ISSN: 0973-7510
E-ISSN: 2581-690X
Thermophilic bacteria live at temperatures above 450 C. Many investigations focused on their potential as sources of highly active enzymes ‘termostable enzyme’ and other products such as antibiotics and compatible solutes. Lake Linow is an active volcanic lake located in Tomohon City, North Sulawesi, Indonesia. Lake Linow becomes the habitat of thermophilic bacteria. A study has been conducted to obtain isolates of thermophilic bacteria and to screen for the potential of thermostable amylase enzymes produced by thermophilic bacteria, Linow lake isolates. Stages of research that have been done are bacterial isolation, thermotolerance test, morphological analysis, biochemical test and amylase activity screening. The results of this research were obtained six isolates that can survive at 450C and three isolates that can survive at 550C. Two bacterial isolates showed amylolytic activity at a temperature of 550C-600C. Thus, isolate thermophilic bacteria from Linow lake, potentially as a source of thermostable amylase enzyme.
Lake Linow, Termophilic bacteria, Amylase
Indonesia is geologically located at the meeting of three major tectonic plates, the European-Asian plate, the Indian-Australian plate and the Pacific plate, which play a role in the process of forming volcanoes in Indonesia (Gorsel, 2016). This geological condition, giving a real contribution to the availability of geothermal energy in Indonesia (Nazhib, 2014). Geothermal manifestations of no less than 244 locations, scattered on the island of Sumatra, Java, Bali, Borneo, the islands of Nusa Tenggara, Maluku Sulawesi Island, Halmahera and Irian Jaya, show how much geothermal energy wealth is stored in it (Ambarsari, 2005 ).
North Sulawesi province, geologically has many volcanic sites. This is because the island of Sulawesi is a meeting of two world volcanic circles, especially the Pacific (Hall and Spakman, 2015). Volcanic sites in North Sulawesi include; Soputan volcano area in Southeast Minahasa, Lokon volcano region, in Tomohon city, the Karagetang volcano region in the Sanger Talaud archipelago. Lake Linow is part of Mount Lokon volcanic region. Lake Linow is located in the town of Tomohon and adjacent to the geothermal exploration area. Lake Linow is a volcanic lake with high sulfur and hot mud activity.
Lake Linow has a special characteristic that changes the color of water, so it is also known as the three color lake. The color change is caused by the difference of volcanic sites and sulfur content in some parts of the lake. Another uniqueness of Lake Linow is around this lake there are endemic animals, among other insects that locals call “sayok” or “komo”. Unique insects that live in the water, but winged and can fly is consumed by the locals (Rigawa, 2013).
One characteristic of the volcanic region is the high temperature. This condition becomes a barrier for living things, to be able to adapt and survive. Thermophilic bacteria are one of the microbes that can survive at high temperatures and grow optimally at 450C – 800C, even thermophilic bacteria, which can survive at 1000C (Lestari, 2000, Ambarsari et.al. 2005; Yuliana & Nuniek 2014). Thermophilic bacteria are able to survive and thrive in high temperature conditions because thermophilic bacteria proteins are more stable and heat resistant than mesophilic because the proteins contained in thermophilic bacterial cells have very strong hydrophobic bonds and ionic bonds (Nelson and Cox, 2005; Gayar et.al. 2017). The cell membrane composition of thermophilic bacteria is composed by saturated fatty acids which can be stable at high temperatures (Ambarsari et al 2005). Thermophilic bacteria can produce thermophile and thermostable enzymes (Nelson and Cox, 2005). Thermophiles are growing optimally between 55 and 80°C, while hyperthermophiles grow above 80 °C (Brock 1978; Bertoldo et al., 2002; Balsam et.al. 2017). They may be Gram positive or Gram negative, spore forming or not, and may exhibit an aerobic or anaerobic metabolism. Their study has become a major domain of research and several new thermophilic genera and species have recently been described (Yoneda et al., 2013; Cihan et al., 2014). In Indonesia, they were intensively studied due to their potential to produce thermostable enzymes (proteases, amylases, lipases, xylanases) (Ginting, 2008, Ambarsari et. al. 2005). Thermo-enzymes are usually not only thermostable, but also active at high salinity and extreme pH (Gomez and Steiner, 2004; Aanniz et.al. 2014).
