ISSN: 0973-7510
E-ISSN: 2581-690X
Research on the isolation and characterization of antibiotics-producing endophytic bacteria from Citrus aurantifolia leaves has been done. The isolation process was carried out by spreading spread plate isolation technique, screening of antibiotic-producing bacteria was done by disc dilution method and characterization of antibiotic-producing bacteria was done by microscopic, macroscopic and 16S rRNA analysis methods. The result of this research is four endophytic bacteria isolates that potentially produces antibiotic compounds. First, isolates with the CA01 code of fermentation fluids have inhibitory activity against Streptococcus mutans bacteria. Second, isolate with the code CA 02 able to inhibit growth of Vibrio chloreae bacteria. Third, isolates with the CA 03 code were able to inhibit bacterial growth of Salmonella thypii and E. faecalis. Fourth, isolate with CA 04 code has inhibitory activity on Salmonella thypii and Salmonella thyposa bacteria. The microscopic, macroscopic and 16S rRNA characterization for antibiotic-producing endophytic bacteria results were Bacillus cereus RNS_01 (code CA01), Pantoea agglomerans ZFJ-15 (Code CA 02), Bacillus subtilis 55C1-1 (Code CA 03) and Bacillus pumilus SH-B11 (Code CA 04).
Endophytic bacteria, Citrus aurantifolia, antibiotic, 16S rRNA
Antibiotics had been used since 1928 for disease preventive and treatment of infectious diseases. Nowadays, the use of antibiotics is increasing along with the increase of new infectious disease cases and resistance increase of some bacteria that cause the infection against the antibiotics used. Most antibiotics that are commercially used are synthetic antibiotics that are prone to trigger resistance to pathogens, especially bacteria1.2,3. This situation led to the need for exploration to find a source of new and more potent natural antibiotics. One of the natural source antibiotics that widely developed recently by various research institutions is the endophytic bacteria that live mutually in the host plant4,5.
Several types of endophytic bacteria that have been successfully isolated and are known to produces active compounds with antibiotic6, antimalaria7 and antifungal8,9 properties. The ability of endophytic bacteria to produce these active compounds is a potential that can be developed to replace the process of discovery of active compounds by extracting plants, especially medicinal plants10. One disadvantage of obtaining an active compound from the plant is it requires longer time and more complex process with smaller yield compared to extracting active compound from bacteria after a fermentation process.
One of the plants that has antibiotic potential is the Citrus aurantofolia plant. Natural content of Citrus aurantifolia plant that it has secondary metabolite in the form of essential oil. It was reported by many researchers that the essential oil of lime leaves has antibacterial and antifungal properties11,12. In society, lime leaves was used to treat skin diseases, sore throat and sprue. Some study also reported that lime leaves used as anti-inflammatory (anti-inflammatory) and mouthwash, because the leaf is known also contains essential oils13,14.
The essential oils antibacterial activity is caused by active compounds contained in essential oils which can inhibit or kill bacterial or fungal growth15,16. The compounds produced by the essential oil of lime leaves include limonene, b pinen, sabinene, (E) -b-Ocimene, a-pinen, myrcene, linalool, geranial, neral, citronellol, geranilacetate, nerilacetate, geraniol, and anti fungal nerol17. Given the potential of active compounds contained in Citrus aurantifolia plants, it was suspected that endophytic bacteria living in citrus plants can also produce the same active compounds or almost the same as those produced by the original plant. There is not much research has been done related to endophytic bacterial isolation on Citrus aurantifolia plant. So this research is done to obtained the endophytic bacteria isolate from Citrus aurantifolia plant which able to produces antibiotic compound, followed by bacterial characterization and antibacterial activity test.
Tools and Materials
Tools used in this research were petri dish, bunsen lights, mortar, stanfer, ose, beaker glass, erlenmeyer, test tube, vortex, autoclave, Laminar Air Flow cabinet, incubator cabinet. Material used were medium Nutrient Broth (NB), Nutrient Agar (NA), disc paper, NaCl 0.85% solution, alcohol, sodium hypochlorite, aquadest.
Sample Collection
Leaves of Citrus aurantifolia Swingle were taken from the fruiting plant, cut with sterile knife and then washed with sterile aquadest, then it was put in plastic bags and placed in refrigerator (cooled temperature ±10oC)18.
