ISSN: 0973-7510
E-ISSN: 2581-690X
The outline of our work delineates the isolation and evaluation of sun screening activity of melanin producers such as Pseudomonas mosselli STGRDS1, Pseudomonas putida STGRDS3, Bacillus amyloliquefaciens STGRDV11, Bacillus subtilis STGRDV5 and Bacillus cereus STGRDT12. All of the isolates were tested against the fungal melanin STGRDM1, which was used as control throughout the study. The Sun Protection Factor (SPF) of formulated creams containing 5% and 10% of melanin was determined with values ranging from 1.96 ± 0.008 to 26.33 ± 0.061; further, the transmission spectroscopy was used to calculate the percentage of protection factor that stipulates the potentiality of pigments showing sunscreen effect.
Melanin, Pseudomonas mosselli, Pseudomonas putida, Bacillus amyloliquefaciens, Bacillus subtilis, Bacillus cereus, SPF, Transmission Spectroscopy
Melanin is classified as polyphenol compounds, which exhibits brown, grey and black pigments in plants, microbes and animals. Foremost groups of melanin includes, Eumelanin that discerns as brown to black in color and this type of melanin is contemplated to be the conventional type that is extensively found in vertebrates and invertebrates. Pheomelanins are found in birds and mammals that are characterized as yellow or red in color. Allomelanins are chiefly found in seeds, fungi and spores. Melanin production has been contemplated in both Prokaryota and Eukaryota.1 Harmful effects are caused by the UV region. Skin that is detriment to elasticity and the collagen fibers of connective tissue is caused by exposure to UV A radiation that results in premature ageing (photo ageing).2 In contrast, acute inflammation (sunburn) and extreme sunburn are caused by exposure to UV B radiation. Before reaching the earth surface, radiation from UV C is conventionally filtered by the atmosphere; radiation from UV B is only partially filtered by the ozone layer. Skin cancers are mainly caused due to UV radiations.3
Melanin plays an essential role in photoprotection, acting as a physical barrier absorbent filter that prevents UV penetration into the epidermis. Early in the 20th century, sunscreen usage became widespread. Initially, sunscreen preparation was done with salicylates, which were reported for allergic, photoallergic reactions, contact dermatitis, severe anaphylactic reactions, photo-toxic, and contact urticaria. Therefore, there is a high demand for natural sunscreen that would be effective with less or no side effects. A regimen that comprehends effective sunscreen and clothing helps in photoprotection. Two major pathways that involves sunscreen activity are (i) absorption (ii) UV energy scattering and reflection.4 Sunscreen efficacy is calculated by Sun Protection Factor (SPF). UV energy gets explicated by SPF that is required in protecting the skin to produce the Minimal erythema dose (MED) divided by the UV energy imparted in unprotected skin to produce the same MED.5 It is assumed that the sunscreen activity of melanin ranges from 1.5 to 2.0 SPF to 4 SPF, indicating that melanin can absorb 50 to 75% of UV radiation.6 Enhancement of SPF study in melanin determines the efficacy in cosmetic applications. The purpose of the present study was to identify the best melanin producers, evaluate their photoprotective activity via in vitro SPF determination, and calculate their average UV-A and UV-B protection factor via transmission spectroscopy.