Applications of amylase enzymes in industry are high, among others in the food industry, health and environment (Leveque et. al. 2000). Amylase can serve as a sugar hydrolysis, which is widely used for the production of glucose syrup or high fructose fructose syrup. The amylase enzyme produced by thermophilic bacteria has been applied to the manufacture of glucose syrup at 600C for 72 hours (Yunianta, 2010), bleaching waste paper at a temperature of 500C, hydrolysis of starch to maltose and glucose in bread and baby food making (Sebayang, 2005). Thus the amylase produced by thermophilic bacteria is very potential and economic value in the field of industry. The search for thermophilic bacterial isolates on volcanic sites in Indonesia in addition to enriching isolates of thermophilic bacteria recorded in Indonesia, also obtained new isolates as a source of thermostable enzymes. Lake Linow is a unique ecosystem, which is strongly believed to store potential thermophilic microbes and has never been explored. No research reports and research publications have been conducted on exploration of thermophilic bacteria at the volcanic site of Lake Linow Tomohon. Thus, the research that has been done is a preliminary study. The research has been conducted to isolate and test the activity of thermophilic bacteria bacteria producing amylase enzyme from Lake Linow North Sulawesi.
Tools and Materials
Equipment used in this research include: sterile bottle, thermometer, universal pH, hot water flask, petri dish, reaction tube, freezer, Mammert incubator, erlenmeyer, beaker glass, analytical balance, measuring cylinders, stirrer rods, hot plate, laminar air flow, vortex, dropper drip, ose needle, electric autoclave, eppendorf micropipette, glass grill, Hirox KH8700 digital microscope, slide term, paper disc, bunsen, cotton, paper label and centrifuge eppendorf. The materials used in this study include: mud samples from North Sulawesi’s linow lake, nutrient agar (NA) Merck, nutrient broth (NB) Merck, yeast extract, peptone (Merkc), MgSO4.7H2O, CaCl2.2H2O, NaCl, agar , sterile aquades, 70% alcohol, lugol, Rose Brand rice flour as starch source, aluminum foil, gauze, iodine solution and McFarland solution scale 1.
Research Methods
The research has been conducted using quantitative descriptive method with laboratory experiments. The research stages are shown in the research flow diagram (Figure 1).
Research Procedure
Sterilization
All tools and media grow bacteria, which have been used, first sterilized. Sterilization tools and bacteria growing media, carried out with automatic autoclave at 121°C and pressure 15 psi for 15 minutes. Equipment and materials in sterilization are tools and materials that are heat resistant and not damaged. Sterilization of the tool aims to avoid contamination of the tools used in the study.
Preparation of bacteria growing media
Preparation of nutrient agar medium (NA)
Medium NA is made by weighing NA as much as 20g. Then put into beaker glass and added sterile aquades until volume 1000 mL gradually. The mixture is heated to boiling, after boiling medium poured into a sterile erlenmeyer then sealed with cotton and aluminum foil. The medium was sterilized in an autoclave at a temperature of 1210C and a pressure of 15 psi for 15 min.
Preparation of media to be selectively amylolytic
Amilolytic selective medium was prepared by weighing yeast extract as much as 2 g, 10 g of starch, pepton of 5 g, MgSO4.7H2O of 0.5 g, NaCl 0.5 g, CaCl2.2H2O of 0.15 g, and for as much as 20 g. Then the ingredients are inserted into a beaker glass and added a sterile aquades to a volume of 750 mL. The mixture is heated to boiling, after boiling medium poured into a sterile erlenmeyer then sealed with cotton and aluminum foil. The medium was sterilized in an autoclave at a temperature of 1210 C and a pressure of 15 psi for 15 min.
Preparation of starch medium
The starch medium is prepared by weighing starch as much as 7.5 g and agar 20 g, then put into beaker glass and added sterile aquades up to 750 mL volume. The mixture is heated to boiling, after boiling medium poured into a sterile erlenmeyer then sealed with cotton and aluminum foil. Medium sterilized in autoclave at 1210C and 15 psi pressure for 15 min.
Sample collection
The sampling of isolates suspected to contain thermophilic bacteria was taken as hot mud from Linow lake, North Sulawesi. Before the samples are taken, the physical and chemical parameters are measured first. The measured physical parameter is the sludge temperature at the sampling point by using a thermometer dipped for 3 minutes into the source of sampling. The measured chemical parameters were pH of the mud at the point of sampling by pH meter dipped to the surface of the sludge, then the color obtained was matched with the pH table listed on the universal pH box. The mud samples were taken as much as 100 mL and put into a sterile thermos to maintain the temperature. Samples were then taken to the bioactivity and molecular biology laboratory, Manado State University and immediately isolated.
Isolation of Bacteria and Pure Culture
Samples in the flask were poured into a 250 ml bottle, shaken for homogenous bacterial isolation by pouring sterile NA medium into sterile petri dishes. After a solid medium of 0.1 mL the sample of hot mud is taken by micropipette and inserted into a petri dish, then dispersed with a drill glass on the agar surface, then incubated at 520C growing temperature for 24-48 hours, so that there are visible bacterial colonies grow. The bacterial colonies grown on NA medium were inoculated into sterile Petri dishes containing NA mediums with quadrant method, then incubated at 520C for 24-48 hours until visible single colonies grew. Each sample is repeated 2 times. Pure isolates that grow are characterized by microscopic observations in the form of colony, colony color, colony edge and colony elevation (Ginting, 2009).