Sterilization of Surface from Plant Organs
Collected leaves was cut for approximately 1 cm2. Leaves cut then desinfected with etanol 70% for 1 minute, natrium hipoklorit 2% for 6 minutes, etanol 70% for 30 seconds to remove the natrium hipoklorit and then washed using sterile aquadest19.
Isolation, Purification and Gram Coloration of Endophytic Bacteria Isolates from Citrus aurantifolia Leaves
Sterile leaves was finely grounded using a sterile mortar and then inserted into a serial dilution of 0.85% NaCl solution and vortex. Each serial dilution was inoculated into TSA medium and benomil fungicide as many as 1 µL mL-1 was added with an antifungal spread plate method18. It was incubated at 270C for 1 to 3 x 24 hours. The colonies of grown bacteria was observed morphologically and purified. Pure bacteria isolates then pass through Gram staining19.
Characterization of Citrus aurantifolia Leaf Endophytic Bacteria Isolates using 16S rRNA
Characterization of bacterial isolate by 16S rRNA method was done at the Laboratory of Industrial Microbiology of LIPI Biotechnology Research Center, Bogor. The base order was checked and edited using the Bioedit Sequence Alignment Editor (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Equality analysis was done by using the Basic Local Alignment Tool at the National Center For Biotechnology Information (http://www.ncbi.nlm.nih.gov). While the evolution analysis was done by using ClustalW2 Phylogenetic Tree (www.ebi.ac.uk).
Antibiotic Activity Test from Fermentation Fluid of Endophytic Bacteria Isolate Citrus aurantifolia Leaf
The test was done towards test bacteria obtained from UAAC Bacteria Culture Center Biotechnology Laboratory, UPT Sumber Daya Hayati, Andalas University, Padang. Test bacteria that were used in this research are Streptococcus mutans ATCC 25175, Staphlococcus aureus ATCC 25923, Eschericia coli ATCC 25922, Enterobacter faecalis ATCC 29212, Micrococcus luteus ATCC 10240, Pseudomonas aeruginosa ATCC 27853, Bacillus subtilis ATCC 6633, Vibrio chloreae INABA, S.epidermidis ATCC 12228, Salmonella thypii, Salmonella thyposa NCTC 786 Salmonella thypimurium ATCC 14028 and Methicillin-Resistant Staphylococcus aureus (MRSA).
Each endophytic bacteria isolate was grown into Nutrient Broth medium for 24 hours at 270C with agitation of 120 rpm. Subsequently, it was centrifuged at 10,000 rpm for 15 min and the supernatant formed was tested for its activity using a diffusion-agar disc paper. Each of the test bacteria was planted into Nutrient Agar medium by spread plate method and disc paper which immersed with bacterial supernatant was placed, incubated at 270C for 48 hours. The inhibition zone formed indicates the formation of antibiotic compounds produced by endophytic bacteria after the fermentation process10.
After isolation and screening of endophytic bacteria from Citrus aurantifolia leaf, as much as seven bacterial colonies were detected to produce antibiotic compounds. The morphology of these seven bacteria turned out to be grouped into four colonies of different bacteria. Based on the results of characterization using Gram staining method and microscopic observation, it was shown that the four isolated bacteria were all in the form of bacillus, three bacteria from Gram positive group and one Gram negative (Table 1). The number of bacteria that grow in the medium is relatively small, this is due to environmental factors and the organ taken only leaves. It also known that the presence of endophytic bacteria in plants is influenced by environmental factors and plant organs3,19.
Table (1):
Macroscopic and microscopic results of antibiotic-producing endophytic bacteria isolate from Citrus aurantifolia leaves.
| No. | Isolate Code | Macroscopic observation of the colony | Microscopic observation of the colony | ||||
|---|---|---|---|---|---|---|---|
| Form | Color | Border | Elevasion | Gram | Cell form | ||
| 1. | CA 01 | Circular | White | Undulate | Convex | Positif | Bacil |
| 2. | CA 02 | Circular | White | Entire | Umbonate | Negative | Bacil |
| 3. | CA 03 | Circular | Cream | Entire | Convex | Positif | Bacil |
| 4. | CA 04 | Circular | Cream | Entire | Umbonate | Positif | Bacil |
The data in Table 1 shows that the macroscopic and microscopic characteristics of antibiotic-producing endophytic bacteria isolates from Citrus aurantifolia leaves are very diverse. This is shows that the presence of bacteria contained in leaf tissue is also relatively large and diverse as well. In Table 1, it was also shown that all isolated antibiotic-producing bacteria colony were in circular form, three endophytic bacterial isolates were Gram-positive i.e CA 01, CA 03, CA 04 whereas CA 03 Gram was negative with all bacil cell foem. It is known that theoretically the colony’s distinction from bacteria is a characteristic of a particular species20,21.
