ISSN: 0973-7510
E-ISSN: 2581-690X
The overall aim of this paper was to divulge the pathogenic and beneficial bacterial flora of cockroaches (Blatta orientalis) living in a hospital area in Annaba city, Algeria. Thirteen cockroaches were randomly apprehended in sterile conditions during March 2022 in two different hospitals in Annaba, “El Bouni” Hospital and the “Sainte-Therese” Hospital. Bacterial strains were isolated from the imprint of the insect on Petri dishes previously inoculated with different media, also from external and internal body part suspensions of the captured specimens. Biochemical identification was established using the analytical profile Index (API) System (Biomerieux, France). The identification of the enteric-screened strain was confirmed using molecular sequencing of the 16S rRNA gene and phylogenetic analysis was performed. The results showed a high prevalence of the pathogenic strains isolated from the oriental cockroach Blatta orientalis (225 strains), such as Serratia liquefaciens, Raoultella ornithinolytica, Pseudomonas luteola, Enterobacter aerogenes, Hafniaalvei and Bacillus sp. Phylogenetic analysis of the enteric bacteria confirmed the affiliation with Shimwellia blattae NCTC10965 (100%), Basonym Escherichia blattae, which is a natural cyanocobalamin producer. Results confirm at the same time the symbiotic relationship between S. blattae and Blatta orientalis, but also point out the underrepresented potential of these insects as a source of strains with biotechnological interest.
Cockroaches, Blatta orientalis, Molecular Identification, Shimwellia blattae, Cyanocobalamin
Cockroaches are the most tenacious and hateable non-biting insects living in hotels, restaurants, hospitals and residential buildings. They are prevalent in hot and humid places especially in kitchens, toilets, drainage systems, even sewers, and in most public places and households.1
Blatta orientalis and Blatella germanica are the most common species found in Algeria.2,3 These pests probably have survived on earth for more than 300 million years because of their behaviours and lifestyles. They eat garbage, rotting food and even the faecal waste of other cockroaches. They transmit contaminants to food, surfaces and all the places they pass through.4
Wild insects such as cockroaches are linked to bacteria at various levels and rates, ranging from simple contaminants due to their swiftly move on surfaces5; entomopathogens,6-8 or endosymbiotic bacteria typically living inside specialised organs.9
Symbiotic relationship between bacteria and cockroaches are widespread conferring them the ability to recycle nitrogen and provide lacking nutrients like amino acids.10 Some studies have reported the biotechnological properties of the cockroach’s gut microflora.11-13
On the one hand, recent studies confirm that these insects are a source of pathogenic bacteria.1,2,5 On the other hand, few studies investigate the ability of symbiotic bacteria to produce metabolic product that have medical and industrial benefits.14
Shimwellia blattae, basonym Escherichia blattae by Burgess et al., was first reported as a symbiotic Gram-negative aerobic bacterium associated with cockroaches isolated from the gut of Blatta orientalis.15 This strain is also known for its production of vitamin B12 also named cyanocobalamin wich is a widely used vitamin in the medical and food industries, essential for normal human health to avoid some serious pathologies such as the syndrome pernicious anaemia and neural tube defects. Its biosynthesis can only occur in few bacteria and archaea and may involve up to 30 different enzyme-mediated steps.16
S. blattae has proved to be a producer within aerobic pathways by a genetic organization named the cob operon, which showed a considerable similarity with genes from Salmonella enterica serovar Typhimurium.17 A few years ago, engineers focused their effort on Escherichia coli as a platform for biotechnology. However, the enteric bacteria S. blattae constitutes a non-pathogenic interesting alternative as a native and standard producer of vitamin B12 by its biochemical pathways.17,18
Thus far, cockroaches’ microflora has not been studied and S. blattae has not been isolated from insects in Algeria. Therefore, this paper aimed to reveal and distinguish the bacterial flora from the external and internal parts of the oriental cockroach isolated from hospital care unit in Annaba city, Algeria and to expose these insects as an underrepresented source of strains with biotechnological properties.