Chemicals and bacterial isolates
In this work, the chemicals L-tyrosine and fungal melanin (Mykotech, Goa) were used. Both are analytical reagent grade chemicals (Hi-media Laboratories Pvt Ltd, Mumbai, Maharastra, India). Soil was the main place where melanin producers were found. They were sorted out using the serial dilution method, and the colonies that made pigment were then grown in tyrosine broth.21 Biochemical analysis enabled the identification of the bacterial isolates, which was followed by 16S rDNA sequencing and analysis. The strains were sequenced and preserved at the National Center for Biotechnology Information (NCBI).7-9
Melanin extraction
Tyrosine basal broth was used as a production medium in the study.10 Primary inoculum, such as melanin-producing culture, was added to 50ml of production medium, which was kept in environmental shaker at 140rpm at 37˚± 2˚C for 180h. After which, the supernatant was acidified to pH 2 using 1N HCL. The purified melanin was extracted by adding acid, water, and ethanol, and then drying the mixture. 11
Determination of sun protection factor
Purified melanin was formulated with 5% and 10% cream by adding 0.5ml and 1ml of bacterial melanin as stock solution. To 10ml of ethanol, 10mg of stock was added and serially diluted to 1000 µg/ml, 500 µg/ml, 250 µg/ml, and 125 µg/ml concentrations as working stock. The absorption was determined from ranges 290nm – 320nm and ethanol was taken as blank. The data evaluated was equated in accordance with the Mansur equation.12
![]() SPF determination by transmission spectrum
SPF determination by transmission spectrum
A polyvinyl chloride (PVC) sheet strip was taken, and the formulated cream was spread on it as a thin film with concentrations of 5% and 10%. This was put inside a UV-Vis cuvette with the clear side facing out, and the transmission spectrum was measured from 290nm to 400nm using air as a standard. Further, UV A and UV B protection factors were elucidated with the following formulas.13
1) Determination of UV A or UVB blocking percentage. 100-T(UVA) or T(UVB) gives % blocking or % protection against the UVA or UVB.
100-T(UVA) or T(UVB) gives % blocking or % protection against the UVA or UVB.
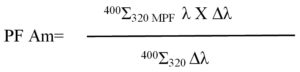
2) Evaluation of average UVA protection factor (PF)
 Statistical analysis
Statistical analysis
All experiments were repeated twice and are expressed as mean ± standard deviation. Microsoft Excel was used for statistical analysis.
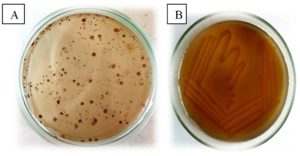
The isolates were treated with primary and secondary screening to check for melanin producers. Whereas with primary screening, the culture is streaked onto tyrosine agar plates (Figure 1) and with secondary screening, melanin producers are inoculated in tyrosine basal broth for its production (Figure 2). STGRDS1 and STGRDS3 were isolated from garden soil, STGRDV11 and STGRDV5 from vermicompost soil, and STGRDT12 was obtained from tomato-yielding soil as part of the preliminary screening for melanin producers in soil. Pigments with a clear zone on tyrosine agar plates were designated as melanin producers, and secondary screening was done to determine the ability of melanin producers to produce in tyrosine basal broth, which serves as the production medium. All the strains showed good production and were therefore selected for further application studies. The cultures were then taken up for molecular identification by 16s rDNA sequencing and the identity of the sequence was searched against the GenBank database using the NCBI BLAST tool and their accession numbers were obtained. STGRDS1 Pseudomonas mosselli (MN967075); STGRDS3 Pseudomonas putida (MT006089); STGRDV11 Bacillus amyloliquefaciens (MW629851); Bacillus subtilis (MW674644); and Bacillus cereus (MW674663) were identified as melanin producers by 16srDNA sequence analysis. Melanin pigment producing dark colonies in Pseudomonas sp were observed in tyrosine basal agar.14 Bacillus cereus melanin production was evaluated on nutrient broths, LB agar, nutrient agar, and T3 agar, which displayed a blackish-brown pigment throughout the medium.15 Compared to previous reports, it was discovered that by optimizing culture conditions, the production of melanin could be increased from a white medium to a dark brown medium upon completion of melanin production.16-18 The extracted melanin was purified and weighed as follows; S1 weighed 0.211g, S3 weighed 0.112g, V11 weighed 0.114g, V5 weighed 0.115g, T12 weighed 0.105g. This purified melanin was used for further application study.