Thermotolerantion Test
Each bacterial isolate was inoculated into 10 ml of Nutrient Broth medium in the test tube. The tube was incubated at an initial temperature of 70 0 C for 48 hours. After the incubation period of each bacterial culture was inoculated by a scratch method, on the surface of the prepared NA medium. Bacterial isolates grown in petri dishes, selected and tested again thermotolerance at higher temperatures. Isolate bacteria that can tolerate temperature 500C – 700C is selected, for testing the activity of thermostable amylase enzyme.
Termostable Amylase Activity Test
The amylase activity test of thermophilic isolates has been carried out following Ginting method (2009). The thermophilic isolates were previously grown on amylolytic selective bacterial media for 24 hours at 60°C. Then the growing isolates were tested in suspension form. The suspension was prepared by taking 1-2 ose bacterial isolates that were 24 hours old on the amylolytic selective medium and fed into a sterile reaction tube containing 0.85% physiological NaCl solution. The mixture homogenised with vortex, turbidity of the mixture compared to turbidity The 1-scale McFarland solution equivalent to 3×108 CFU / mL. If the turbidity level is comparable then further 0.1 mL bacterial suspension is dripped onto the disc paper on starch medium, then incubated for 72 hours at 50 ° C. Bacterial isolates grown spilled with iodine solution to select the amylase-producing bacteria. Isolates that produce amylase are indicated by a clear zone around bacterial colonies. The dimensions of the formed bacterial zones are measured using a sliding range.
Observation
Observations have been made that is macroscopic observation. Macroscopic observation is the observation of colony morphology, including shape, edges, elevation and colony color. Observation of amylase enzyme activity by looking at clear zone formed on isolate thermophilic bacteria producing amylase enzyme obtained from Lake Linow hot mud source of North Sulawesi.
Data Analysis
Data of research result that is characteristic of bacteria isolate was analyzed descriptively. Test the thermostable enzyme activity qualitatively by looking at the diameter of the clear zone formed.
Physiochemical characteristics of hot mud samples from the Linow lake volcanic site
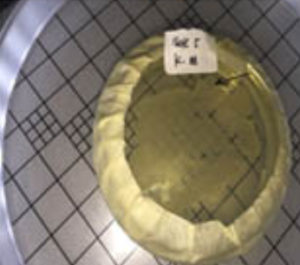
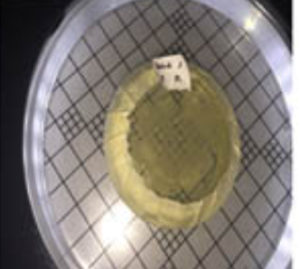
Six isolated thermophilic bacteria have been isolated, in hot mud samples from Linow lake, Lahendong village, Tomohon city (Table 1, Figure 1). Thermophilic bacteria are grown on nutrient media to in vitro in the laboratory, can survive in the temperature range 450C up to 700C. At temperatures above 550C, oxygen can still dissolve well, so the bacteria inside are aerobic bacteria. At each station, bacteria isolates are obtained, with different amounts. Differences in the number of isolates obtained in each station were influenced by site site characteristics (Figure 1). These characteristics, namely physicochemical characteristics. To maintain the temperature conditions from the sampling site to the laboratory, a sample of hot mud, preserved in a thermos (Fig. 2 and Fig. 3).
Table (1):
The physiochemical characteristics
| Statiun | The physiochemical characteristics | |
|---|---|---|