Capturing the cockroaches
Cockroaches were captured in two different places in Annaba city (Algeria), “El-Bouni” and “Sainte-Therese” hospitals. The sampling was performed during a six-day period in March 2022 after a chemical treatment of all hospital institutes in the city. The remaining alive specimens were randomly caught in sterile conditions and transported to the laboratory for identification up to species level using the published keys.19,20
Isolation and identification of bacteria
Each specimen was briefly maintained in the freezer and then directly placed in Petri dishes containing different culture media (MacConkey, Hecktoen, Nutrient agar, Chapman and EMB agar) to obtain the imprint of the cockroaches. Subsequently, dissection was performed using dissection set instruments to separate each part of the cockroach (head, leg and abdomen) into test tubes containing 1ml sterile physiological water and homogenised with a vortex. Suspensions of each specimen were inoculated in the above-mentioned media according to Mehainaoui et al.2 Plates were incubated at 37°C for 24 to 48 hours. Pure culture isolates were identified using the analytical profile Index (API) system (Biomerieux, France).
Molecular identification of the enteric bacteria
Molecular identification was carried out on the non-pathogenic Gram-negative enteric bacteria isolated from the gut of B. orientalis. Genomic DNA was extracted from the previously identified isolate by a commercial DNA extraction kit (Solis Biodyne, Estonia). PCR amplification was achieved using the primer set of 16S rRNA gene (27F: 5’ – AGA GTT TGA TCC TGG CTC AG – 3’ and 1492R: 5’– CCG TCA ATT CCT TTG AGT TT- 3’).21
The PCR reaction mixture contained 50µl of master mix (1.25 U Hot Start Taq DNA Polymerase (Solis Biodyne, Estonia), 25-50 ng/µl of DNA template, 0.3µM/µl of each primer, 1.5 µM magnesium chloride (Solis Biodyne, Estonia), made up to 50µl reaction volume with distilled H20. The PCR cycles ran as follows: Initial denaturation at 94°C (12 min), denaturation at 94°C (1 min), annealing at 55°C (1 min) and extension at 72°C (1 min). The amplification was repeated for 30 cycles followed by a final extension at 72°C (7 min). PCR was conducted using a thermocycler (iCycler Bio-Rad, USA). The DNA concentrations were checked using a Nanodrop Spectrophotometer (NanoDropTM 2000, USA).
For agarose gel electrophoresis: the PCR product was separated into a 1.5% agarose gel (Sigma-Aldrich, USA). A one hundred base pair (100 bp) DNA ladder (Solis Biodyne, Estonia) was used as the DNA molecular weight markers. Electrophoresis was done at 80 V for 1 h 30 min, and the gel was viewed under UV light after staining with Midori Green Advance (Nippon Genetics, Japan) and inspected with a UV transilluminator.
DNA sequences were then aligned using MEGA 7.0.22 The unrooted phylogenetic tree was made using the maximum likelihood analyses of the closely related strains of the Genbank Blast (bootstrap =1000 replicates).
During the sampling, thirteen specimens were found alive in both locations: the hallways of the “Sainte-Therese” Hospital, and the nursery service of “El-Bouni” Hospital.
The collected specimens were identified as eight males B. germanica; two cockroaches were identified as B. orientalis, with one adult male and one adult female, one adult male Periplaneta americana and one adult male Supella longipalpa.
Of all those insects, a total of 225 strains were isolated from the imprint and the external and internal body parts of the cockroaches. Most of the isolates were Gram-negative bacilli (GNB) (188 isolates), five Gram-positive bacilli (Bacillus sp.), thirty-three isolates were Gram positive Cocci of which thirty Streptococcus sp. and three isolates were identified as Staphylococcus aureus.
On this basis, as shown in Table, thirty-two isolates were isolated from the two B. orientalis. Fourteen (44%) were from the imprint, two (6%) from the head, six (19%) were isolated from the legs and finally ten(31%)isolates were found in the gut of the specimens. Results of the screening with the API system (Table) allowed the identification of various pathogenic bacteria such as Staphylococcus aureus, Raoultella ornithinolytica, Serratia liquefasciens, Enterobacter aerogenes, Pseudomonas luteolla and Hafniaalvei.