Figure 1. Primary screening for melanin producing bacteria showing formation of colonies of very dark brown colour (a) and Melanin producing isolate streaked in tyrosine basal agar medium showing utilization of tyrosine (b)
The ability of the Sun Protection Factor (SPF) was checked with the cream formulated with different concentrations, such as 5% and 10%, by purifying bacterial melanin in accordance with the Mansur equation method. (Table 1) in the range of 290nm to 400nm. Melanin producers such as S1, S3, V11, V5 and T12 were compared to the standard melanin M1. Determination of SPF of all purified melanin such as S1, S3, V11, V5 and T12 has compared with standard M1, proving that all the melanin producers showed enhanced protection in both 5% and 10% formulations (Table 2). All the bacterial melanin samples used in the study were observed to enhance the SPF values of commercial creams, thereby providing more protection against harmful radiations such as UV radiation. Enhancement of SPF values was reported in Bacillus safensis, Cinnamomum burmannii and Osmanthus fragrans.19 It was previously reported that Dietzia schimae obtained SPF of about 20.22, Pseudomonas koreensis strain expressed 61.55.20,21 In another study, Sun protection factor of fungal melanin was compared with pure cream that showed 1.0 and melanin blended SPF showed 2.5, showing its photoprotection ability.22 SPF values of various concentrations such as 120µg/ml, 40 µg/ml and 60 µg/ml of water fraction, ethanol extract and n- butanol fraction of Chromolaena odarata leaves were evaluated, which showed SPF ranges of about 2 to 4 and with lesser concentrations, SPF values were lesser than 2.23 Various coffea such as Coffea Arabica, Canephora and Liberica were formulated and they were screened for Sun Protection activity which showed very good protection; 36.087 ± 0.0005; 35.007 ± 0.0005; 36,867 ± 0.0005 respectively.24 Melanin formulated in cream was further checked by transmission spectroscopy in vitro activity with UV – Vis spectrometer ranging from 290nm to 400nm to calculate the percentage of protection (Table 3). Formulated cream containing various concentrations of the leaves extract of Butea monosperma was determined through the transmission spectroscopy method, proving that with the increase in the concentration of extract, there is an increase in the protection from the UV radiation and average UV – A protection factor.25-27 The SPF classification table shows the ranges of protection (Table 4). In previous reports, synthetic skin was used to assess the sunscreen efficacy by employing the assessment of transmission spectroscopy, that showed reduced transmission spectrum with increasing concentrations after 2h of application.28 In a study, the correlation of absorbance and transmittance were used to evaluate the SPF of sunscreen and blockage of UV radiation and the results acclaimed that the SPF of various ranges from (15, 20, 24, 30, 50 and 60) revealed that the SPF and sunscreen absorption had a direct relationship.29 According to a study, the imaging of a sunscreen was evaluated by transmission spectroscopy within the emulsion that made it possible to move outside the spectral region of the visible light and with UV, image with different SPF ingredients within the formulations were done first time with optical microscopy.30 In recent study, an active ingredient melanin/TiO2 nanoparticles were used in formulating sunscreen that achieved SPF of about 116.9 and 162.4 with about 10 percentage of weight and 15 percentage of weight.31 Also, ethyl acetate of Padina boergesenii proved to be a great potential as a natural UV filter in a specific sunscreen formulation.32 The formulation of cream of Polycladia myrica with five percentage of ethyl acetate fraction expressed a high SPF of ranges 31.79 ± 4.73, UVA/PF (24.67 ± 4.03), critical wavelength (383.2 ± 0.1nm) and UVA and UVB ratio (0.98 ± 0.01) revealed that these extracted formulation proved to be valuable sun protective emulsion.33
Table (1):
Normalized Product Function Used in SPF Calculation.
