ISSN: 0973-7510
E-ISSN: 2581-690X
Abnormal vaginal discharge is one of the frequent complaints of women of reproductive age group. This study was carried out to determine the prevalence of vulvovaginal candidiasis(VVC) among the patients attending the tertiary care hospital with complaints of suggestive of vaginitis. This study was done in a tertiary care hospital, Chennai for a period of 1 year from January 2017 to December 2017. The study included 160 women of the age group 15 – 65 years with complaints suggestive of vaginitis. High vaginal swabs were taken and subjected to direct microscopy, cultured onto Sabouraud Dextrose Agar (SDA) and Hichrome Candida differential agar. Candida species were determined by standard microbiological methods and the results were confirmed by automated VITEK2 compact. Candida species were isolated from 56 patients which included C.albicans (25), C.tropicalis (20), C.glabrata (6), C.parapsilosis(4), C.krusei(1). Our study shows higher prevalence of non albicans Candida causing VVC. Hence, we recommend that the investigations up to species identification of Candida may be routinely followed in the microbiology laboratories.
Candida species, vulvovaginal candidiasis, risk factors.
Fungal infections have become an alarming problem over the last ten years mainly because of global increase in the number of immunocompromised patients, who are highly susceptible to opportunistic infections, including mycoses1. According to a recent study, the incidence of Candidaemia is 6.9 per 1,000 ICU patients of which 7.5% received antifungal therapy2. Among the women, vaginal candidiasis has become a common finding worldwide and up to 75% of them have symptomatic vaginal candidiasis at least once3. Vulvovaginal candidiasis (VVC) is defined as signs and symptoms of inflammation of the vulva and vagina in the presence of Candida spp. and in the absence of other infectious etiology4. The clinical features of VVC include pruritus, hyperemia, vaginal discomfort and leucorrhea, burning, soreness, dyspareunia and vaginal or vulvar erythema, which may cause a problem in marital and sexual relations5.
Candida albicans appears to be the cause for 80 to 92 percent of vulvovaginal candidiasis6. There is an increase in frequency of other candida species nowadays, specifically of C. glabrata, may be because of increased use of over-the counter drugs, long-term use of azoles, and the use of short courses of antifungal drugs7. The prevalence of candida in India is estimated to be 30%8. There are various predisposing factors of VVC, few of which are, hormonal fluctuations in pregnancy, luteal phase of menstrual cycle, use of oral contraceptives, and hormone replacement therapy9.
Candida albicans is considered as an important fungal pathogen among humans because of its varying virulence factors that leads to candidiasis like phenotypic switching, phospholipase, proteinase and hemolytic activity. 85-90% of all cases of vulvovaginal candidiasis shows C.albicans followed by C.glabrata (5-10%), C. tropicalis (3-5%) and other species10. Though VVC is one of the most common fungal disease worldwide, the information about the distribution and etiology of it is scarcely known to us, because microbiology tests to identify the species of Candida and their antifungal susceptibility testing are not routinely performed in most of the laboratories11. Hence, this study is undertaken to determine the species specific prevalence and the risk factors associated with occurrence of VVC.
Cyperus rotundus L. (family: Cyperaceae) is regarded as a traditionally medicinal herb that has been used for treating numerous clinical ailments such as diarrhea, diabetes, inflammation, malaria, and belly and bowel disorders. Phytochemical and pharmacological research printed that C. rotundus includes an excessive concentration of vital oils, ascorbic acids, phenolic acids, and flavonoids demonstrating its antibacterial, antioxidant, anti-inflammatory, anti-cancerous, and antimalarial4.
Nuclear magnetic resonance (NMR) considered the most advanced technique for the profiling and identifying the metabolites in complex matrices such as plant extracts. The technique is broadly used due to its ease in pattern instruction and the outcomes received are reproducible5. The NMR technique in combination with multivariate statistics evaluation (MVA) has been employed appreciably in recent studies for metabolites profiling for the discrimination of samples of same origin subjected to different processes6. Therefore, this study objective was to determine the antibacterial and antioxidant activities of C. rotundus L Fermented by lactic acid bacteria. Moreover, the metabolites in charge for the antibacterial and antioxidant activities of C. rotundus were identify by using 1H-NMR spectroscopy.