Table :
Origin and percentage of the pathogenic flora isolated from B. orientalis
| Origin | Strain percentage | Species |
|---|---|---|
| Imprint | 44% | Serratia liquefasciens |
| Pseudomonas luteola | ||
| Enterobacter aerogenes | ||
| Staphylococcus aureus | ||
| Bacillus sp. | ||
| Streptococcus sp. | ||
| Abdomen | 31% | Raoultella ornithinolytica |
| Hafnia alvei | ||
| Staphylococcus sp. | ||
| Klebsiella oxytoca | ||
| Legs | 19% | Bacillus sp. |
| Streptococcus sp. | ||
| Serratia marcescens | ||
| Head | 6% | Staphylococcus aureus |
| Staphylococcus sp. |
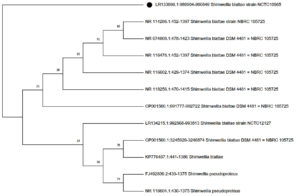
The successfully amplified 946 pbrDNA fragment shared 100% similarity with S. blattae and phylogenetic analyses (Figure) confirmed that the enteric isolate belongs to S. blattae previously named Escherichia blattae by Burgess et al.,15 a strain known as a natural cyanocobalamin producer.
In Algeria, several studies reported the German cockroach as the most abundant cockroach in the urban and hospital environment,2,3,23 while little data are available about the oriental cockroach B. orientalis.
When effectively utilised, chemical treatment (pesticides) can control pest infestations. Still, the random use of those toxic molecules constitutes an obvious risk for the medical staff and vulnerable patients due to the chemical and toxicological effects they possess.24
To describe the significance and the role of bacteria associated with hospital cockroaches; this study reported the isolation of many pathogenic bacteria that can induce a real contamination risk of surfaces and food. Most studies on cockroach-associated bacteria brought on the evidence that those insects carry pathogens and resistant bacteria.1,5,25
However, this study led to the isolation and identification of S. blattae from the imprint (on the Petri dish) and the gut of the oriental cockroach. The non-pathogenic enteric bacterium known as a cobalamin producer seems to provide a real opportunity for biotechnological exploitations. This is the first report of symbiotic strains related to cockroaches with biotechnological interest in Algeria. Recently, a few authors reported the biological properties of gut bacteria from cockroaches, considering these insects as a new source of metabolites of interest.11,14,26
Cyanocobalamin is one of nature’s most interesting biomolecules with two roles; it is a cofactor for two enzymes, the methionine synthase and the methylmalonyl-CoA mutase. In humans, B12 deficiency impedes the formation of methyl groups causing severe pathologies like abnormal brain development and pernicious anaemia.16 Cobalamin biosynthesis is only present in a few prokaryote strains, involving two alternative pathways, de novo and the salvage pathway.27
The genome of the enteric bacterium S. blattae revealed the pathways of the B12 and B12-dependant reactions with a potential gene transfer from S. enterica serovar Typhimurium. The strain showed a critical similarity of the 20 genes that are mainly clustered in a single operon, the cob operon. While S. blattae produce Vit B12 de novo in both aerobic and anaerobic conditions.17
The isolated enteric bacteria was previously classified as Escherichia blattae by Burgess et al,15 isolated from the hindgut of the oriental cockroach Blatta orientalis captured from wild sources, the strain was identified with the following reactions: acid production from lactose, positive mannitol, indole negative but the gluconate was positive compared to E.coli.15
The genus Shimwellia gen. nov. was established by further detailed taxonomic investigations, especially DNA-DNA hybridization studies that provide the evidence of two biogroups of the Obesumbacterium proteus strains that was first isolated by Shimwell et al.28 and wrongly classified as Flavobacterium proteus. Investigations subsequently revealed two distinct groups in O. proteus, the biogroup 2 shared 93% similarity with E. blattae. This led to the assimilation of all these strains into a new genus named Shimwellia gen. nov.29
Cyanocobalamin is a very important vitamin for humans, and its biosynthesis is limited to few bacterial strains. While engineers have focused on traditional strategies like mutagenesis of E. coli and other known bacteria, it is also imperative to provide strains with natural metabolic systems such as S. blattae. Homology in the genome organization of the cob operon of S. blattae and S. enterica serovar Typhimurium was provided in previous study, confirming the novo synthesis of vitamin B12.