Wavelength ((λ nm) |
EE(λ) x I(λ) (normalized) |
|---|---|
290 |
0.0150 |
295 |
0.0817 |
300 |
0.2874 |
305 |
0.3278 |
310 |
0.1864 |
315 |
0.0839 |
320 |
0.0180 |
Total |
1 |
Table (2):
Determination of Sun Protection Factor at various concentrations for 5% and 10% formulated cream of melanin
| Concentration (µg/ml) |
M1 |
S1 |
S3 |
V11 |
V5 |
T12 |
|
|---|---|---|---|---|---|---|---|
|
5% |
125 | 1.96 ± 0.008aA | 2.75 ± 0.047aA | 4.05 ± 0.038cA | 4.00 ± 0.051bA | 6.39 ± 0.482cA | 2.77±0.071bA |
| 250 | 3.76 ± 0.019bA | 3.65 ± 0.050bA | 7.23 ± 0.124cA | 7.12 ± 0.050bA | 7.89 ± 0.906dB | 3.64 ± 0.034aA | |
| 500 | 5.45 ± 0.225bA | 4.69 ± 0.043bA | 15.10±0.041bA | 15.02±0.016bA | 12.07±1.022bB | 4.02 ± 0.898aB | |
| 1000 | 7.70 ± 0.029bA | 5.85 ± 0.050bA | 21.75±0.050cA | 21.68±0.076bA | 14.25 ±1.165bC | 5.84 ± 0.034aC | |
|
10% |
125 | 3.69 ± 0.177aA | 7.52 ±0.041bA | 4.33 ± 0.041bA | 4.26 ± 0.060aA | 8.45 ± 0.066cA | 7.54 ± 0.059cA |
| 250 | 15.76±0.067cA | 10.58±0.048cA | 8.33 ± 0.054bA | 8.27 ± 0.041aA | 17.19 ±6.917dB | 10.57±0.034bA | |
| 500 | 17.10±0.048dA | 14.97±0.115aA | 17.05±0.112cA | 16.94±0.050bA | 23.79±10.409eC | 14.99±0.086bA | |
| 1000 | 19.10±0.053aA | 23.57±0.074aA | 26.06±0.067cA | 26.00 ± 0.055bA | 26.33 ± 0.061cD | 23.58 ± 0.095bA | |
Each value in the table is represented as mean ± SD (n=3).a,b,c,d,e Values in rows with different letters are significantly different at p≤ 0.005. Values in the same column within concentrations are followed by different letter (a-c) are significantly different at p≤ 0.005.
Table (3):
Determination of percent protection of formulated cream against UV rays analysis.
| Culture | Formulation | % protection against UV A | % protection against UV B | Average UV A protection factor | Average UV B protection factor |
|---|---|---|---|---|---|
| M1 |
5% |
84.55 ± 0.141A | 91.01 ± 0.162D | 15.45 | 8.99 |
| S1 | 54.52 ± 0.021A | 87.69 ± 0.014D | 45.48 | 12.31 | |
| S3 | 63.67 ± 0.029A | 90.17 ± 0.090D | 36.33 | 9.83 | |
| V11 | 62.29 ± 0.212A | 97.27 ± 0.101A | 37.71 | 2.73 | |
| V5 | 74.87 ±0.399A | 91.10 ± 0.029C | 25.13 | 8.9 | |
| T12 | 23.90 ± 0.062A | 92.06 ± 4.721B | 76.1 | 7.94 | |
| M1 |
10% |
87.94 ± 0.014A | 95.03 ± 0.028A | 12.06 | 4.97 |
| S1 | 82.98 ± 0.014A | 88.92 ± 0.035A | 17.02 | 11.08 | |
| S3 | 51.43 ± 1.158A | 86.57 ± 0.085A | 48.57 | 13.43 | |
| V11 | 53.62 ± 0.382A | 87.52 ±0.121A | 46.38 | 12.48 | |
| V5 | 92.18 ± 0.282A | 72.42 ± 0.101A | 7.82 | 27.58 | |
| T12 | 89.36 ± 0.116A | 79.15 ± 0.191A | 10.64 | 20.85 |
Each value in the table is represented as mean ± SD (n=3). Values in the same column within concentrations are followed by different letter (A,B,C,D) are significantly different at p≤ 0.005.