| at Linow Lake | at Laboratorium | |
| I | Temperature : 90°C Colour : black / grey Texture : semi solid |
Temperature : 37.3°C pH : 7,08 Oxygen dissolved : 7,16 mg/l Salinity: 0,3 Colour : hitam Texture : semi solid |
| II | Temperature : 110°C Colour : hitam Texture : semi solid |
Temperature : 36°C pH : 7,10 Oxygen dissolved: 7,40 mg/1 Salinity : 1,4 Colour : coklat TeXture : semi solid |
| III | Suhu : 90°C Warna lumpur : hitam Tekstur : semi solid |
Temperature : 37.3°C pH : 8,35 Oxygen dissolved : 7,69mg/l Salinity : 1,5 Colour : hitam Texture : semi solid |
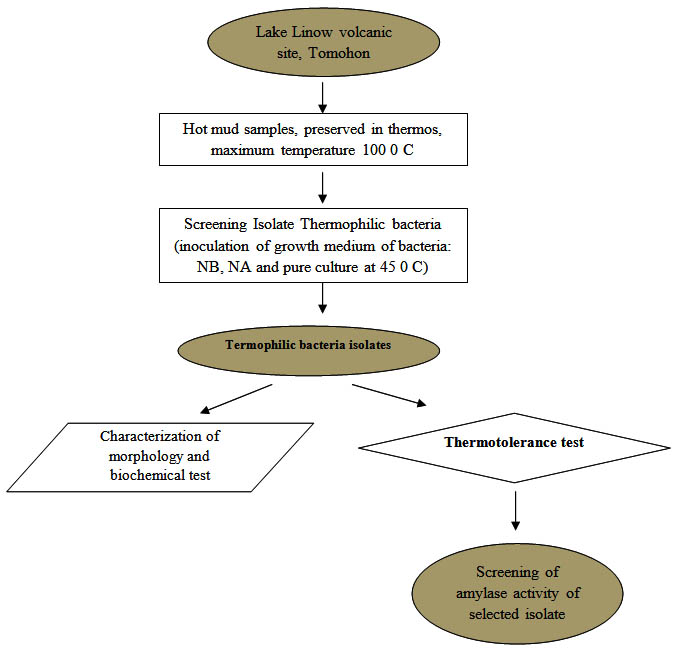
Fig. 1. Diagram of the research flow
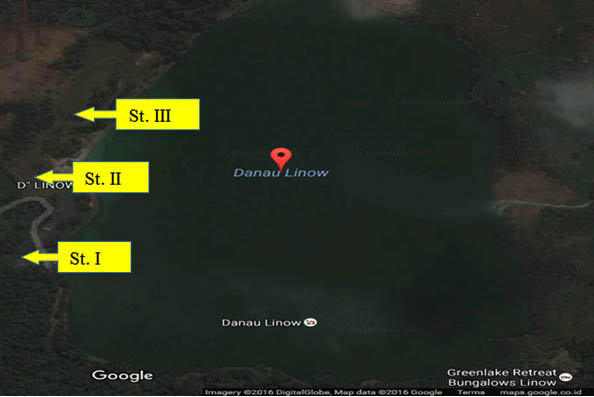
Fig. 2. Sampling sites at Linow Lake, Tomohon (St = station)

Fig. 3. Location of sampling, hot mud, in Linow lake, Tomohon
The physiochemical characteristics of the sample, at station I, at the sampling site, the temperature of the hot mud is 90 0 C. Semi solid mud form, bright black mud color and smell of sulfur, while the physiochemical characteristics of samples in laboratory that has a temperature of 400C; pH: 7.10; oxygen content: 7.40 mg / l; salinity 1.4; brown sample color and semi solid texture. Characteristics of samples at station II, the location of sampling has a temperature of 1100C with the color of gray mud, semi-solid texture and smell of sulfur. The physicochemical characteristics of the lab samples had a temperature of 400C; pH: 7.10; oxygen contents: 7.40 mg / l; salinity 1.4; brown sample color and semi solid texture. Physiochemical characteristics of samples at station III, where sampling has a mud temperature of 900C with gray mud color, semi-solid texture and sulfur smell. While the physiochemical characteristics of the samples in laboratory were 380C; pH: 8.35; oxygen content: 7.69 mg / l; salinity 1.5; brown sample color and semi-solid texture (Table 1).
Isolate thermophilic bacteria
Inoculation was done on nutrient agar medium, with incubation time 2 x 24 hours at
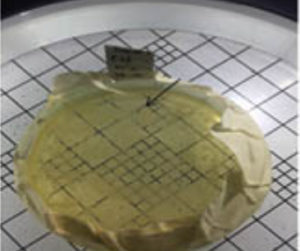
450 C. Samples from station I, obtained 2 isolates, samples from station II, obtained 2 isolates while samples from station III, obtained 2 isolates (Figure 4).

Fig. 4. Hot mud samples, preserved in a thermos, are directly inoculated on NA media in the laboratory
Mophology and Biochemistry Characteristics of Termophilic Bacteria Isolates
Morphological characteristics
Bacterial isolates were grown on nutrient agar medium with a pH between 7.0 and 7.2. Characteristic of isolate morphology obtained by colony form: irregular and spread, round; Colony color: white, clear white, yellowish white and beige; the edges of the colony: irregular, serrated and slippery; and colony elevations: such as crater, arise, convex and flat (Table 2). Isolation of thermophilic bacteria, carried out by the method of dilution of the casting plate and the scratch method. Samples from station I, yielding 2 isolates, samples from station II, yielding 2 isolates and samples from station III, yielding 2 isolates. The isolate obtained can be distinguished by its morphological characteristics (Table 2 and Figure 4). Screening of thermophilic bacteria on NA medium was started at incubation temperature of 450C. Pure culture of each isolate at 450C showed consistent growth for 2×24 hours.