Table (2):
Profile of antibiotic activity of the bacterial fermentation of endophytic bacterial isolates from Citrus aurantifolia leaves.
| No | Isolate Code |
Antibiotic activity against bacaterial test | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. thypii |
S. thyposa NCTC 786 | S. thypimurium ATCC 14028 | E.coli ATCC 25922 | E.faecalis ATCC 29212 |
S.mutan ATCC 25175 | M.luteus ATCC 10240 | V.chloreae INABA | B.subtilis ATCC 6633 | MRSA | P. aeruginosa ATCC 27853 | S. epidermidis ATCC 12228 | S. aureus ATCC 25923 | ||
| 1. | CA 01 | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 2. | CA 02 | – | – | – | – | – | – | – | + | – | – | – | – | – |
| 3. | CA 03 | + | – | – | – | + | – | – | – | – | – | – | – | + |
| 4. | CA 04 | + | + | – | – | – | – | – | – | – | – | – | – | – |
Furthermore, Table 2 shows the results of antibiotic activity test from antibiotic-producing endophytic bacteria from Citrus aurantifolia leaf. It is demonstrated that endophytic bacterial isolate may inhibit the growth of some test bacteria. Inhibitory power of each isolate bacteria is different, as CA1 isolate bacteria positive to be able to inhibit Streptococcus mutans bacteria, CA 02 isolate bacteria can inhibit Vibrio chloreae bacteria, CA 03 can inhibit E. faecalis bacteria and CA 04 can inhibit Salmonella thypii and Salmonella thyposa bacteria. It is characterized by the activity of each isolate that have the same ability with its host plant. This ability is antibacterial, because the host plant have secondary metabolite compound that is essential oil which have ability as antibacterial. It has been reported by previous researchers that Citrus aurantifolia plant in addition to benefits for health, it also containing essential oil with antibacterial and antifungal properties12.
Table (3):
Result of endophytic bacteria 16S rRNA sequence analysis.
No. |
Isolate Code |
16S rRNA Sequence |
BLAST NCBI Result |
Homology (%) |
|---|---|---|---|---|
1. |
CA 01 |
AGAGCTTGCTCTTATGAAGTTAGCGGCGGACGGGTG AGTAACACGTGGGTAACCTGCCCATAAGACTGGGAT AACTCCGGGAAACCGGGGCTAATACCGGATAACAT TTTGAACCGCATGGTTCGAAATTGAAAGGCGGCT TCGGCTGTCACTTATGGATGGACCCGCGTCGCA TTAGCTAGTTGGTGAGGTAACGGCTCACCAAGG CAACGATGCGTAGCCGACCTGAGAGGGTGAT CGGCCACACTGGGACTGAGACACGGCCCAG ACTCCTACGGGAGGCAGCAGTAGGGAATCTTCCG CAATGGACGAAAGTCTGACGGAGCAACGCCGCG TGAGTGATGAAGGCTTTCGGGTCGTAAAACTCT GTTGTTAGGGAAGAACAAGTGCTAGTTGAATA AGCTGGCACCTTGACGGTACCTAACCAGAAAGCCACG GCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGTG GCAAGCGTTATCCGGAATTATTGGGCGTAAAGCGCGCG CAGGTGGTTTCTTAAGTCTGATGTGAAAGCCCACGGCTC AACCGTGGAGGGTCATTGGAAACTGGGAGACTTGAGTG CAGAAGAGGAAAGTGGAATTCCATGTGTAGCGGTGAAA TGCGTAGAGATATGGAGGAACACCAGGTGGCGAAGGCG ACTTTTCTGGTCTGTAACTGAACACTGAAGGCCGCGAAA GCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCC ACGCCGTAAACGATGAGTGCTAAGGGTTAGAGGGGTTT CCGCCCCTTAGTGCTGAAGTTAACGCATTAAGCACTCCC CCTGGGGAGTACGGCCCCAAGCCTGAAACCTCAAAGAA ATTGACCGGGGCCCCCAACAAGCGGGTGGAGCATGTG GTTTAATCCGAAGCAACGCGGAAGAACCTTACCCAG GTCTTGACATCCTCTGACAAACCCTAGAGGATAGGCC TCTCTCCTTGGGGACCAGAGGGCCAGGTGGGCCATG GTGGCGGTCACTCGTGGTGGTGAGATGTTGGGTTAA TTCCGGCACCGAGGCCAACCTTTGTCCTTAGTGCCCA CCATTAAGTTGGCCATCCTAAGGTGATGCCCGGGGC CAACCCGGAGGAAGGTGGGGAGGAGGTCAATTCTTC ATCCCCCTTATGCCTTGGCTTCCCCACGTCTTCCAAT GGACGGTCCAAAGAGCTCCAGGACCGGGAGGGGGA GTTATTTTCATAAACCCTTTTTCAGTTGGGATTTTGGG CTCCAATTGCCTTCCAGGAAGCGGGAATCCTTGGTAA TCGGGGATCACCAGGCCGCGTGGAATAGGTTCCCGG CCCTGTTCCCCCCCCCCGGCCCCACCCGGGGATTTG GTACCACCGGAAGTGGGTGGGGTACCTTTTTGGAGC CAGCCGCCTAAGG |
Bacillus cereus RNS_01 (KT380683.1) |
94 |
2. |
CA 02 |
GCAGTCGAGCGGAGTTGACGGAAAGCTTGCTTTC CTGATACTTAGCGGCGGACGGGTGAGTAACACG TAGGCAACCTGCCCTCAAGCTTGGGACAACTAC CGGAAACGGTAGCTAATACCGAATACTTGTTTT CTTCGCCTGAAGGAAACTGGAAAGACGGAGCAA TCTGTCACTTGGGGATGGGCCTGCGGCGCATTA GCTAGTTGGTGAGGTAACGGCTCACCAAGGCGA CGATGCGTAGCCGACCTGAGAGGGTGATCGGCC ACACTGGGACTGAGACACGGCCCAGACTCCTAC GGGAGGCAGCAGTAGGGAATCTTTCCGCAATGG GCGAAAGCCTGACGGAGCAATGCCGCGTGAGT GATGAAGGTTTTCGGATCGTAAAGCTCTGTTGCC AGGGAAGAACGCTTGGGAGAGTAACTGCTCCCA AGGTGACGGTACCTGAGAAGAAAGCCCCGGCTA ACTACGTGCCAGCAGCCGCGGTAATACGTAGGG GGCAAGCGTTGTCCGGAATTATTGGGCGTAAAG CGCGCGCAGGCGGTCATGTAAGTCTGGTGTTTA ATCCCGGGGCTCAACCCCGGATCGCACTGGAAA CTGCGTGACTTGAGTGCAGAAGAGGAGAGTGGA ATTCCACGTGTAGCGGTGAAATGCGTAGAGATG TGGAGGAACACCAGTGGCGAAGGCGACTCTCTG GGCTGTAACTGACGCTGAGGCGCGAAAGCGTG GGGAGCAAACAGGATTAGATACCCTGGTAGTCC ACGCCGTAAACGATGAATGCTAGGTGTTAGGGG TTTCGATACCCTTGGTGCCGAAGTTAACACATTA AGCATTCCGCCTGGGGAGTACGGTCGCAAGACT GAAACTCAAAGGAATTGACGGGGACCCGCACAA GCAGTGGAGTATGTGGTTTAATTCGAAGCAACG CGAAGAACCTTACCAGGTCTTGACATCCAACTAA CGAGGCAGAGATGCGTTAGGTGCCCTTCGGGG AAAGTTGAAACAGGTGGTGCATGGTTGTCGTCA GCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGC AACGAGCGCAACCCTTATATTTAGTTGCCAGCAT TTCGGATGGGCACTCTAAATAGACTGCCGGTGA CAAACCGGAGGAAGGTGGGGATGACGTCAAATC ATCATGCCCCTTATGACCTGGGCTACACACGTAC TACAATGGCCGGTACAACGGGCAGTGAAGCCGC GAGGTGGAACCAATCCTAAAAAGCCGGTCTCAG TTCGGATTGCAGGCTGCAACTCGCCTGCATGAA GTCGGAATTGCTAGTAATCGCGGATCAGCATGC CGCGGTGAATACGTTCCCGGGTCTTGTACACAC CGCCCGTCACACCACGAGAGTTTATAACACCCG AAGTCGGTGGGGTAACCGCAAGGAGCCAGCCG CCGAAGGTGGGATAGAT |