Table (4):
SPF classification table.
Protection level |
SPF value |
|---|---|
Low protection |
6, 10 |
Medium protection |
15, 20, 25 |
High protection |
30, 40 |
Very high protection |
50+ |
This study revealed the screening of melanin producers and sun protection factors by purified melanin producers. This study could be of use to provide information before beginning with in vivo studies. In the future, this potential melanin producers to develop higher SPF creams or lotions that could provide efficient photoprotective activity.
ACKNOWLEDGMENTS
The authors would like to thank Dr. G. R. Damodaran College of Science, Coimbatore, for all the means of research support throughout the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All data sets generated and analyzed during the study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Smith DG, Davis RJ, Rorick-Kehn L, et al. Melanin- concentrating hormone-1 receptor modulates neuroendocrine, behavioral, and corticolimbic neurochemical stress responses in Neuropsychopharmacology. 2006;1135-1145.
Crossref - Dutra EA, Kedor-Hackmann ERM, Santoro Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev. Bras Cienc Farm. 2004;40(3):381-385.
Crossref - Wilson BD, Moon S, Armstrong F. Comprehensive review of ultraviolet radiation and the current status on J Clin Aesthet Dermatol. 2012;5(9):18-23 PMCID: PMC3460660
- Shaath NA. Sunscreens (regulations and commercial development). Cosmetic Science and Technology 2005;17.
- Brenner M, Hearing The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84(3):539-549.
Crossref - Karadag A, Ozcelik B, Saner S. Review of methods to determine antioxidant Food Analytical Methods. 2009;2(1):41-60.
Crossref - Suryawanshi RK, Patil CD, Borase HP, et al. Towards an understanding of bacterial metabolites prodigiosin and violacein and their potential for use in commercial Int J Cosmet Sci. 2015;37(1):98-107.
Crossref - Sayre RM, Agin PP, LeVee GJ, Marlowe A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem Photobiol. 1979;29(3):559-566.
Crossref - Mansur JDS, Breder MNR, Mansur MCDA, Azulay Determinacao do fator de protecao solar porespectrofotometria. Ann Bras Dermatol. 1986;61(3):121-124.
- Yabuuchi E, Ohyama Characterization of “pyomelanin”-producing strains of Pseudomonas aeruginosa. Int J Syst Evol Microbiol. 1972;22(2):53-64.
Crossref - More BH, Sakharwade SN, Tembhurne SV, Sakarkar Evaluation of Sunscreen activity of Cream containing Leaves Extract of Butea monosperma for Topical application. International Journal of Research in Cosmetic Science. 2013;3(1):1-6.
- Drewnowska JM, Zambrzycka M, Kalska-Szostko B, Fiedoruk K, Swiecicka Melanin-like pigment synthesis by soil Bacillus weihenstephanensis isolates from Northeastern Poland. PloS one. 2015;10(4):E125428.
Crossref - Turick CE, Tisa LS, Caccavo Jr F. Melanin production and use as a soluble electron shuttle for Fe (III) oxide reduction and as a terminal electron acceptor by Shewanella algae Appl Environ Microbiol. 2002;68(5):2436-2444.
Crossref - Surwase SN, Jadhav SB, Phugare SS, Jadhav Optimization of melanin production by Brevundimonas sp. SGJ using response surface methodology. 3 Biotech. 2013; 3(3):187-194.
Crossref - Kiran GS, Dhasayan A, Lipton AN, Selvin J, Arasu MV, Al-Dhabi NA. Melanin-templated rapid synthesis of silver nanostructures. J Nanobiotechnol. 2014;12-18.
Crossref - Tarangini Korumilli and Sumitha Mishra. Production of Melanin by Soil Microbial Isolate on Fruit Waste Extract: Two Step Optimization of Key Parameters. Biotechnol 2014;4:39-146.
Crossref - Priyanka S, Inala MSR, Nandini HS, Kutty AVM, Kiranmayee A Pilot study on Sun Protection Factor of plant extracts: An Observational study. Asian J Pharm Clin Res. 2017;11(4).