Table (2):
Morphology characteristics of termophylic bacteria isolate from Lake Linow
Location |
Code |
Early temperature |
Optimum temperature |
Colony form |
Colour |
The edges of the colony |
Elevation |
|---|---|---|---|---|---|---|---|
Station I |
St.1.1 |
45°C |
70°C |
Tidak beraturan dan menyebar |
Putih bening |
Tidak beraturan |
Datar |
St.1.3 |
45°C |
70°C |
Bundar |
Putih kekuningan |
Licin |
Seperti kawah |
|
Station II |
St.2.1 |
45°C |
65°C |
Bundar |
Putih bening |
Licin |
Datar |
St.2.2 |
45°C |
65°C |
Tidak beraturan dan menyebar |
Putih kekuningan |
Bergerigi |
Cembung |
|
Station III |
St.3.1 |
45°C |
65°C |
Tidak beraturan dan menyebar |
Putih kekuningan |
Bergerigi |
Cembung |
St.3.2 |
45°C |
65°C |
Tidak beraturan dan menyebar |
Putih kekuningan |
Bergerigi |
Cembung |
Table (3):
Biochemical test results of thermophilic bacteria isolates
Isolate |
catalase |
Metil Red |
Voges Proskeaur |
Indol |
Ornithine |
citrat |
|---|---|---|---|---|---|---|
St.1.1 |
+ |
+ |
– |
– |
+ |
– |
St.1.3 |
– |
– |
– |
+ |
+ |
+ |
St.2 |
+ |
+ |
+ |
+ |
– |
+ |
St.2.2 |
+ |
+ |
+ |
– |
– |
+ |
St.3. |
+ |
+ |
+ |
+ |
– |
– |
St.3.1 |
– |
– |
– |
+ |
– |
– |
Description : (+) = result of test positive, (-) = result of test negative
Gram Staining
Gram staining was performed to obtain to obtain morphological and cell wall characteristics, isolates of thermophilic bacteria. From the results of the obtained Gram staining, four isolates of the thermophilic bacteria (isolates of St. 1.3., Isolates St. 2.1, isolates St. 2.2 and isolates St. 3.1.), were classified to the Gram positive. Meanwhile, two isolates of thermophilic bacteria (isolates St.1.1 and isolates St. 3.2.), were classified as gram negative (Figure 5). Microscopic observation, acquired the characteristics of a single colony of bacteria, with the shape of the stem cells (Bacillus).
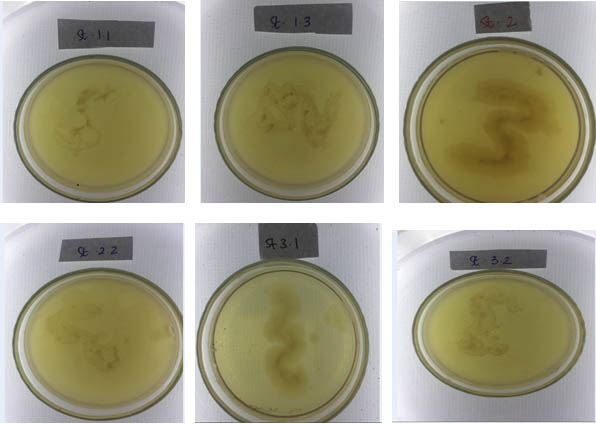
Fig. 5. Isolates of thermophilic bacteria, obtained from 3 sampling stations on the volcanic site of Lake Linow Tomohon. (St. 1.1: Station 1 isolate 1, St. 1.3: station 1 isolate 3, St. 2.1: Station 2 isolates 1, St.2.2.: Station 2 isolate 2, St. 3.1 station 3 isolates 1 and St. 3.2: Station 3 isolates 2
Biochemical characteristics of thermophilic bacterial isolates
Biochemical tests of thermophilic bacterial isolates show different response to substrate that has been used. Isolates St1.1 showed positive activity on catalase, methyl red and ornitin tests while negative activity on Voges Proskeaur, Indol and Citrate tests. Of the nine isolates obtained, the isolates of St.1.3 showed a positive response almost in all the biochemical tests performed except the ornithine test. While isolate St. 1.1, showed a negative response almost on all biochemical tests except indol test. Samples from station II (St.2.1 and St.2.2) showed the results of biochemical tests with the most positive responses compared to the other samples. The results of this study obtained a biochemical test of isolates showed a varied response on six test parameters performed (Table 4).
Table (4):
Results of thermotolerantion test of termophilic bacteria isolates from Linow Lake at 45°C
Isolate Code |
Characteristic |
Isolate |
|---|---|---|
St.1.1.PB |
There is a growth of colonies, isolates of clear white |
|
St.1.3.PM |
There is a growing colony, a clear white isolate |
|
St.3.PB |
There is a growing colony, a clear white isolate |
|
St.3.2KB |
There is a growing colony, a clear white isolate |
Thermotolerance Test
The isolate obtained, tested thermotolerance to obtain the optimum temperature in which the isolate of thermophilic bacteria can survive. The thermotolerance test is carried out according to the method used by Ambrasari et al. (2005), with scratching technique on agar nutrient media. Incubation is carried out for 2 x 24 hours at a constant temperature. The result of thermotolerance test at constant temperature 450C, obtained two isolates from station I, namely isolate St.1.1 and St. 1.3, can live 2 x 24 hours at constant temperature 450C. While isolate from station II obtained isolate of St. 3.1. and St. 3.2. which can live 2 x 24 hours at a constant temperature of 450C (Table 1). The thermotolerance test was continued at a temperature of 550C using a test isolate at a temperature of 450C subcultured. Test results at a constant temperature of 55 0 C for 2 x 24 hours obtained isolates St. 1.1, St. 3.1., And St. 3.2. able to live, characterized by bacterial colony growth (Table 5). Thus, only 3 isolates can survive at a temperature of 550C.