Pantoea agglomerans ZFJ-15 (EU931554.1) |
99 |
3. |
CA 03 |
GCAGTCGAGCGGACAGATGGGAGCTTGCTC CCTGATGTTAGCGGCGGACGGGTGAGTAAC ACGTGGGTAACCTGCCTGTAAGACTGGGAT AACTCCGGGAAACCGGGGCTAATACCGGAT GGTTGTTTGAACCGCATGGTTCAAACATAAA AGGTGGCTTCGGCTACCACTTACAGATGGAC CCGCGGCGCATTAGCTAGTTGGTGAGGTAA CGGCTCACCAAGGCAACGATGCGTAGCCGA CCTGAGAGGGTGATCGGCCACACTGGGACT GAGACACGGCCCAGACTCCTACGGGAGGCA GCAGTAGGGAATCTTCCGCAATGGACGAAA GTCTGACGGAGCAACGCCGCGTGAGTGATG AAGGTTTTCGGATCGTAAAAGCTCTGTTGTT TAGGGAAGAACCAAGTACCGTTCGGAATAG GGGCGGTACCTTGACCGGTACCTAACCCAG AAAGCCACGGGCTAACTACGTGCCAGCAGC CGCGGTAATACGTAGGTGGCAAGCGTTGTC CGGAAATTATTGGGCGTAAAGGGCTCGCAG GCGGTTTCTTAAGTCTGATGTGAAAGCCCCC GGCTCAACCGGGGAGGGTCATTGGAAACTG GGGAACTTGAGTGCAGAAGAGGAGAGTGGA ATTCCACGTGTAGCGGTGAAATGCGTAGAG ATGTGGAGGAACACCAGTGGCGAAGGCGAC TCTCTGGTCTGTAACTGACGCTGAGGAGCGA AAGCGTGGGGAGCGAACAGGATTAGATACC CTGGTAGTCCACGCCGTAAACGATGAGTGC TAAGTGTTAGGGGGTTTCCGCCCCTTAGTGC TGCAGCTAACGCATTAAGCACTCCGCCTGGG GAGTACGGTCGCAAGACTGAAACTCAAAGG AATTGACGGGGGCCCGCACAAGCGGTGGAG CATGTGGTTTAATTCGAAGCAACGCGAAGAA CCTTACCAGGTCTTGACATCCTCTGACAATC CTAGAGATAGGACGTCCCCTTCGGGGGCAG AGTGACAGGTGGTGCATGGTTGTCGTCAGC TCGTGTCGTGAGATGTTGGGTTAAGTCCCGC AACGAGCGCAACCCTTGATCTTAGTTGCCAG CATTCAGTTGGGCACTCTAAGGTGACTGCCG GTGACAAACCGGAGGAAGGTGGGGATGACG TCAAATCATCATGCCCCTTATGACCTGGGCT ACACACGTGCTACAATGGACAGAACAAAGG GCAGCGAAACCGCGAGGTTAAGCCAATCCC ACAAATCTGTTCTCAGTTCGGATCGCAGTCT GCAACTCGACTGCGTGAAGCTGGAATCGCTA GTAATCGCGGATCAGCATGCCGCGGTGAATA CGTTCCCGGGCCTTGTACACACCGCCCGTCA CACCACGAGAGTTTGTAACACCCGAAGTCGG TGAGGTAACCTTTTAGGAGCCAGCCGCCGAA GGTGGGACAGATGAT |
Bacilus subtilis 55C1-1 (JN366797.1) |
99 |
4. |
CA 04 |
GCTTGCTCCCGGATGTTAGCGGCGGAC GGGTGAGTAACACGTGGGTAACCTGCC TGTAAGACTGGGATAACTCCGGGAAACC GGAGCTAATACCGGATAGTTCCTTGAAC CGCATGGTTCAAGGATGAAAGACGGTTT CGGCTGTCACTTACAGATGGACCCGCGG CGCATTAGCTAGTTGGTGAGGTAACGGC TCACCAAGGCGACGATGCGTAGCCGACC TGAGAGGGTGATCGGCCACACTGGGACT GAGACACGGCCCAGACTCCTACGGGAGG CAGCAGTAGGGAATCTTCCGCAATGGAC GAAAGTCTGACGGAGCAACGCCGCGTGA GTGATGAAGGTTTTCGGATCGTAAAGCTC TGTTGTTAGGGAAGAACAAGTGCAAGAG TAACTGCTTGCACCTTGACGGTACCTAAC CAGAAAGCCACGGCTAACTACGTGCCAG CAGCCGCGGTAATACGTAGGTGGCAAG CGTTGTCCGGAATTATTGGGCGTAAAGG GCTCGCAGGCGGTTTCTTAAGTCTGATG TGAAAGCCCCCGGCTCAACCGGGGAGG GTCATTGGAAACTGGGAAACTTGAGTGC AGAAGAGGAGAGTGGAATTCCACGTGT AGCGGTGAAATGCGTAGAGATGTGGAG GAACACCAGTGGCGAAGGCGACTCTCT GGTCTGTAACTGACGCTGAGGAGCGAA