In this study, we report the isolation and the identification of the enteric non-pathogenic bacterium S.blattae basonym E. blattae, a vitamin B12 producer from B. orientalis isolated from hospital care units of Annaba, Algeria. Symbiotic bacterial species isolated from insects such as cockroaches deserve to be studied for their biotechnological applications and metabolic pathways.
ACKNOWLEDGMENTS
The authors would like to thank all staff and facility workers in the Biochemistry Department of the “Badji Mokhtar” University for their contributions, assistance and support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SB accomplished the study. AL and AM collected the data and contributed in the experimental part of the study. DGK revised and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Chehelgerdi M, Ranjbar R. Virulence factors and antibiotic resistance properties of Streptococcus species isolated from hospital cockroaches. 3 Biotech. 2021;11(7):321.
Crossref - Mehainaoui A, Menasria T, Benouagueni S, Benhadj M, Lalaoui R, Gacemi-Kirane D. Rapid screening and characterization of bacteria associated with hospital cockroaches (Blattella germanica L.) using MALDI-TOF mass spectrometry. J Appl Microbiol. 2021;130(3):960-970.
Crossref - Menasria T, Moussa F, El-Hamza S, Tine S, Megri R, Chenchouni H. Bacterial load of German cockroach (Blattella germanica) found in hospital environment. Pathogens and Global Health. 2014;108(3):141-147.
Crossref - Atiokeng Tatang RJ, Tsila HG, Wabo Pone J. Medically Important Parasites Carried by Cockroaches in Melong Subdivision, Littoral, Cameroon. J Parasitol Res. 2017;7967325.
Crossref - Luckyjane Molewa M, Barnard T, Naicker N. A potential role of cockroaches in the transmission of pathogenic bacteria with antibiotic resistance: A scoping review. J Infect Dev Ctries. 2022;16(11):1671-1678.
Crossref - Arredondo D, Castelli L, Porrini MP, et al. Lactobacillus kunkeei strains decreased the infection by honey bee pathogens Paenibacillus larvae and Nosema ceranae. Beneficial Microbes. 2018;9(2):279-290.
Crossref - Corsaro D, Thomas V, Goy G, Venditti D, Radek R, Greub G. ‘Candidatus Rhabdochlamydia crassificans’, an intracellular bacterial pathogen of the cockroach Blatta orientalis (Insecta: Blattodea). Syst Appl Microbiol. 2007;30(3):221-228.
Crossref - Rossoni RD, dos Santos Velloso M, Figueiredo LMA, Martins CP, Jorge AOC, Junqueira JC. Clinical strains of Lactobacillus reduce the filamentation of Candida albicans and protect Galleria mellonella against experimental candidiasis. Folia Microbiol. 2018;63(3):307-314.
Crossref - Vicente CSL, Mondal SI, Akter A, Ozawa S, Kikuchi T, Hasegawa K. Genome analysis of new Blattabacterium spp., obligatory endosymbionts of Periplaneta fuliginosa and P. japonica. Munderloh UG, ed. PLoS ONE. 2018;13(7):e0200512.
Crossref - Tokuda G, Elbourne LDH, Kinjo Y, et al. Maintenance of essential amino acid synthesis pathways in the Blattabacterium cuenoti symbiont of a wood-feeding cockroach. Biol Lett. 2013;9(3):20121153.
Crossref - Akbar N, Siddiqui R, Iqbal M, Sagathevan K, Khan NA. Gut bacteria of cockroaches are a potential source of antibacterial compound(s). Lett Appl Microbiol. 2018;66(5):416-426.
Crossref - Chen Z, Ou P, Liu L, Jin X. Anti-MRSA Activity of Actinomycin X2 and Collismycin A Produced by Streptomyces globisporus WA5-2-37 From the Intestinal Tract of American Cockroach (Periplaneta americana). Front Microbiol. 2020;11:555.