Microorganisms and culture conditions
This study was conducted in a tertiary care hospital in Chennai over a period of 1 year. The present study enrolled 160 patients attending the Obstetrics and Gynaecology Out patients department and Inpatients admitted in the wards with symptoms suggestive of vaginitis. The study has been approved by institutional ethical committee and Informed consent was obtained from all the subjects.
Sterile vaginal swabs were used for collection of vaginal samples from the patients. Two high vaginal swab samples were collected aseptically from the posterior vaginal fornix using speculum and posterior vaginal wall retractor. The swabs were transferred to the microbiology laboratory and processed immediately.
One of the swabs was used for Gram stain and direct wet mount microscopy using 10% Potassium hydroxide solution to determine the presence of yeast cells in the sample. The second swab was streaked on Sabouraud Dextrose agar (SDA) ( HiMedia, India ) plates containing chloramphenicol and incubated at 37oC for 24-48 hours. The colonies from SDA plate were subjected to Gram stain to confirm the growth of candida and were further subtyped by streaking on Hichrome Candida differential agar ( HiMedia, India ) and incubated at 37oC for 24-48 hours. The pigmented colonies were further examined for assimilation of various sugars and candida species were identified using standard microbiological methods. The colonies from the SDA plate were again processed in automated VITEK2 compact (bioMérieux) and the results were documented.
The colonies from SDA plate were also subjected to germ tube test to check for the production of germ tubes. Germ tube test is done by mixing 3-4 colonies in 0.5 ml human serum and incubated at 37oC for 2-4 hours and examined under microscope for the formation of germ tube. Colonies suggestive of C.albicans were confirmed by this germ tube test.
Preparation of Cyperus rotundus
C. Rotundus were bought from Alkadhemia city, Baghdad. The tubers were washed with water, air dried and cut into strips. A quantity of 300 g Cyperus rotundus and 1000 mL water were mixed and the mixture blended using home blander. The mixture was then filtered using a cotton cloth filter, autoclaved at 121°C for 30 min.
Fermentation process
The initial pH of the mixture from Section 100 mL was inoculated with 10% (v/v) of L. plantarum and incubated at 37°C for 48 h. The cells counts and pH values were determined after 48 h of fermentation. Non-fermented samples were subjected to same conditions but without starter culture.
Antioxidant Activity of Fermented Cyperus rotundus
DPPH Assay
The Radical Scavenging Activity (DPPH) determines how potent the antioxidant capacity of the fermented mixture8. The assay was performed based on the method from previous study using 2, 2-diphenyl-1-picrylhydrazyl radicals9. Briefly, in 96 wells plate, 0. 25 mL of C. rotundus extract was mixed with 1. 75 mL DPPH solution that was prepared by diluting DPPH in methanol. The plate was incubated in dark place at room temperature for 30 min and absorption was taken at 515 nm using microtiter plate reader (Biotek, EL 800) and DPPH percentage was calculated as the following:
% DPPH = [(control 515 – sample 515) / control 515] *100
FRAP Assay
Ferric reducing antioxidant power (FRAP) assay is a method used to estimate the antioxidant capacity of the fermented mixture that count on reducing the ferric-tripyridyltriazine10. FRAP reagent was prepared by mixing 2, 4, 6-tripyridyl-s-triazine (TPTZ) and FeCl3 solutions in (pH 3. 6) buffer acetic acid at the ratio of 1: 1: 10 (v / v / v). In 96 microtiter plate, 20µL sample was mixed with FRAP reagent (200µL) and incubated for 30 min at room temperature. The absorbance was measured at 593 nm and the calculations were determined using different concentrations from 0. 1 to 1 mM of ferrous sulfate as standard, and expressed as mmol Fe (II) / g DW.