AGCGTGGGGAGCGAACAGGATTAGATA CCCTGGTAGTCCACGCCGTAAACGATG AGTGCTAAGTGTTAGGGGGTTTCCGCC CCTTAGTGCTGCAGCTAACGCATTAAG CACTCCGCCTGGGGAGTACGGTCGCA AGACTGAAACTCAAAGGAATTGACGGG GGCCCGCACAAGCGGTGGAGCATGTG GTTTAATTCGAAGCAACGCGAAGAAC CTTACCAGGTCTTGACATCCTCTGACA ACCCTAGAGATAGGGCTTTCCCTTCG GGGACAGAATGACAGGTGGTGCATG GTTGTCGTCAGCTCCTGTCCTGAGAT GTTGGGTTAAGTCCCCCAACGAGCGC AACCCTTGATCTTAGTTGCCAGCATTC AGTTGGGCACTCTAAGGTGACTGCCG GTGACAAACCGGAGGAAGGTGGGGAT GACGTCAAATCATCATGCCCCTTATGAC CTGGGCTACACACGTGCTACAATGGAC AGAACAAAGGGCTGCGAGACCGCAAG GTTTAGCCAATCCCACAAATCTGTTCTC AGTTCGGATCGCAGTCTGCAACTCGAC TGCGTGAAGCTGGAATCGCTAGTAATC GCGGATCAGCATGCCGCGGTGAATACG TTCCCGGGCCTTGTACACACCGCCCGT CACACCACGAGAGTTTGCAACACCCGA AGTCGGTGAGGTAACCTTTATGGAGCC AGCCGCCGAAG |
Bacillus pumilus SH-B11 (CP010997.1) |
99 |
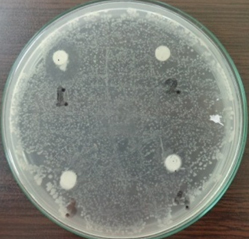
Figure 1 showed the ability of the inhibitory power of the endophytic bacteria isolate fermentation fluid to one of the test bacteria, Streptococcus mutans. The results of this experiment showed that each endophytic bacterial isolate produces antibiotic compounds that different capabilities towards the test bacteria used (Table 2). This allegedly caused by the differences in the active compound and molecular structure of the resulting antibiotic compounds. Concerning this condition, further research on the purification and determination of the molecular structure of each antibiotic compound produced is needed. In another study it was reported that the essential oils and antioxidant content of Citrus aurantifolia leaf with volatile oils concentrations of 20%, 10%, 5%, 1% was able to inhibited the growth of Staphylococcus aures, Bacillus subtilis, Enterobacter faecalis, Salmonella paratypii, E.coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Serratia marcescens23.
Fig. 1. Observation of endophytic bacterial fermentation fluid inhibitory zone towards Streptococcus mutans test bacteria
In Figure 2 it can be seen that endophytic bacterial isolates that have a large inhibitory ability was the CA 01 bacterial isolate that able to inhibit the Streptococcus mutans. This condition is shown by the clear zone formed. The clear zone was showed that the activity of CA 01 bacterial isolate has the content of bioactive compounds which is similar to the leaves of citrus aurantifolia. Ardani (2010) also mentioned that the essential oil contained in C. aurantifolia can inhibit the bacterium Streptococcus mutans for 0.06%22.