Table (5):
Observation of thermotolerance test of thermophilic bacteria from lake Linow Station II, at temperature 55°C, yield 3 isolates
Isolate Code |
Characteristics |
Isolate |
|---|---|---|
St.1.1 PB |
There is a growing colony, a clear white isolate |
|
St.3.P |
There is a growing colony, a clear white isolate |
|
St.3.2PB. |
There is a growing colony, a clear white isolate |
Activity of amylase enzyme from thermophilic bacterial isolate
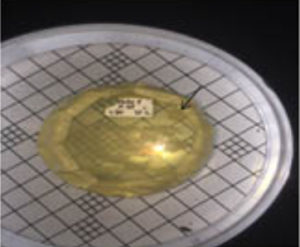
From isolation result, to six isolates of thermophilic bacteria, only two isolates were able to produce clear zone, in amylase activity test. The amylase activity of the isolate is characterized by the formation of clear zone on the enriched media with the substrate of starch. The presence of the starch substrate on the media so that the amylase enzyme present in the bacteria will catalyze the reaction of starch into disaccharides and monosaccharides (Nelson and Cox, 2005, Pelzar and Chan, 2003). Quantitatively the amylase activity of thermophilic bacteria isolates was measured based on the diameter of the clear zone formed (mm). The bacterial isolates tested were bacterial isolates that had passed through the thermotolerance test stage and stabilized to grow for 48 hours at a temperature of 550C. The two selected isolates tested were isolates of St. 3.2 and St. Isolates 3.1. Both isolates are from Station III. Diameter of clear zone of isolate St. 3.2. is 28.3 mm whereas the isolate St.3.1 is 20.2 mm (Figure 6).
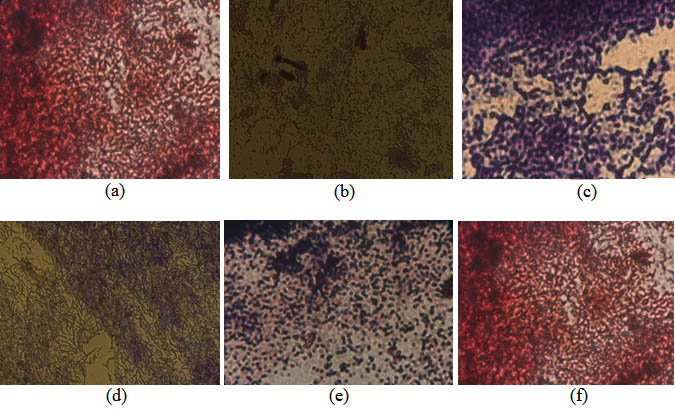
Fig. 6. Gram stain, isolate thermophilic bacteria from the volcanic site of Linow Lake, Tomohon. (a) Isolate St. 1.1. (b) Isolate St. 1.3. (c). Isolate St. 2.1. (d). Isolate St. 2.2. (e). Isolate St. 3.1. and (f). Isolate St.3.2
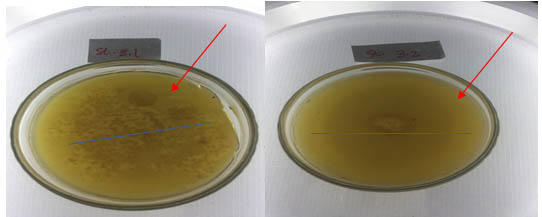
Fig. 7. Clear zone on amylase activity test, isolate thermophilic bacteria
Lake Linow is an active volcanic lake, characterized by hot mud on several sites, around the lake. The in situ mud temperature is 90 0 C – 110 0 C with semi-solid texture, with mud color: black and gray. The sludge conserved in the thermos, in the laboratory has decreased the temperature to 36 0 C – 45 0 C. However, isolation of thermophilic bacteria was successfully performed and bacterial isolates were able to live on a laboratory scale up to 70 0C. Thus, there were bacterial isolates thermophilic, which lives on the hot mud of Lake Linow Tomohon. The content of oxygen, classified as high between 7.16 mg / L – 7.69 mg / L, so that bacteria successfully isolated classified as aerobic bacteria.