Antibacterial Activity of Fermented Cyperus rotundus
The antibacterial activity of the fermented mixture was determined in following the method described by Aween et al.,11. The fermented mixture was tested against Pseudomonas aeruginosa ATCC10415, Bacillus subtilis ATCC11778 and Escherichia coli ATCC11229. Fermented mixture 100 µL was added to the wells and 100 µL nutrient broth containing 106 of each selected pathogens. The control was nutrient broth with the pathogenic bacteria only. The plates were incubated at 37°C for 24 h and the growth inhibition was measured at 600 nm by using microtiter plate reader. The antibacterial activity was calculated by subtracting the 0 h readings from the 24 h readings, then using the following mathematical equation.
1H-NMR metabolomics analysis
The method used for the extraction was adopted from Abas et al., (2013)12. Ten mg of the fermented and samples were mixed with CH3OH-d4 (0. 375 mL) and KH2PO4 buffer (0. 375 mL) in D2O (pH 6) containing 0. 1 % TSP as standard. The mixed samples were subjected to sonication for 15 min at 30°C. The samples were centrifuged at 13,000 rpm for 5 min and 600µL transferred into NMR tubes13. The NMR works on the INOVA 500 MHz variable spectrometer and at 499. 887 MHz at room temperature 26°C with 64 scans were done on every sample and verified, the acquisition time was 193 s, 3. 75µL pulse width and 1. 0 sec relaxation delay. Each sample was measurement was repeated six times. The residual water region (d 4. 70 to 4. 96) and methanol region (d 3. 28 to 3. 33) were excepted from the analysis.
Statistical analysis
MINITAB version 16 was used to analysis the Data. Statistical measures were used to evaluate the results of these samples and their controls, where one-way analysis of variance (ANOVA). The multivariate analysis was done using SIMCA-P software (Umetrics, Sweden).
A total of 160 women with complaints suggestive of vulvovaginal infections were enrolled in the present study. Their age ranged from 15 – 65 years (table -1). In our study 51 women presented with complaints of white discharge (32%), 45 women (28%) had complaints of itching and 18 women (11%) had pain.
Table (1):
Agewise distribution of the study subjects.
Age in years |
No. of patients (n=160) |
Percentage (%) |
|---|---|---|
<20 |
2 |
1.2 |
20-40 |
124 |
77.5 |
40-60 |
31 |
19.4 |
>60 |
3 |
1.9 |
Table (2):
Risk factors associated with the study subjects.
Risk factor of VVC |
No. of patients |
|---|---|
Pregnancy |
20 |
Antibiotic usage |
4 |
Oral contraceptive pills usage |
4 |
Diabetes |
6 |
Tuberculosis |
1 |
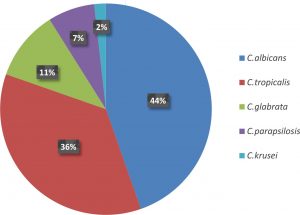
Out of the 160 high vaginal swab samples, Candida were isolated from 56 samples. The isolated candida was processed for species identification. The Various candida species isolated in the current study were shown in the table.
Table (3):
Species distribution of candida.
Candida species |
No. of isolates |
|---|---|
C.albicans |
25 |
C.tropicalis |
20 |
C.glabrata |
6 |
C.parapsilosis |
4 |
C.krusei |
1 |
Vaginitis is a universal problem affecting millions of women globally. Vulvovaginal candidiasis (VVC) is defined as signs and symptoms of inflammation of the vulva and vagina in the presence of Candida spp. and in the absence of other infectious etiology12.
It is an established fact that C.albicans can convert into a disease causing pathogen from a commensal, when there is a change in the host environment13. The increasing antifungal resistance leading to treatment failures and added mortality warrant the need for identification of species in candida. The incidence is more common in women with diabetes where high blood sugars favour growth of candida. Other common associations for the predisposition of candida include pregnancy, antibiotic use and rarely infections like Tuberculosis.
In our study of 160 women, 56 high vaginal swabs (35%) were culture positive and grew candida species. This data is similar to reports by Kumari et al (30.6%), but lower than reports from Namrata Kalia et al (47%) and ranks second as the cause for vulvo vaginal infections14.