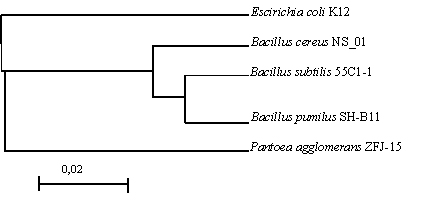
Fig. 2. Phylogenetic tree of 16S rRNA sequence of isolated antibiotic-producing endophytic bacterial in this research
The evolutionary analysis was performed using Clustal W2 Phylogenetic Tree (www.ebi.ac.uk). In this analysis, the outgroup used was Escherichia coli K12. The results showed that Bacillus subtilis 55-C1-1 usually has very close genetic relationship with Bacillus pumilus SH-B11 (Figure 3). From the results of Pairwise Sequence Alignment analysis both bacteria have the similarity of 93.9%. In this study, most of the bacteria obtained is from the Bacillus genera, this is because Bacillus existence is very abundant in the environment Citrus aurantifolia and Bacillus bacteria have the ability to penetrate into plant tissue23,24.
As much as four endophytic bacterial isolates that could potentially produce antibiotic compounds was obtained. First, isolates with CA01 code has fermentation liquids with inhibitory activity against Streptococcus mutans bacteria. Second, isolate with code CA02 was able to inhibit the growth of Vibrio chloreae bacteria. Third, isolate with the CA03 code was able to inhibit bacterial growth, Salmonella thypii and E. faecalis. Fourth, isolate with CA04 code has inhibitory activity on Salmonella thypii and Salmonella thyposa bacteria. The results of microscopic, macroscopic and 16S rRNA characterization, the four endophytic bacteria that potentially producing the antibiotic were Bacillus cereus RNS_01 (code CA01), Pantoea agglomerans ZFJ-15 (Code CA 02), Bacillus subtilis 55C1-1 (Code CA 03) and Bacillus pumilus SH-B11 (Code CA 04).
ACKNOWLEDGMENTS
The author would like to say special thanks to The Rector of Andalas University for his support this research under Professor Research Grant Project, Andalas University 2017, with Contract Number: 17/UN.16.17PP.HGB/LPPM/2017.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
- Ruhe JJ, Monson T, Bradsher, RW, Menon A. Use of Long-Acting Tetracyclines for Methicillin-Resistant Staphylococcus aureus Infections: Case Series and Review of the Literature. Clin. Inf. Dis. 2005; 40: 1429–1434.
- Kiyomizu K, Yagi T, Yoshida H, Minami R, Tanimura A, Karasuno T, Hiraoka A. Fuliminant septicemia of Bacillus cereus resistant to carbapenem in a patient with biphenotypic acute leukemia. J. Infect. Chemother. 2008; 14: 361–367.
- Rivai, H, Asia A, Rina W, Alen Y, Handayani D, Aldi Y, Marlina, and Djamaan A., Isolation of Endophytic Bacteria from Bark, Leaf, and Pericarp of Mangosteen (Garcinia mangostana L.) and Testing of The Antimicrobial Activity, Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2016; 7(1): 1910-1920
- Savini V, Catavitello C, Talia M, Febbo F, Balbinot A, Pompilio A, Et Al. Misidentification of Ampicillin-Sulbactam Heteroresistance In Acinetobacter baumannii Strains From ICU Patients. J. Infect, 2009; 58(4):316–7
- Djamaan, A, Mhd Riza Marjoni, and Friardi Ismed, The Influence of Pretreatment Time, Type and the Concentration of Yeast on Ethanol Production from Rice Straw. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2015, 6(3): 583-591.
- Castillo U, et al. Kakadumycins, novel antibiotics from Streptomyces sp.NRRL 30566, an endophyte of Grevillea pteridifolia. FEMS Microbiol. Lett. 2003; 224: 180-190.
- Simanjuntak. P., Bustanussalam., Otovina. D. M., Rahayuningsih. M and Said. E. G. Isolation and identification of artemisinin from endophytic microbial cultivation of the Artemisia annua plant. study of plant endophytic microbes Artemisia spp. Pharmaceutical Magazine Indonesian, 2004; 15 (2), 68- 74.
- Beck HC, Hansen AM, Lauritsen FR. Novel pyrazine metabolites found in polymyxin biosynthesis by Paenibacillus polymyxa. FEMS Microbiol Lett, 2003; 220: 67–73.