Crossref - Kurian NK, Nair HP, Bhat Evaluation of anti- inflammatory property of melanin from marine Bacillus spp. BTCZ31. Evaluation. 2015;8(2):251-255.
- Ghadge V, Kumar P, Singh S, et al. Natural melanin produced by the endophytic Bacillus subtilis 4NP-BL Associated with the Halophyte Salicornia J Agric Food Chem. 2020;68(25):6854-6863.
Crossref - Eskandari S, Etemadifar Biocompatibility and radioprotection by newly characterized melanin pigment and its production from Dietzia schimae NM3 in optimized whey medium by response surface methodology. Ann Microbiol. 2021;71-17.
Crossref - Eskandari S, Etemadifar Melanin biopolymers from newly isolated Pseudomonas koreensis strain UIS 19 with potential for cosmetics application, and optimization on molasses waste medium. J Appl Microbiol. 2021;131(3):1331-1343.
Crossref - Oh J-J, Kim JY, Son SH, et al. Fungal melanin as a biocompatible broad-spectrum sunscreen with high antioxidant RSC Advances. 2021;11(32):19682- 19689.
Crossref - Tahir KA, Miskat UA, Djawad K, et Tyrosinase Enzymes Activities and Sun Protection Factor of Ethanol Extract, Water Fraction, and n-Butanol Fraction of Chromolaena odorata L. Leaves. Open Access Maced J Med Sci 2021:493-498.
Crossref - Latief M, Muhaimin, Heriyanti, Tarigan IL, Sutrisno. Determination Antioxidant Activity of Coffea Arabica, Coffea Canephora, Coffea Liberica and Sunscreens Cream Formulation for Sun Protection Factor (SPF). Pharmacognosy 2022;14(2):253-261.
Crossref - Lopusiewicz Isolation, characterisation and biological activity of melanin from Exidianigricans. World Scientific News. 2018;91:111-129.
- Korumilli T, Mishra Production, characterization and analysis of melanin from isolated marine Pseudomonas sp. using vegetable waste. Res J Eng Sci. 2013;9472.
- Reddy GSN, Prakash JSS, Matsumoto GI, Stackebrandt E, Shivaji, Arthrobacterroseus sp. nov., a psychrophilic bacterium isolated from an antarctic cyanobacterial mat sample. Int J Syst Evol Microbiol. 2002;52(3):1017- 1021.
Crossref - Al-Saeedi FMAA, Dahmash In Vitro Assessment of Sunscreen Efficacy Using Fourier Transform Infrared (FTIR) Spectroscopy on Synthetic Skin. AAPS Pharm Sci Tech. 2022;23(2):73.
Crossref - Baby N, Chakraborty Determination of Sun Protection Factor (SPF) for Various Sunscreens by UV Spectrophotometry. YMER. 2022;0044-0477.
- Crowther JM, Schutz R, Jurgen Vollhardt. Ultraviolet transmission microscopy for the imaging of topical sunscreen emulsions. Int J Cosmet Sci. 2022;44(6):663- 671.
Crossref - Li S, Wang Y, Li T, et Skin bioinspired anti-ultraviolet melanin/TiO 2 nanoparticles without penetration for efficient broad-spectrum sunscreen. Colloid and Polymer Science. 2021;299(11):1797-1805.
Crossref - Soleimani S, Yousefzadi M, Nezhad SBM, Pozharitskaya ON, Shikov Potential of the Ethyl Acetate Fraction of Padina boergesenii as a Natural UV Filter in Sunscreen Cream Formulation. Life. 2023;13(1):239.
Crossref - Soleimani S, Yousefzadi M, Nezhad SBM, Pozharitskaya Evaluation of fractions extracted from Polycladia myrica: Biological activities, UVR protective effect, and stability of cream formulation based on it. J Appl Phycol. 2022;34(3):1-15.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.