Screening isolates of thermophilic bacteria originating from 3 stations, yielded six isolates that survived at a constant temperature of 450C for 2 x 24 hours. The obtained isolate was successfully purified and kept stable growing at a constant temperature of 450C. Thus, the isolates of bacteria obtained are thermophilic bacteria (Pelzar and Chan, 2006). Initial temperature conditions at station I slurry sample laboratory: 37.3 0 C; Station II 45 0 C; and Station III 37.3 0C. Each contains thermophilic bacteria capable of living at a constant temperature of 45 0 C, for 48 hours. Thus, the decrease in temperature in the laboratory compared with the in situ mud temperatures does not kill the thermophilic bacteria present in the hot mud samples. Thermophilic bacteria are capable of forming endospores at ambient temperatures that do not support. Thermophilic bacteria were successfully isolated with nutrient agar medium with pH 7.0 – pH 7.2. All isolates obtained were identified and characterized by morphology, microscopic, and biochemical tests. From the test results, it is suspected that the isolates obtained are genus Bacillus sp. Genus Bacillus sp. has the characteristic of a straight rod-shaped cell, measuring between 0.5-2, 5 x 1.2-10 ¼m and often clustered.
Gram staining was performed on isolates of thermophilic bacteria. Gram staining is the first step for bacterial identification (Pelzar and Chan, 1996). Gram staining aims to determine the properties of gram and morphology of the identified bacteria. From the results of Gram staining can be known characteristics of bacterial cell cell walls that have been obtained. Gram-positive cell wall cells consist of a thick layer of peptidoglycan while gram-negative bacterial cells have a thick lipid content (Pelzar and Chan, 1996; Simanjuntak and Mokosuli, 2015). The addition of crystalline violet solution causes the gram-positive or gram-negative cell walls to absorb the dye. However, when given alcohol, violet crystals in gram negative will fade. This is due to the structure of Gram negative cell wall cells that are largely composed of lipids. When given an alkaline fuchsin or a second dyestuff, the gram negative bacteria cell wall will reabsorb so that the gram-negative bacteria coloring results will be red while the gram-positive bacteria will remain purple, although given a second substance. Gram-positive cell wall cells are composed of thick layers of peptidoglycan that can not be washed by alcohol (Pelzar and Chan, 1996; Simanjuntak and Mokosuli, 2015). The discovery of gram-negative isolates on lake linow volcanic sites may be associated with environmental conditions. Gram-negative bacteria require relatively simpler nutrients compared to gram-positive. This means the ability of this group of bacteria to grow in a higher environment than gram positive.
The thermophilic bacterial isolates obtained from Lake Linow, were able to grow at a temperature range of 45 0C – 70 0C. The growing ability of thermophilic bacterial isolates over the temperature range was also produced in Habibie et.al. 2015, isolates from Lampindo hot mud. Thermophilic bacterial isolates, positive as amylase producer, if isolates are able to form clear zone, after iodine sequestration on starch agar medium. The clear zone does not get stained by iodine solution, because in that zone the starch is already hydrolyzed into simpler compounds such as disaccharides or monosaccharides (Star, 2008). Amylase is produced by bacteria to hydrolyze the starch polysaccharide complex compounds, which exist in the outer environment of cells to be simpler compounds, in order to be used by bacteria as an energy source, for growth and regeneration (Pelzar and Chan, 2006; Brock, 2001). Amylase is an extracellular enzyme, produced from within cells, and expelled to the fermentation medium (Nelson and Cox, 2005). Outside the cell, this enzyme degrades polysaccharide polymers into soluble compounds that can be absorbed by cell walls, as a source of energy in their life. In this case, starch is used as a source of carbon for energy sources for thermophilic bacteria, and the result of starch reshuffle due to amylase activity is characterized by the formation of clear zones around thermophilic bacteria.
The ability of amylolytic isolates of thermophilic bacteria, different by type of isolates (Fatoni, 2014). The diameter of the clear zone that can be produced by isolates grown on starch media is 20.2 mm – 28.3 mm. The largest clear zone diameter was produced by isolate St.3.2; whereas the smallest zoned diameter was generated by the isolates of St.3.1. Both isolates with the largest diameter and the smallest diameter produced, originated from the same sample location of the station 3. Bacterial isolates from Rimbo Anti Pasaman hot spring, West Sumatera resulted in the largest mean diameter of the cleft zone of 28.1 mm, at a temperature of 50 0 C (Indawati, 2011). Thus, screening of amylase activity produced by Linow lake bacteria isolate, has a larger clear zone. Activity of amylolytic enzyme produced by isolate at temperature of 55 0C. Temperature influence amylolitic activity of isolate. In laboratory scale, starch-containing media was incubated in the temperature range 45 – 70 0 C. The amylolytic activity of the optimum bacterial isolates of thermophilic at temperature distribution of 55 0 C was also produced by Ginting (2009), Muharni (2009) and Indawarti (2012), respectively -mases in isolates of thermophilic bacteria isolated in hot springs. The ability of bacteria to produce different enzyme amylase (Nelson and Cox, 2005). Thus the diameter of the amylolytic activity of the resulting isolates shows the ability of the isolates to produce an amylolytic enzyme.