- Zam. S. I., Samsyuardi., A. Agustien., M. Jannah., Y. Aldi., A. Djamaan. Isolation, Characterization of Endophytic Bacteria from Citrus aurantifolia Swingle Leaves and Testing of Antifungal Activity Towards Fusarium oxysporum. J. Der Pharmachia Lettre, 2016; 8(11): 83-89.
- Alen Y, Nufika Y., Suharti N, Nakajima S, Djamaan A., The determination of profenofos insecticide residue cabbage (Brassica oleracea), Der Pharmacia Lettre, 2016; 8(8): 137-140. GC- MS analysis and antibacterial activity of cultivated Satureja cuneifolia Ten. Essential oil. Turk. J.Chem. 30: 253-259.
- Arias. B. A., Laca. L.R. Pharmacological Properties of Citrus And Their Ancient And Medieval Uses In The Mediterranean Region. Journal of Ethnopharmacology, 2005; 97: 89–95
- Chanthaphon, S., Chanthachum, S., & Hongpattarakere, T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. against food-related microorganisms. Songklanarin Journal of Science and Technology, 2008; 30(1): 125–131.
- Patil. JR., Chidambara. M. KN., Jayaprakasha. GK., Chetti. MB., Patil. BS. Bioactive Compounds From Mexican Lime (Citrus aurantifolia ) Juice Induce Apoptosis In Human Pancreatic Cells. J. Agricultural and Food Chemical Article, 2009; 57(22): 10933–10942.
- Nwauzoma. A. B., Dappa. M. S. Ethnobotanical Studies of Port Harcourt Metropolis, Nigeria. J. International Scholarly Research Notices. 2013.
- Kan, Y., U. S. Ucan, M. Kartal, M. L. Altun, S. Aslan, E. Sayar, and T. Ceyhan. GC-MS Analysis and Antibacterial Activity Of Cultivated Satureja Cuneifolia Ten Essential Oil. Turkish Journal of Chemistry, 2006; 30: 253- 259.
- Ngaisah, S. Identification and Test of Antibacterial Activity of Essential Oil of Red Betel Leaf (Piper crocatum Ruiz & Pav) Origin of Magelang, Skripsi, 2010; Faculty Of Math and Natural Science University of Sebelas Maret. Surakarta, p. 57.
- Lota, Marie-Laure, Serra, D. de R., Tomi, F. and Casanova, J. Chemical variability of peel and leaf essential oils of mandarins from Citrus reticulata Blanco Biochemical Systematics and Ecology, 2000; 28: 61-78.
- De Melo. , F. M. P., Fiore, M. F., de Morales, L. A. B., Silva-Stenico, M. E., Scramin, S., Teixeira, M. A., and de Melo, I. S. Antifungal compound produced by the cassava endophyte Bacillus pumilus MAIIIM4A. Sci. Agric, 2009; 66(5): 583-592.
- Araujo, W. L., Maccheroni, W. Jr., Aguilar-Vildoso, C. I., Barroso, P. A. V., Saridakis, H. O., and Azevedo, J. L. Can. J. Microbiol. 2001; 47: 229-236.
- Artasasta, M. A., Yanwirasti., Djamaan, A., Handayani, D. Cytotoxic activity screening of ethyl acetate fungal extracts derived from the marine sponge Neopetrosia chaliniformis AR-01. Journal of Applied Pharmaceutical Science, 2017; 7(12), pp. 174-178.
- Harley- Prescott. 2002. Laboratory Exercies in Microbiology, Fith Edition. The McGraw- Hill Companies, New York.
- Tamura, K., Stecher, K., Peterson, D., Filpski, A., dan Kumar, S. MEGA 6: Molecular Evolutionary Genetics Analysis Version 6.0. Journal Molecular Biology Evolution 2013; 30(12): 2725-2729 .
- Ibukun, A., Adenipekun, T., Adelowoton, T., Ogunsanya, T., Odugbemii, T. Evaluation Of The Antimicrobial Properties Of Different Parts Of Citrus aurantifolia (Lime Fruit) As Used Locally. Afr. J. Trad. CAM 2007; 4(2): 185 – 190.
- Ehigbai, I. O., Ehimwenma S., Omoregie., Faith E. Oviasogie., Kelly Oriakhi. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Journal Food Science & Nutrition; 2016; 4(1): 103–109.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.