Among the 56 women with positive cultures, 20 were pregnant (35%), 6 women were diabetic (11%), 4 had a history of antibiotic use (7%), and 4 were taking Oral contraceptive pills (OCP) (7%). In our study, there was significant association of positive cultures with pregnancy which could be attributed to high levels of reproductive hormones inducing higher glycogen content in vaginal epithelial cells favouring growth of candida. Some studies say that estrogens have a direct effect on the growth of Candida and its adherence to the vaginal epithelium which explain the increased incidence in women using OCPs 15,16.
The other major risk factor is diabetes where the likely reasons could be due to uncontrolled blood sugars and also the use of unsuitable antifungal agents17. The use of antibiotics acts as a short term risk factor for the symptomatic vulvovaginal candidiasis due to the loss of lactobacilli and other normal flora in the vaginal surface18.
Most patients who seek medical attention have complaints of white discharge, itching and pain. In our study 51 women had complaints of white discharge (32%) which was the major complaint. It was followed by complaints of itching which was seen in 45 women (28%) and complaints of pain by 18 women (11%). The clinical presentation was slightly different from reports by Latha ragunathan et al19, where itching (31%) was the major complaint followed by white discharge (29.4%) and pain (15.6%).
In our study identification of candida species was done by conventional methods and confirmed by Vitek 2 compact. There were various candida species detected in our study with highest being C. albicans (44%), and non albicans (56%). Our study showed results similar to study by Kumari et al12 and studies from other parts of the world where there was a higher prevalence of non albicans species20,21,22. This shows the increasing trend of non albicans species, which could be due to environmental variation. The increasing frequency warrants the need to identify the species and test the anti-fungal susceptibility to commonly used antifungal drugs.
The prevalence of non albicans species in our study were as follows C.tropicalis – 20 (36%), C.glabrata – 6 (11%), C.parapsilosis – 4 (7%), C.krusei – 1 (2%). Among the NAC (Non albicans Candida) C.tropicalis was the predominant species followed by C.glabrata which was similar to studies by Jayalakshmi et al23 and Sundar Khadka et al24, but there was a slight difference in the study by Faraji et al17 where candida was isolated from diabetic women only. The predominant Non albicans species was C.glabrata followed by C.tropicalis in the study by Sundar Kadka et al24. The other species C.parapsilosis, C.krusei have been reported less frequently in patients with vulvovaginitis similar to our study23.
Thus, this study shows a changing trend in the causative agents of VVC. Increasing emergence and spread of various non albicans candida is a major concern in the management of VVC.
Candida albicans was found to be the predominant species isolated in the current study followed by C.tropicalis. Yet, there is higher prevalence of non albicans candida species in the study. Hence, screening of all women with vulvovaginal infections for different species of candida would be helpful in providing better care. Thus, complete identification of causative agent of Vulvovaginal candidiasis upto species level in all the microbiology laboratories is highly recommended in order to study the emergence and spread of non albicans candida in the community. More intensive studies are needed to determine the optimal treatment of antifungal drugs for VVC caused by non albicans species.
Conflict Of Interest
The authors declare that there is no conflict of interest.
- Kumari V, Banerjee T, Kumar P, et al. Emergence of non-albicans Candidaamong candidal vulvovaginitis cases and study of their potential virulence factors, from a tertiary care center, North India. Indian J. Pathol. Microbiol. 2013; 56:144-7.
- Pahwa N, Kumar R, Nirkhiwale S, et al. Species distribution and drug susceptibility of candida in clinical isolates from a tertiary care centre at Indore. Indian J. Med. Microbiol. 2014; 32:44-8.
- Latha Ragunathan, G.K Poongothai, Annie Rofeena Sinazer, et al. Phenotypic characterization and antifungal susceptibility pattern to fluconazole in candida species isolated from vulvovaginal candidiasis in a tertiary care hospital. Journal of Clinical and Diagnostic Research. 2014 ; 5: DC01 – DC04
- Achkar JM, Fries BC. Candidainfections of the genitourinary tract. ClinMicrobiol Rev. 2010; 23:253-73.
- Moreira D, Paula CR. Vulvovaginal candidiasis. Int. J .Gynaecol Obstet. 2006; 92:266-7.
- Odds, FC. Candidosis of the genitalia. In: Odds, FC. Candida and candidosis: A review and bibliography, 2nd ed, Bailliére Tindall, London 1988, p. 124.
- Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J. Clin Pharmacol. 1992; 32:248-55.
- Thulkar, J., Kriplani, A., Agarwal, N., et al. Aetiology & risk factors of recurrent vaginitis & its association with variouscontraceptive methods. Indian J.Med. Res. 2010; 131: 83 87.
- Geiger AM, Foxman B, Gillespie BW. The epidemiology of Vulvovaginal candidiasis among university students Am. J. Public Health.1995; 85:1146-8.
- Peters RB, Bahn AN, Barens G. Candida albicans in the oral cavities of diabetics. J Dent. Res. 1966; 45:771-777
- Paulitsch A, Weger W, Ginter-Hanselmayer G, et al. A 5-year (2000-2004) epidemiological survey of Candida and non-Candida yeast species causing vulvovaginal candidiasis in Graz, Austria. Mycoses 2006; 49:471-5.
- Kumari V, Banerjee T, Kumar P, et al. Emergence of non-albicans Candida among candidal vulvovaginitis cases and study of their potential virulence factors, from a tertiary care center, North India. Indian J. Pathol Microbiol. 2013; 56:144-7
- Sharma Y, Chumber SK, Kaur M. Studying the prevalence, species distribution, and detection of in vitro production of phospholipase from Candida isolated from cases of invasive candidiasis. J. Global Infect Dis. 2017; 9:8-11
- Namarta Kalia, Jatinder Singh, Sujata Sharma, Sukhdev Singh Kamboj, Hardesh Arora, Manpreet Kaur. Prevalence of Vulvovaginal Infections and Species Specific Distribution of Vulvovaginal Candidiasis in Married Women of North India. IJCMAS. 2015; 4(8):253-266
- Drake TE, Maibach HI, Candida and candidiasis: cultural conditions, epidemiology, and pathogenesis. Postgrad Med.1973; 53:83-7.
- Atousa Aminzadeh, Ali Sabeti Sanat, and Saeed Nik Akhtar. Frequency of Candidiasis and Colonization of Candida albicans in Relation to Oral Contraceptive Pills. Iran Red Crescent Med J. 2016 October; 18(10):e38909.
- Reza Faraji, Mehr Ali Rahimi, Fatemeh Rezvanmadani, et al. Prevalence of vaginal candidiasis infection in diabetic women. African Journal of Microbiology Research. 6(11): 2773-2778
- Ahmad A, Khan AU. Prevalence of Candida species and potential risk factors for vulvovaginal candidiasis in Aligarh, India. Eur J Obstet Gynecol Reprod Biol.2009; 144(1):68-71.
- Latha Ragunathan, G.K Poongothai, Annie Rofeena Sinazer, et al. Phenotypic Characterization and Antifungal Susceptibility Pattern to Fluconazole in Candida species Isolated from Vulvovaginal Candidiasis in a Tertiary Care Hospital. Journal of Clinical and Diagnostic Research. 2014; 5: DC01 – DC04
- Méan M, Marchetti O, Calandra T. Bench to bedside review: Candida infections in the intensive care unit. Crit Care. 2008; 12:204.
- Mokaddas EM, Al Sweih NA, Khan ZU. Species distribution and antifungal susceptibility of Candida bloodstream isolates in Kuwait: A 10 year study. J. Med. Microbiol. 2007; 56:255 9.
- Jain N, Mathur P, Misra MC, et al. Rapid identification of yeast isolates from clinical specimens in critically ill trauma ICU patients. J. Lab. Physicians. 2012; 4:30 4.
- Jayalakshmi L, RatnaKumari G, Samson SH. Isolation, Speciation and Antifungal Susceptibility Testing of Candida from Clinical Specimens at a Tertiary Care Hospital. Sch. J. App. Med. Sci., 2014; 2(6E):3193-3198
- Sundar Khadka, Jeevan Bahadur Sherchand, Bharat Mani Pokhrel, et al. Isolation, speciation and antifungal susceptibility testing of Candida isolates from various clinical specimens at a tertiary care hospital, Nepal. BMC Res Notes. 2017; 10:218
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.