Crossref - Guzman J, Vilcinskas A. Bacteria associated with cockroaches: health risk or biotechnological opportunity? Appl Microbiol Biotechnol. 2020;104(24):10369-10387.
Crossref - Alkhalifah DHM. Evaluation of antimicrobial activity of bacterial symbionts isolated from wild field cockroach Blattella vaga from Saudi Arabia. Saudi J Biol Sci. 2021;28(11):6239-6244.
Crossref - Burgess NRH, McDermott SN, Whiting J. Aerobic bacteria occurring in the hind-gut of the cockroach, Blatta orientalis. J Hyg. 1973;71(1):1-8.
Crossref - Andres S, Wiezer A, Bendfeldt H, Waschkowitz T, Toeche-Mittler C, Daniel R. Insights into the Genome of the Enteric Bacterium Escherichia blattae: Cobalamin (B12) Biosynthesis, B12-Dependent Reactions, and Inactivation of the Gene Region Encoding B12-Dependent Glycerol Dehydratase by a New Mu-Like Prophage. Microb Physiol. 2004;8(3):150-168.
Crossref - Smith AD, Warren MJ, Refsum H. Vitamin B12. Adv Food Nutr Res. 2018;83:215-279.
Crossref - Brzuszkiewicz E, Waschkowitz T, Wiezer A, Daniel R. Complete Genome Sequence of the B12 -Producing Shimwellia blattae Strain DSM 4481, Isolated from a Cockroach. J Bacteriol. 2012;194(16):4436-4436.
Crossref - Choate PM. A Dichotomous Key for the Identification of the Cockroach Fauna (Insecta: Blattaria) of Florida. Depart-ment of Entomology and Nematology, University of Florida. 2009.
- Roth LM. Some Cockroaches from Africa and Islands of the Indian Ocean, with Descriptions of Three New Species (Blattaria). Transactions of the American Entomological Society. 2003;129(1):167-186.
- Edwards U, Rogall T, Blocker H, Emde M, Bottger EC. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989;17(19):7843-7853.
Crossref - Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33(7):1870-1874.
Crossref - Loucif L, Gacemi-Kirane D, Cherak Z, Chamlal N, Grainat N, Rolain JM. First Report of German Cockroaches (Blattella germanica) as Reservoirs of CTX-M-15 Extended-Spectrum-β-Lactamase- and OXA-48 Carbapenemase-Producing Enterobacteriaceae in Batna University Hospital, Algeria. Antimicrob Agents Chemother. 2016;60(10):6377-6380.
Crossref - Gilden RC. Pesticides Use in Hospitals: Health Protection, Health Hazards, and Viable Alternatives. Nurs Adm Q. 2010;34(4):320-326.
Crossref - Jalil N, Keyhani A, Hasan MKS, Mahdi M, Monireh M, Atefeh B. Cockroaches’ bacterial infections in wards of hospitals, Hamedan city, west of Iran. Asian Pac J Trop Dis. 2012;2(5):381-384.
Crossref - Akbar N, Siddiqui R, Iqbal M, Khan NA. Antibacterial Activities of Selected Pure Compounds Isolated from Gut Bacteria of Animals Living in Polluted Environments. Antibiotics. 2020;9(4):190.
Crossref - Fang H, Kang J, Zhang D. Microbial production of vitamin B12: a review and future perspectives. Microb Cell Fact. 2017;16(1):15.
Crossref - Shimwell JL. Obesumbacterium, a New Genus for the Inclusion of “Flavobacterium proteus.” J Inst Brew. 1964;70(3):247-248.
Crossref - Priest FG, Barker M. Gram-negative bacteria associated with brewery yeasts: reclassification of Obesumbacterium proteus biogroup 2 as Shimwellia pseudoproteus gen. nov., sp. nov., and transfer of Escherichia blattae to Shimwellia blattae comb. nov. Int J Syst Evol Microbiol. 2010;60(4):828-833.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.