geochemistry and Health, 2010; 32: 415-430.
- Jarup, L., Berglund, M., Elinder, C. G., Nordberg, G., Vahter, M., Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand. J. Work Environ. Health, 1998; 24: 1–52.
- Kumar, A., Bisht, B. S. and Joshi, V. D. Bioremediation potential of three acclimated bacteria with reference to heavy metal removal from waste. International Journal of Environmental Sciences, 2011; 2: 896-908.
- Nies, D. H., Microbial heavy-metal resistance. Applied Microbiology and Biotechnology, 1999; 51: 730-750.
- Aswathanarayana, U., Geoenvironment: an introduction, Rotterdam, the Netherlands, A.A. Balkema Publishers, 1995; pp: 270.
- Govil, P.K., Reddy, G.L.N., and Krishna A.K., Soil contamination due to heavy metals in Patancheru industrial development area Environmental Geology, 2001; 41: pp: 461-469.
- Salem, H.M.; Eweida, E.A.; Farag, A. Heavy metals in drinking water and their environmental impact on human health. In ICEHM 2000; Cairo University: Giza, Egypt, 2000; pp. 542–556.
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, Article 20
- Padmavathiamma, P.K.; Li, L.Y. Phyto remediation technology: Hyper accumulation metals in plants. Water Air Soil Poll. 2007, 184: 105–126.
- Gadd, G.M. Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr. Opin. Biotechnol. 2000, 11: 271–279.
- Singh Jiwan; Kalamdhad Ajay S. Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life: Int. J. Res. Chem. Environ. 2011; 1(2); 15-21.
- Doyle R, Matthews T, Streips U. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J Bacteriol, 1980; 143: 471-480.
- Wang L, Chua H, Zhou Q et al. Role of cell surface components on Cu2+ adsorption by Pseudomonas putida 5-x isolated from electroplating effluent. Water Res,. 2003; 37: 561-568.
- Nakamura K., Hagimine M., Sakai M. and Furukawa K. Removal of mercury from mercury-contaminated sediments using a combined method of chemical leaching and volatilization of mercury by bacteria. Biodegradation, 1999; 10: 443-447.
- Okino, S., Iwasaki, K., Yagi, O. and Tanaka, H. Removal of mercuric chloride by immobilized cells of genetically engineered mercury- volatilizing bacterium Pseudomonas putida Pp Y101/pS134. Bill. Environ. Contam. Toxicol. 2002; 68: 712-719.
- Sinha, K. R., Valani, D., Sinha, S., Singh, S. and Herat, S. Bioremediation of contaminated sites: A low-cost nature biotechnology for environmental clean-up by versatile microbes, plants and earthworms. In: Solid waste management and environmental remediation. Timo Faerber and Johann Herzog. 2009; ISBN: 978-1-60741-761-3.
- Calomoris JJ, Armstrong TL & Seidler RJ. Association of metal- tolerance with multiple antibiotic resistances of bacteria isolated from drinking water. Appl Environ Microbiol., 1984; 47: 1238-1242.
- Shakibaie, M. R., Dhakephallker, P. K., Kapadnis, B. P., Chopade, B. A., Removal of Silver from Photographic waste Water effluents by Acinetobaeter baumnannii BL54: Can. J. Microbiol, 1999; 45: 995-1000.
- Bartha R & Atlas RM. The microbiology of aquatic oil spills. Adv Appl Microbiol. 1977; 22: 225-266.
- Ramamoorthy S & Kushnor DJ. Binding of mercuric and other metal ions by microbial growth media. Microbial Ecol. 1975; 2: 162-175. 16.
- Washington II JA, Snyder RJ, Kohner PC, Wiltse CG, Itstrup DM & McCall JT. Effect of cation content of agar on the activity of gentamycin, tobramycin and amikacin against Pseudomonas aeruginosa. J. Infectious Diseases. 1978; 137: 103-111.
- Shivakumar et al., C. K. Shivakumar, B. Thippeswamy, M. Krishnappa Optimization of heavy metals bioaccumulation in Aspergillus niger and Aspergillus flavus Int. J. Environ. Biol., 2014 ; 4(2): pp. 188-195.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.