This is the first investigation of thermophilic bacteria in Lake Linow, North Sulawesi, Indonesia. Six bacterial isolates were grown at a constant temperature of 450 C – 55 0 C, from Lake Linow hot mud. The decrease in the temperature of hot mud samples to half the temperature of the in situ mud, does not kill the thermophilic bacteria contained in the sludge sample. The thermophilic bacteria isolate obtained consisted of 2 Gram positive bacteria and 4 gram negative bacteria. The activity of amylase enzyme two isolates of thermophilic bacteria was stable up to temperature of 55 0 C.
ACKNOWLEDGMENTS
This research can be done, because the financing from DRPM Kemristekdikti through college grant scheme, 2017. Therefore, thank you to DRPM Kemristekdikti through research institute and community service, Manado State University. Thank the students for the master biology program of State University of Manado, Marcella and Marcelin who have participated in the research. Thank you, to the manager of Lake Linow, Tomohon City that allows sampling.
- Aanniz T, Ouadghiri M, Melloul M, Swings J, Elfahime E, Ibijbijen J, Ismaili M and Amar M. 2014. Termophilic Bacteria in Moroccan hot springs, salt marches and desert soils. Brazilian Journal of Microbiology, 2015; 46(2): 443-453.
- Balsam T. Mohammad,Hala I. Al Daghistani, Atef Jaouani, Saleh Abdel-Latif, and Christian Kennes. 2017. Isolation and Characterization of Thermophilic Bacteria from Jordanian Hot Springs: Bacillus licheniformis and Thermomonas hydrothermalis Isolates as Potential Producers of Thermostable Enzymes. Int J Microbiol. 2017; 2017: 6943952. Published online 2017 Oct 15. doi: 10.1155/2017/6943952
- Fatoni A and Zusfahair. Isolation and Partial Purification of New Protease form Thermophilic Bacteria Pseudomonas otitidis WN 1 obtained from Indonesian Hot Spring. 2016; Conference Paper. DOI: 10.13140/RG.2.1.1975.9844 https://www.researchgate.net/publication/281078206.
- Gayar KEE, Abboud MAA and Essa AMM. Characterization of Thermophilic Bacteria Isolatedfrom two Hot Springs in Jazan, Saudi Arabia. J. Pure Appl. Microbio, 2017; 11(2): pp 1-10. JUNE 2017.
- Ginting, Y. Isolasi Bakteri dan Uji Aktivitas Amilase Termofil Kasar dari Sumber Air Panas Semangat Gunung Sumatera Utara. 2009; Tesis: USU Medan
- Gorsel JTV. 2016. Bibliography of the geology of indonesia. www.vangorselslist.com/pdf/BIG_I_Regional.pdf
- Hall, R., and W. Spakman, 2015, Mantle Structure and Tectonic History of SE Asia: Tectonophysics v. 658, p. 14–45, https://doi.org/10.1016/j.tecto.2015.07.003
- Habibi FM, Sigres DV, Islami LN. Isolasi Dan Identifikasi Molekuler Mikroorganisme Termofilik Penghasil Xilanase Dari Lumpur Panas Lapindo. Jurnal Pangan dan Agroindustri Vol. 2 No 4 p.231-238, Oktober 2014
- Indawati 2011. Isolasi Bakteri Termofilik Penghasil Amilase Dari Sumber Air Panas Rimbo Panti, Pasaman. Laporan Penelitian. Universitas Negeri Padang.
- Nelson and Cox. Principle of Biochemistry. WH Freeman Company, New York 2005.
- Pelzar and Chan. Pengantar Mikrobiologi. UI Press 2006.
- Muharni. Isolasi dan Identifikasi Bakteri Penghasil Kitinase dari Sumber Air Panas Danau Ranau Sumatera Selatan. Jurnal Penelitian Sains, 2009; 9(1): 12-15.
- Rahmawati A dan Yulianti E. Eksplorasi Bakteri Termofilik Pasca Erupsi Merapi Sebagai Penghasil Enzim Ekstraseluler. J. Penelitian Saintek, 2010; 17(1): pp1-12.
- Simanjuntak S dan Mokosuli YS. 2016. Penuntun Praktikum Mikrobiologi. Jurusan Biologi FMIPA Universitas Negeri Manado.
- Yuanita DN dan Wikandari PR. Screening Bakteri Proteolitik Termofilik Dari Sumber Air Panas Singgahan Tuban. UNESA Journal of Chemistry, 2014; 3(3), pp 49-54.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.